Effects of gp120 Inner Domain (ID2) Immunogen Doses on Elicitation of Anti-HIV-1 Functional Fc-Effector Response to C1/C2 (Cluster A) Epitopes in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Immunogen Expression, Isolation and Preparation
2.2. Mice and Immunization Protocol
2.3. Immunogen Specific ELISA
2.4. RFADCC and ADCC
2.5. Cell Surface Staining
2.6. Competition ELISA
2.7. Statistics
3. Results
3.1. The Overall ID2 IgG Titer in Mice Sera Increases with ID2 Immunization Dose
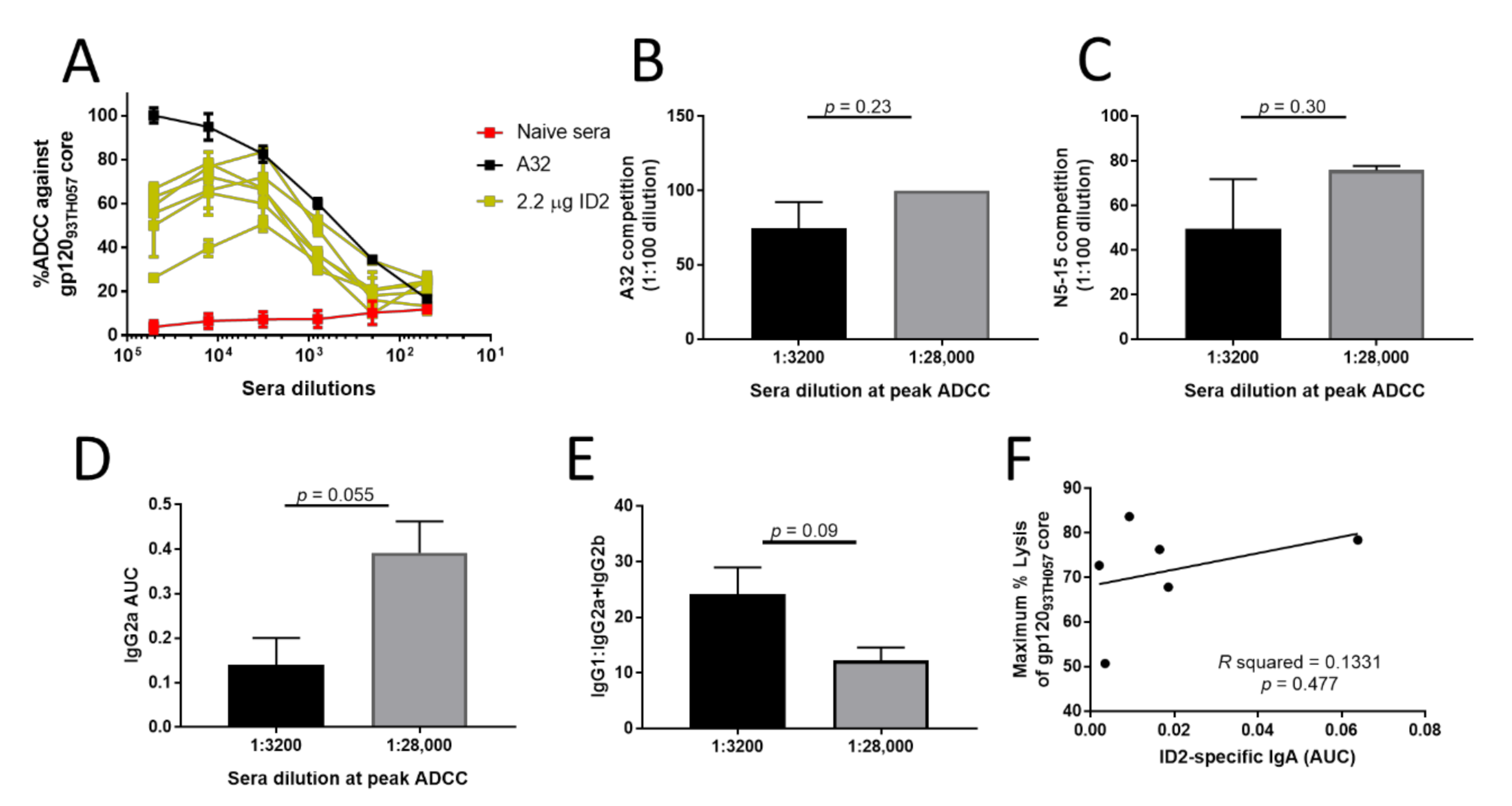
3.2. Higher ID2 Immunization dose Fails to Induce Sera Antibodies with Potent ADCC Activity
3.3. Higher ID2 Immunization dose Failed to Induce Sera Antibody Responses with Increased C1/C2 Epitope Specificity
3.4. Higher ID2 Immunization dose Induces IgG Isotypes with Lower Fc-Functionality
3.5. Isotype Specificity and Antibody Affinity in the 2.2 μg dosing Group Affects ADCC Potency
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
Disclosure
References
- Barbaro, G.; Iacobellis, G. Metabolic syndrome associated with HIV and highly active antiretroviral therapy. Curr. Diabetes Rep. 2009, 9, 37–42. [Google Scholar] [CrossRef]
- Delicio, A.M.; Lajos, G.J.; Amaral, E.; Cavichiolli, F.; Polydoro, M.; Milanez, H. Adverse effects in children exposed to maternal HIV and antiretroviral therapy during pregnancy in Brazil: A cohort study. Reprod. Health 2018, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Maddali, M.V.; Dowdy, D.W.; Gupta, A.; Shah, M. Economic and epidemiological impact of early antiretroviral therapy initiation in India. J. Int. AIDS Soc. 2015, 18, 20217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gresele, P.; Falcinelli, E.; Momi, S.; Francisci, D.; Baldelli, F. Highly active antiretroviral therapy-related mechanisms of endothelial and platelet function alterations. Rev. Cardiovasc. Med. 2014, 15 (Suppl. 1), S9–S20. [Google Scholar]
- Rolland, M. HIV-1 immune evasion—A threat to effective vaccines? Nat. Med. 2016, 22, 580–581. [Google Scholar] [CrossRef] [PubMed]
- Guha, D.; Ayyavoo, V. Innate Immune Evasion Strategies by Human Immunodeficiency Virus Type 1. ISRN AIDS 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moldt, B.; Rakasz, E.G.; Schultz, N.; Chan-Hui, P.-Y.; Swiderek, K.; Weisgrau, K.L.; Piaskowski, S.M.; Bergman, Z.; Watkins, D.I.; Poignard, P.; et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, 18921–18925. [Google Scholar] [CrossRef] [Green Version]
- Gautam, R.; Nishimura, Y.; Pegu, A.; Nason, M.C.; Klein, F.; Gazumyan, A.; Golijanin, J.; Buckler-White, A.; Sadjadpour, R.; Wang, K.; et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 2016, 533, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Barouch, D.H.; Whitney, J.B.; Moldt, B.; Klein, F.; Oliveira, T.Y.; Liu, J.; Stephenson, K.E.; Chang, H.-W.; Shekhar, K.; Gupta, S.; et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 2013, 503, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Landais, E.; Moore, P.L. Development of broadly neutralizing antibodies in HIV-1 infected elite neutralizers. Retrovirology 2018, 15, 61. [Google Scholar] [CrossRef] [Green Version]
