HIV-1 Entry and Prospects for Protecting against Infection
Abstract
:1. Introduction
2. Viral and Host Factors Modulating HIV-1 Entry
2.1. Viral Factors Modulating HIV-1 Mucosal Transmission and Infection
2.1.1. Phenotypes of Transmitted Founder Viruses
2.1.2. Identification of HIV-1 Target to Inhibit Viral Cell Binding and Entry
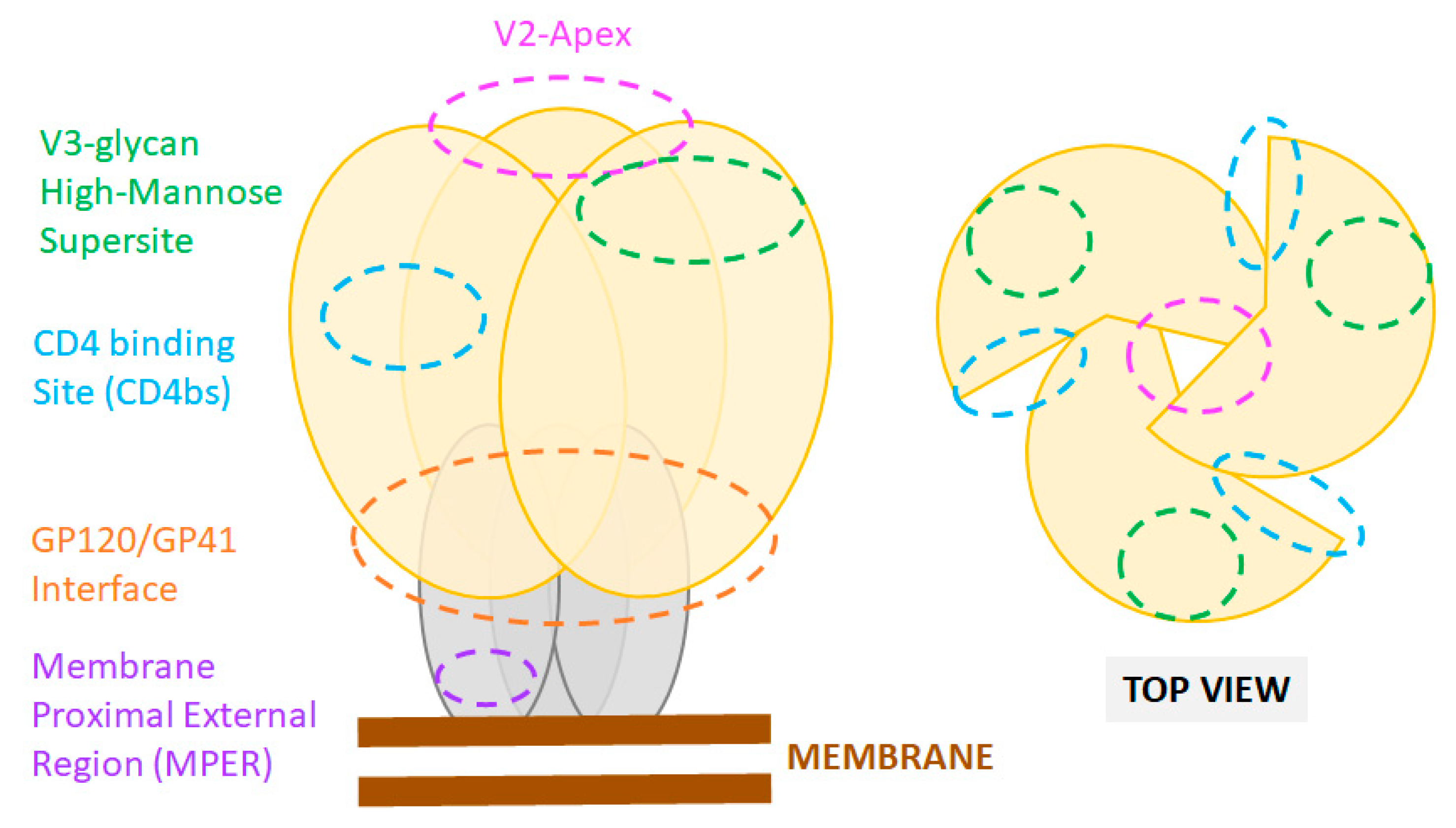
2.2. Host Factors Modulating HIV-1 Mucosal Transmission and Infection
2.2.1. Early Cells Targeted by HIV-1 in the Mucosa
2.2.2. Factors Associated with Inflammation
2.2.3. Mucosal Correlates of Protection
3. Early Prevention of HIV-1 Infection: Passive and Active Immunization Targeting HIV Env-Mediated Cell Entry
3.1. Neutralizing Antibodies: Epitopes and Functions
3.2. Targeting the Transmitted Founder Virus
3.3. Systemic and Mucosal Antibody-Mediated Protection agaisnt HIV-1
3.3.1. Mucosal Protection by Passive Immunization
3.3.2. Mucosal Protection Induced by Active Immunization
4. Treatment of HIV-1 Infection with Entry Inhibitors
4.1. Gp120 Inhibitors
4.2. Gp41-Inhibitors
4.3. CD4-Modulators
4.4. Coreceptor Inhibitors
4.4.1. CCR5
4.4.2. CXCR4-Inhibitors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohn, L.B.; Chomont, N.; Deeks, S.G. The Biology of the HIV-1 Latent Reservoir and Implications for Cure Strategies. Cell Host Microbe 2020, 27, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Scully, E.P.; Weld, E.D.; Blankson, J.N. Challenges in optimizing preexposure prophylaxis development, engagement, and access for HIV prevention. J. Clin. Investig. 2019, 129, 5071–5073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prator, C.A.; Donatelli, J.; Henrich, T.J. From Berlin to London: HIV-1 Reservoir Reduction Following Stem Cell Transplantation. Curr. HIV/AIDS Rep. 2020, 17, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.M.; Aguilar-Jimenez, W.; Su, R.-C.; Rugeles, M.T. Mucosa: Key Interactions Determining Sexual Transmission of the HIV Infection. Front. Immunol. 2019, 10, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muldoon, K.A.; King, R.; Zhang, W.; Birungi, J.; Nanfuka, M.; Tibengana, S.; Afolabi, O.; Moore, D.M. Sexual Health Consequences of Forced Sexual Debut Among Ugandan Women in HIV Serodiscordant Partnerships: Results from the HAARP Study. J. Interpers. Violence 2018, 33, 1731–1747. [Google Scholar] [CrossRef] [PubMed]
- Mwinnyaa, G.; Gray, R.H.; Grabowski, M.K.; Ssekasanvu, J.; Ndyanabo, A.; Ssekubugu, R.; Kagaayi, J.; Kigozi, G.; Nakigozi, G.; Serwadda, D.M.; et al. Age-Disparate Relationships and HIV Prevalence among Never Married Women in Rakai, Uganda. J. Acquir. Immune Defic. Syndr. 2018, 79, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Adimora, A.A.; Hughes, J.P.; Wang, J.; Haley, D.F.; Golin, C.E.; Magnus, M.; Rompalo, A.; Justman, J.; Del Rio, C.; El-Sadr, W.; et al. Characteristics of Multiple and Concurrent Partnerships Among Women at High Risk for HIV Infection. JAIDS J. Acquir. Immune Defic. Syndr. 2014, 65, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Fried, S.; Harrison, B.; Starcevich, K.; Whitaker, C.; O’Konek, T. Integrating interventions on maternal mortality and morbidity and HIV: A human rights-based framework and approach. Health Hum. Rights 2012, 14, 14. [Google Scholar]