- Su, B.; Dispinseri, S.; Iannone, V.; Zhang, T.; Wu, H.; Carapito, R.; Bahram, S.; Scarlatti, G.; Moog, C. Update on Fc-Mediated Antibody Functions Against HIV-1 Beyond Neutralization. Front. Immunol. 2019, 10, 2968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, J.P.; Sattentau, Q.J.; Yoshiyama, H.; Thali, M.; Charles, M.; Sullivan, N.; Poon, S.W.; Fung, M.S.; Traincard, F.; Pinkus, M. Probing the structure of the V2 domain of the human immunodeficiency virus type 1 surface glycoprotein gp120 with a panel of eight monoclonal antibodies: The human immune response to the V1 and V2 domains. J. Virol. 1993, 67, 6136–6151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, G.; Pollara, J.; Kozink, D.; Harms, T.; Drinker, M.; Freel, S.; Moody, M.A.; Alam, S.M.; Tomaras, G.D.; Ochsenbauer, C.; et al. An HIV-1 gp120 Envelope Human Monoclonal Antibody That Recognizes a C1 Conformational Epitope Mediates Potent Antibody-Dependent Cellular Cytotoxicity (ADCC) Activity and Defines a Common ADCC Epitope in Human HIV-1 Serum. J. Virol. 2011, 85, 7029–7036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veillette, M.; Coutu, M.; Richard, J.; Batraville, L.-A.; Dagher, O.; Bernard, N.; Tremblay, C.; Kaufmann, D.E.; Roger, M.; Finzi, A. The HIV-1 gp120 CD4-Bound Conformation Is Preferentially Targeted by Antibody-Dependent Cellular Cytotoxicity-Mediating Antibodies in Sera from HIV-1-Infected Individuals. J. Virol. 2014, 89, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Veillette, M.; Coutu, M.; Prévost, J.; Scharf, L.; Bjorkman, P.J.; Ferrari, G.; Robinson, J.E.; Stürzel, C.; Hahn, B.H.; et al. A Highly Conserved Residue of the HIV-1 gp120 Inner Domain Is Important for Antibody-Dependent Cellular Cytotoxicity Responses Mediated by Anti-cluster A Antibodies. J. Virol. 2015, 90, 2127–2134. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.P.; Prévost, J.; Baril, S.; Richard, J.; Medjahed, H.; Chapleau, J.-P.; Tolbert, W.D.; Kirk, S.; Smith, A.B.; Wines, B.D.; et al. Two Families of Env Antibodies Efficiently Engage Fc-Gamma Receptors and Eliminate HIV-1-Infected Cells. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [Green Version]
- Gohain, N.; Tolbert, W.D.; Acharya, P.; Yu, L.; Liu, T.; Zhao, P.; Orlandi, C.; Visciano, M.L.; Kamin-Lewis, R.; Sajadi, M.M.; et al. Cocrystal Structures of Antibody N60-i3 and Antibody JR4 in Complex with gp120 Define More Cluster A Epitopes Involved in Effective Antibody-Dependent Effector Function against HIV-1. J. Virol. 2015, 89, 8840–8854. [Google Scholar] [CrossRef] [Green Version]
- Acharya, P.; Tolbert, W.D.; Gohain, N.; Wu, X.; Yu, L.; Liu, T.; Huang, W.; Huang, C.-C.; Kwon, Y.D.; Louder, R.K.; et al. Structural Definition of an Antibody-Dependent Cellular Cytotoxicity Response Implicated in Reduced Risk for HIV-1 Infection. J. Virol. 2014, 88, 12895–12906. [Google Scholar] [CrossRef] [Green Version]
- Mengistu, M.; Ray, K.; Lewis, G.K.; DeVico, A.L. Antigenic Properties of the Human Immunodeficiency Virus Envelope Glycoprotein Gp120 on Virions Bound to Target Cells. PLoS Pathog. 2015, 11, e1004772. [Google Scholar] [CrossRef] [Green Version]
- Mengistu, M.; Tang, A.-H.; Foulke, J.S.; Blanpied, T.A.; Gonzalez, M.W.; Spouge, J.L.; Gallo, R.C.; Lewis, G.K.; DeVico, A.L. Patterns of conserved gp120 epitope presentation on attached HIV-1 virions. Proc. Natl. Acad. Sci. USA 2017, 114, E9893–E9902. [Google Scholar] [CrossRef] [Green Version]
- Ray, K.; Mengistu, M.; Yu, L.; Lewis, G.K.; Lakowicz, J.R.; DeVico, A.L. Antigenic Properties of the HIV Envelope on Virions in Solution. J. Virol. 2014, 88, 1795–1808. [Google Scholar] [PubMed] [Green Version]
- Lewis, G.K.; Guan, Y.; Kamin-Lewis, R.; Sajadi, M.; Pazgier, M.; DeVico, A.L. Epitope target structures of Fc-mediated effector function during HIV-1 acquisition. Curr. Opin. HIV AIDS 2014, 9, 263–270. [Google Scholar] [PubMed]
- Lewis, G.K.; Pazgier, M.; Evans, D.T.; Ferrari, G.; Bournazos, S.; Parsons, M.S.; Bernard, N.F.; Finzi, A. Beyond Viral Neutralization. AIDS Res. Hum. Retrovir. 2017, 33, 760–764. [Google Scholar] [PubMed]
- Pollara, J.; Bonsignori, M.; Moody, M.A.; Pazgier, M.; Haynes, B.F.; Ferrari, G. Epitope Specificity of Human Immunodeficiency Virus-1 Antibody Dependent Cellular Cytotoxicity [ADCC] Responses. Curr. HIV Res. 2013, 11, 378–387. [Google Scholar]
- Ding, S.; Gasser, R.; Gendron-Lepage, G.; Medjahed, H.; Tolbert, W.D.; Sodroski, J.; Pazgier, M.; Finzi, A. CD4 Incorporation into HIV-1 Viral Particles Exposes Envelope Epitopes Recognized by CD4-Induced Antibodies. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Veillette, M.; Désormeaux, A.; Medjahed, H.; Gharsallah, N.-E.; Coutu, M.; Baalwa, J.; Guan, Y.; Lewis, G.; Ferrari, G.; Hahn, B.H.; et al. Interaction with Cellular CD4 Exposes HIV-1 Envelope Epitopes Targeted by Antibody-Dependent Cell-Mediated Cytotoxicity. J. Virol. 2013, 88, 2633–2644. [Google Scholar]
- Veillette, M.; Richard, J.; Pazgier, M.; Lewis, G.K.; Parsons, M.S.; Finzi, A. Role of HIV-1 Envelope Glycoproteins Conformation and Accessory Proteins on ADCC Responses. Curr. HIV Res. 2016, 14, 9–23. [Google Scholar]
- Prévost, J.; Richard, J.; Medjahed, H.; Alexander, A.; Jones, J.; Kappes, J.C.; Ochsenbauer, C.; Finzi, A. Incomplete Downregulation of CD4 Expression Affects HIV-1 Env Conformation and Antibody-Dependent Cellular Cytotoxicity Responses. J. Virol. 2018, 92, e00484-18. [Google Scholar]
- Finnegan, C.M.; Berg, W.; Lewis, G.K.; DeVico, A.L. Antigenic properties of the human immunodeficiency virus envelope during cell-cell fusion. J. Virol. 2001, 75, 11096–11105. [Google Scholar]
- Finnegan, C.M.; Berg, W.; Lewis, G.K.; DeVico, A. Antigenic Properties of the Human Immunodeficiency Virus Transmembrane Glycoprotein during Cell-Cell Fusion. J. Virol. 2002, 76, 12123–12134. [Google Scholar]