- Carlos, S.; Burgo, C.L.-D.; Burgueño, E.; Martínez-González, M.A.; Osorio, A.; Ndarabu, A.; Passabosc, C.; De Irala, J. Male condom use, multiple sexual partners and HIV: A prospective case-control study in Kinshasa (DRC). AIDS Care 2016, 29, 772–781. [Google Scholar] [CrossRef] [Green Version]
- Memiah, P.; Mu, T.A.; Prevot, K.; Cook, C.K.; Mwangi, M.M.; Mwangi, E.W.; Owuor, K.; Biadgilign, S. The Prevalence of Intimate Partner Violence, Associated Risk Factors, and Other Moderating Effects: Findings from the Kenya National Health Demographic Survey. J. Interpers. Violence 2018, 886260518804177. [Google Scholar] [CrossRef]
- Evens, E.; Lanham, M.; Santi, K.; Cooke, J.; Ridgeway, K.; Morales, G.; Parker, C.; Brennan, C.; De Bruin, M.; Desrosiers, P.C.; et al. Experiences of gender-based violence among female sex workers, men who have sex with men, and transgender women in Latin America and the Caribbean: A qualitative study to inform HIV programming. BMC Int. Health Hum. Rights 2019, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, E.; Otwombe, K.; Dietrich, J.J.; Andrasik, M.P.; Morgan, C.A.; Kublin, J.G.; Gray, G.E.; Isaacs, A.J.; Laher, F. Vaginal practices among women at risk for HIV acquisition in Soweto, South Africa. S. Afr. J. HIV Med. 2019, 20, 866. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, M.L.; Rodriguez, V.J.; Fischl, M.A.; Jones, D.L.; Weiss, S.M. Addressing intravaginal practices in women with HIV and at-risk for HIV infection, a mixed methods pilot study. Int. J. Women’s Health 2017, 9, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagar, M. Origin of the transmitted virus in HIV infection: Infected cells versus cell-free virus. J. Infect. Dis. 2014, 210, S667–S673. [Google Scholar] [CrossRef] [Green Version]
- Pedro, K.D.; Henderson, A.J.; Agosto, L.M. Mechanisms of HIV-1 cell-to-cell transmission and the establishment of the latent reservoir. Virus Res. 2019, 265, 115–121. [Google Scholar] [CrossRef]
- Dufloo, J.; Bruel, T.; Schwartz, O. HIV-1 cell-to-cell transmission and broadly neutralizing antibodies. Retrovirology 2018, 15, 51. [Google Scholar] [CrossRef] [Green Version]
- Len, A.C.L.; Starling, S.; Shivkumar, M.; Jolly, C. HIV-1 Activates T Cell Signaling Independently of Antigen to Drive Viral Spread. Cell Rep. 2017, 18, 1062–1074. [Google Scholar] [CrossRef] [Green Version]
- Nijmeijer, B.M.; Geijtenbeek, T.B.H. Negative and Positive Selection Pressure During Sexual Transmission of Transmitted Founder HIV-1. Front. Immunol. 2019, 10, 1599. [Google Scholar] [CrossRef]
- Novitsky, V.; Moyo, S.; Wang, R.; Gaseitsiwe, S.; Essex, M. Deciphering Multiplicity of HIV-1C Infection: Transmission of Closely Related Multiple Viral Lineages. PLoS ONE 2016, 11, e0166746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterrett, S.; Learn, G.H.; Edlefsen, P.T.; Haynes, B.F.; Hahn, B.H.; Shaw, G.M.; Bar, K.J. Low Multiplicity of HIV-1 Infection and No Vaccine Enhancement in VAX003 Injection Drug Users. Open Forum Infect. Dis. 2014, 1, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akand, E.H.; Maher, S.J.; Murray, J.M. Mutational networks of escape from transmitted HIV-1 infection. PLoS ONE 2020, 15, e0243391. [Google Scholar] [CrossRef]