- Guan, Y.; Pazgier, M.; Sajadi, M.M.; Kamin-Lewis, R.; Al-Darmarki, S.; Flinko, R.; Lovo, E.; Wu, X.; Robinson, J.E.; Seaman, M.S.; et al. Diverse specificity and effector function among human antibodies to HIV-1 envelope glycoprotein epitopes exposed by CD4 binding. Proc. Natl. Acad. Sci. USA 2012, 110, E69–E78. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.L.; Cortez, V.; Dingens, A.S.; Gach, J.S.; Rainwater, S.; Weis, J.F.; Chen, X.; Spearman, P.; Forthal, N.N.; Overbaugh, J. HIV-specific CD4-induced Antibodies Mediate Broad and Potent Antibody-dependent Cellular Cytotoxicity Activity and Are Commonly Detected in Plasma From HIV-infected humans. EBioMedicine 2015, 2, 1464–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupuy, F.P.; Kant, S.; Barbé, A.; Routy, J.-P.; Bruneau, J.; Lebouché, B.; Tremblay, C.; Pazgier, M.; Finzi, A.; Bernard, N.F. Antibody-Dependent Cellular Cytotoxicity-Competent Antibodies against HIV-1-Infected Cells in Plasma from HIV-Infected Subjects. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruel, T.; Guivel-Benhassine, F.; Lorin, V.; Lortat-Jacob, H.; Baleux, F.; Bourdic, K.; Noël, N.; Lambotte, O.; Mouquet, H.; Schwartz, O. Lack of ADCC Breadth of Human Nonneutralizing Anti-HIV-1 Antibodies. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, J.; Prévost, J.; Baxter, A.E.; Von Bredow, B.; Ding, S.; Medjahed, H.; Delgado, G.G.; Brassard, N.; Stürzel, C.M.; Kirchhoff, F.; et al. Uninfected Bystander Cells Impact the Measurement of HIV-Specific Antibody-Dependent Cellular Cytotoxicity Responses. mBio 2018, 9, e00358-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milligan, C.; Richardson, B.A.; John-Stewart, G.; Nduati, R.; Overbaugh, J. Passively acquired antibody-dependent cellular cytotoxicity (ADCC) activity in HIV-infected infants is associated with reduced mortality. Cell Host Microbe 2015, 17, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Naiman, N.E.; Slyker, J.; Richardson, B.A.; John-Stewart, G.; Nduati, R.; Overbaugh, J. Antibody-dependent cellular cytotoxicity targeting CD4-inducible epitopes predicts mortality in HIV-infected infants. EBioMedicine 2019, 47, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Von Bredow, B.; Arias, J.F.; Heyer, L.N.; Moldt, B.; Le, K.; Robinson, J.E.; Zolla-Pazner, S.; Burton, D.R.; Evans, D.T. Comparison of Antibody-Dependent Cell-Mediated Cytotoxicity and Virus Neutralization by HIV-1 Env-Specific Monoclonal Antibodies. J. Virol. 2016, 90, 6127–6139. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Gajjar, M.R.; Yu, J.; Padte, N.N.; Gettie, A.; Blanchard, J.L.; Russell-Lodrigue, K.E.; Liao, L.E.; Perelson, A.S.; Huang, Y.; et al. Quantifying the contribution of Fc-mediated effector functions to the antiviral activity of anti–HIV-1 IgG1 antibodies in vivo. Proc. Natl. Acad. Sci. USA 2020, 117, 18002–18009. [Google Scholar]
- Yates, N.L.; Liao, H.-X.; Fong, Y.; DeCamp, A.; Vandergrift, N.A.; Williams, W.T.; Alam, S.M.; Ferrari, G.; Yang, Z.-Y.; Seaton, K.E.; et al. Vaccine-Induced Env V1-V2 IgG3 Correlates with Lower HIV-1 Infection Risk and Declines Soon After Vaccination. Sci. Transl. Med. 2014, 6, 228ra39. [Google Scholar] [CrossRef] [Green Version]
- Chung, A.W.; Ghebremichael, M.; Robinson, H.; Brown, E.; Choi, I.; Lane, S.; Dugast, A.-S.; Schoen, M.K.; Rolland, M.; Suscovich, T.J.; et al. Polyfunctional fc-effector profiles mediated by igg subclass selection distinguish rv144 and vax003 vaccines. Sci. Transl. Med. 2014, 6, 228ra38. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.F.; Gilbert, P.B.; McElrath, M.J.; Zolla-Pazner, S.; Tomaras, G.D.; Alam, S.M.; Evans, D.T.; Montefiori, D.C.; Karnasuta, C.; Sutthent, R.; et al. Immune-correlates analysis of an hiv-1 vaccine efficacy trial. N. Engl. J. Med. 2012, 366, 1275–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonsignori, M.; Pollara, J.; Moody, M.A.; Alpert, M.D.; Chen, X.; Hwang, K.-K.; Gilbert, P.B.; Huang, Y.; Gurley, T.C.; Kozink, D.M.; et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an hiv-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J. Virol. 2012, 86, 11521–11532. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.-X.; Bonsignori, M.; Alam, S.M.; McLellan, J.S.; Tomaras, G.D.; Moody, M.A.; Kozink, D.M.; Hwang, K.-K.; Chen, X.; Tsao, C.-Y.; et al. Vaccine Induction of Antibodies against a Structurally Heterogeneous Site of Immune Pressure within HIV-1 Envelope Protein Variable Regions 1 and 2. Immunity 2013, 38, 176–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Yates, N.L.; Shen, X.; Bonsignori, M.; Moody, M.A.; Liao, H.-X.; Fong, Y.; Alam, S.M.; Overman, R.G.; Denny, T.N.; et al. Infectious Virion Capture by HIV-1 gp120-Specific IgG from RV144 Vaccinees. J. Virol. 2013, 87, 7828–7836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollara, J.; Bonsignori, M.; Moody, M.A.; Liu, P.; Alam, S.M.; Hwang, K.-K.; Gurley, T.C.; Kozink, D.M.; Armand, L.C.; Marshall, D.J.; et al. HIV-1 Vaccine-Induced C1 and V2 Env-Specific Antibodies Synergize for Increased Antiviral Activities. J. Virol. 2014, 88, 7715–7726. [Google Scholar] [CrossRef] [Green Version]
- Prévost, J.; Zoubchenok, D.; Richard, J.; Veillette, M.; Pacheco, B.; Coutu, M.; Brassard, N.; Parsons, M.S.; Ruxrungtham, K.; Bunupuradah, T.; et al. Influence of the Envelope gp120 Phe 43 Cavity on HIV-1 Sensitivity to Antibody-Dependent Cell-Mediated Cytotoxicity Responses. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [Green Version]
- Easterhoff, D.; Pollara, J.; Luo, K.; Tolbert, W.D.; Young, B.; Mielke, D.; Jha, S.; O’Connell, R.J.; Vasan, S.; Kim, J.; et al. Boosting with ALVAC-HIV and AIDSVAX B/E enhances Env constant region 1 and 2 antibody-dependent cellular cytotoxicity. bioRxiv 2019, 632844. [Google Scholar] [CrossRef]
- Akapirat, S.; Karnasuta, C.; Vasan, S.; Rerks-Ngarm, S.; Pitisuttithum, P.; Madnote, S.; Savadsuk, H.; Rittiroongrad, S.; Puangkaew, J.; Phogat, S.; et al. Characterization of HIV-1 gp120 antibody specificities induced in anogenital secretions of RV144 vaccine recipients after late boost immunizations. PLoS ONE 2018, 13, e0196397. [Google Scholar] [CrossRef] [Green Version]
- Tolbert, W.D.; Van, V.; Sherburn, R.; Tuyishime, M.; Yan, F.; Nguyen, D.N.; Stanfield-Oakley, S.; Easterhoff, D.; Bonsignori, M.; Haynes, B.F. Recognition Patterns of the C1/C2 Epitopes Involved in Fc-Mediated Response in HIV-1 Natural Infection and the RV114 Vaccine Trial. mBio 2020, 11. [Google Scholar] [CrossRef]
- Tolbert, W.D.; Gohain, N.; Veillette, M.; Chapleau, J.-P.; Orlandi, C.; Visciano, M.L.; Ebadi, M.; DeVico, A.L.; Fouts, T.; Finzi, A.; et al. Paring Down HIV Env: Design and Crystal Structure of a Stabilized Inner Domain of HIV-1 gp120 Displaying a Major ADCC Target of the A32 Region. Structure 2016, 24, 697–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visciano, M.L.; Gohain, N.; Sherburn, R.; Orlandi, C.; Flinko, R.; Dashti, A.; Lewis, G.K.; Tolbert, W.D.; Pazgier, M. Induction of Fc-Mediated Effector Functions Against a Stabilized Inner Domain of HIV-1 gp120 Designed to Selectively Harbor the A32 Epitope Region. Front. Immunol. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolbert, W.D.; Sherburn, R.; Van, V.; Pazgier, M. Structural Basis for Epitopes in the gp120 Cluster A Region that Invokes Potent Effector Cell Activity. Viruses 2019, 11, 69. [Google Scholar] [CrossRef] [Green Version]
- Richard, J.; Veillette, M.; Batraville, L.-A.; Coutu, M.; Chapleau, J.-P.; Bonsignori, M.; Bernard, N.; Tremblay, C.; Roger, M.; Kaufmann, D.E.; et al. Flow cytometry-based assay to study HIV-1 gp120 specific antibody-dependent cellular cytotoxicity responses. J. Virol. Methods 2014, 208, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin-Bussières, G.; Prévost, J.; Gendron-Lepage, G.; Melillo, B.; Chen, J.; Iii, A.B.S.; Pazgier, M.; Finzi, A. Elicitation of Cluster A and Co-Receptor Binding Site Antibodies Are Required to Eliminate HIV-1 Infected Cells. Microorganisms 2020, 8, 710. [Google Scholar] [CrossRef]
- Orlandi, C.; Flinko, R.; Lewis, G.K. A new cell line for high throughput HIV-specific antibody-dependent cellular cytotoxicity (ADCC) and cell-to-cell virus transmission studies. J. Immunol. Methods 2016, 433, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Alsahafi, N.; Bakouche, N.; Kazemi, M.; Richard, J.; Ding, S.; Bhattacharyya, S.; Das, D.; Anand, S.P.; Prévost, J.; Tolbert, W.D.; et al. An Asymmetric Opening of HIV-1 Envelope Mediates Antibody-Dependent Cellular Cytotoxicity. Cell Host Microbe 2019, 25, 578–587. [Google Scholar] [CrossRef] [Green Version]
- Prévost, J.; Richard, J.; Ding, S.; Pacheco, B.; Charlebois, R.; Hahn, B.H.; Kaufmann, D.E.; Finzi, A. Envelope glycoproteins sampling states 2/3 are susceptible to ADCC by sera from HIV-1-infected individuals. Virology 2018, 515, 38–45. [Google Scholar] [CrossRef]
- Tolbert, W.D.; Gohain, N.; Alsahafi, N.; Van, V.; Orlandi, C.; Ding, S.; Martin, L.; Finzi, A.; Lewis, G.K.; Ray, K.; et al. Targeting the Late Stage of HIV-1 Entry for Antibody-Dependent Cellular Cytotoxicity: Structural Basis for Env Epitopes in the C11 Region. Structure 2017, 25, 1719–1731.e4. [Google Scholar] [CrossRef] [Green Version]
- Prechl, J. A generalized quantitative antibody homeostasis model: Maintenance of global antibody equilibrium by effector functions. Clin. Transl. Immunol. 2017, 6, e161. [Google Scholar] [CrossRef] [Green Version]
- Andersson, B. Studies on the regulation of avidity at the level of the single antibody-forming cell. The effect of antigen dose and time after immunization. J. Exp. Med. 1970, 132, 77–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; De Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Tomaras, G.D.; Ferrari, G.; Shen, X.; Alam, S.M.; Liao, H.-X.; Pollara, J.; Bonsignori, M.; Moody, M.A.; Fong, Y.; Chen, X.; et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc. Natl. Acad. Sci. USA 2013, 110, 9019–9024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, J.E.; Elliott, D.H.; Martin, E.A.; Micken, K.; Rosenberg, E.S. High frequencies of antibody responses to CD4 induced epitopes in HIV infected patients started on HAART during acute infection. Hum. Antibodies 2005, 14, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Fouda, G.G.; Yates, N.L.; Pollara, J.; Shen, X.; Overman, G.R.; Mahlokozera, T.; Wilks, A.B.; Kang, H.H.; Salazar-Gonzalez, J.F.; Salazar, M.G.; et al. HIV-Specific Functional Antibody Responses in Breast Milk Mirror Those in Plasma and Are Primarily Mediated by IgG Antibodies. J. Virol. 2011, 85, 9555–9567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ampol, S.; Pattanapanyasat, K.; Sutthent, R.; Permpikul, P.; Kantakamalakul, W. Comprehensive Investigation of Common Antibody-Dependent Cell-Mediated Cytotoxicity Antibody Epitopes of HIV-1 CRF01_AE gp120. AIDS Res. Hum. Retroviruses 2012, 28, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.W.; Isitman, G.; Navis, M.; Kramski, M.; Center, R.J.; Kent, S.J.; Stratov, I. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc. Natl. Acad. Sci. USA 2011, 108, 7505–7510. [Google Scholar] [CrossRef] [Green Version]
- Burton, D.R.; Hessell, A.J.; Keele, B.F.; Klasse, P.J.; Ketas, T.A.; Moldt, B.; Dunlop, D.C.; Poignard, P.; Doyle, L.A.; Cavacini, L.; et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc. Natl. Acad. Sci. USA 2011, 108, 11181–11186. [Google Scholar] [CrossRef] [Green Version]
- Moog, C.; Dereuddre-Bosquet, N.; Teillaud, J.-L.; Biedma, M.E.; Holl, V.; Van Ham, G.; Heyndrickx, L.; Van Dorsselaer, A.; Katinger, D.; Vcelar, B.; et al. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol. 2013, 7, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Santra, S.; Tomaras, G.D.; Warrier, R.; Nicely, N.I.; Liao, H.-X.; Pollara, J.; Liu, P.; Alam, S.M.; Zhang, R.; Cocklin, S.L.; et al. Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques. PLoS Pathog. 2015, 11, e1005042. [Google Scholar] [CrossRef]
- Van Erp, E.A.; Luytjes, W.; Ferwerda, G.; Van Kasteren, P.B. Fc-Mediated Antibody Effector Functions During Respiratory Syncytial Virus Infection and Disease. Front. Immunol. 2019, 10, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Ferrari, G.; Alter, G.; Forthal, D.N.; Kappes, J.C.; Lewis, G.K.; Love, J.C.; Borate, B.; Harris, L.; Greene, K.; et al. Diversity of Antiviral IgG Effector Activities Observed in HIV-Infected and Vaccinated Subjects. J. Immunol. 2016, 197, 4603–4612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, M.Z.; Wiehe, K.; Pollara, J. Antibody-Dependent Cellular Phagocytosis in Antiviral Immune Responses. Front. Immunol. 2019, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.; Hammond, S.A.; Oberst, M.; Wilkinson, R.W. The role of Fc gamma receptors in the activity of immunomodulatory antibodies for cancer. J. Immunother. Cancer 2014, 2, 29. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, Y.; Lubeck, M.D.; Steplewski, Z.; Koprowski, H. Induction of mouse IgG2a- and IgG3-dependent cellular cytotoxicity in human monocytic cells (U937) by immune interferon. Cancer Res. 1984, 44, 5127–5131. [Google Scholar] [PubMed]
- Wong, J.; Layton, D.; Wheatley, A.K.; Kent, S.J. Improving immunological insights into the ferret model of human viral infectious disease. Influ. Other Respir. Viruses 2019, 13, 535–546. [Google Scholar] [CrossRef]
- Kramski, M.; Schorcht, A.; Johnston, A.P.R.; Lichtfuss, G.; Jegaskanda, S.; De Rose, R.; Stratov, I.; Kelleher, A.; French, M.A.; Center, R.; et al. Role of monocytes in mediating HIV-specific antibody-dependent cellular cytotoxicity. J. Immunol. Methods 2012, 384, 51–61. [Google Scholar] [CrossRef]
- Tolbert, W.D.; Subedi, G.P.; Gohain, N.; Lewis, G.K.; Patel, K.R.; Barb, A.W.; Pazgier, M. From Rhesus macaque to human: Structural evolutionary pathways for immunoglobulin G subclasses. mAbs 2019, 11, 709–724. [Google Scholar] [CrossRef]
- Chan, Y.N.; Boesch, A.W.; Osei-Owusu, N.Y.; Emileh, A.; Crowley, A.R.; Cocklin, S.L.; Finstad, S.L.; Linde, C.H.; Howell, R.A.; Zentner, I.; et al. IgG Binding Characteristics of Rhesus Macaque FcgammaR. J. Immunol. 2016, 197, 2936–2947. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherburn, R.; Tolbert, W.D.; Gottumukkala, S.; Beaudoin-Bussières, G.; Finzi, A.; Pazgier, M. Effects of gp120 Inner Domain (ID2) Immunogen Doses on Elicitation of Anti-HIV-1 Functional Fc-Effector Response to C1/C2 (Cluster A) Epitopes in Mice. Microorganisms 2020, 8, 1490. https://doi.org/10.3390/microorganisms8101490
Sherburn R, Tolbert WD, Gottumukkala S, Beaudoin-Bussières G, Finzi A, Pazgier M. Effects of gp120 Inner Domain (ID2) Immunogen Doses on Elicitation of Anti-HIV-1 Functional Fc-Effector Response to C1/C2 (Cluster A) Epitopes in Mice. Microorganisms. 2020; 8(10):1490. https://doi.org/10.3390/microorganisms8101490
Chicago/Turabian StyleSherburn, Rebekah, William D. Tolbert, Suneetha Gottumukkala, Guillaume Beaudoin-Bussières, Andrés Finzi, and Marzena Pazgier. 2020. "Effects of gp120 Inner Domain (ID2) Immunogen Doses on Elicitation of Anti-HIV-1 Functional Fc-Effector Response to C1/C2 (Cluster A) Epitopes in Mice" Microorganisms 8, no. 10: 1490. https://doi.org/10.3390/microorganisms8101490
APA StyleSherburn, R., Tolbert, W. D., Gottumukkala, S., Beaudoin-Bussières, G., Finzi, A., & Pazgier, M. (2020). Effects of gp120 Inner Domain (ID2) Immunogen Doses on Elicitation of Anti-HIV-1 Functional Fc-Effector Response to C1/C2 (Cluster A) Epitopes in Mice. Microorganisms, 8(10), 1490. https://doi.org/10.3390/microorganisms8101490