- Sanders, R.W.; Vesanen, M.; Schuelke, N.; Master, A.; Schiffner, L.; Kalyanaraman, R.; Paluch, M.; Berkhout, B.; Maddon, P.J.; Olson, W.C.; et al. Stabilization of the Soluble, Cleaved, Trimeric Form of the Envelope Glycoprotein Complex of Human Immunodeficiency Virus Type 1. J. Virol. 2002, 76, 8875–8889. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Sharma, S.K.; Cottrell, C.; Guenaga, J.; Tran, K.; Wilson, R.; Behrens, A.-J.; Crispin, M.; De Val, N.; Wyatt, R.T. Structure-Guided Redesign Improves NFL HIV Env Trimer Integrity and Identifies an Inter-Protomer Disulfide Permitting Post-Expression Cleavage. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; He, L.; De Val, N.; Vora, N.; Morris, C.D.; Azadnia, P.; Sok, D.; Zhou, B.; Burton, D.R.; Ward, A.B.; et al. Uncleaved prefusion-optimized gp140 trimers derived from analysis of HIV-1 envelope metastability. Nat. Commun. 2016, 7, 12040. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Li, W.; Lu, M.; Bess, J.; Chao, C.W.; Gorman, J.; Terry, D.S.; Zhang, B.; Zhou, T.; Blanchard, S.C.; et al. Subnanometer structures of HIV-1 envelope trimers on aldrithiol-2-inactivated virus particles. Nat. Struct. Mol. Biol. 2020, 27, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Rantalainen, K.; Berndsen, Z.T.; Antanasijevic, A.; Schiffner, T.; Zhang, X.; Lee, W.-H.; Torres, J.L.; Zhang, L.; Irimia, A.; Copps, J.; et al. HIV-1 Envelope and MPER Antibody Structures in Lipid Assemblies. Cell Rep. 2020, 31, 107583. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, L.; Liang, J.; Dong, G.-H.; Xia, Y.-L.; Fu, Y.-X.; Liu, S.-Q. Molecular dynamics simulations reveal distinct differences in conformational dynamics and thermodynamics between the unliganded and CD4-bound states of HIV-1 gp120. Phys. Chem. Chem. Phys. 2020, 22, 5548–5560. [Google Scholar] [CrossRef] [Green Version]
- Rolland, M.; Tovanabutra, S.; Dearlove, B.; Li, Y.; Owen, C.L.; Lewitus, E.; Sanders-Buell, E.; Bose, M.; O’Sullivan, A.; Rossenkhan, R.; et al. Molecular dating and viral load growth rates suggested that the eclipse phase lasted about a week in HIV-1 infected adults in East Africa and Thailand. PLoS Pathog. 2020, 16, e1008179. [Google Scholar] [CrossRef]
- Klein, K.; Nankya, I.; Nickel, G.; Ratcliff, A.N.; Meadows, A.A.J.; Hathaway, N.; Bailey, J.A.; Stieh, D.J.; Cheeseman, H.M.; Carias, A.M.; et al. Deep Gene Sequence Cluster Analyses of Multi-Virus-Infected Mucosal Tissue Reveal Enhanced Transmission of Acute HIV-1. J. Virol. 2020, 95, e01737-20. [Google Scholar] [CrossRef]
- Joseph, S.B.; Swanstrom, R.; Kashuba, A.D.M.; Cohen, M.S. Bottlenecks in HIV-1 transmission: Insights from the study of founder viruses. Nat. Rev. Genet. 2015, 13, 414–425. [Google Scholar] [CrossRef] [Green Version]
- Peters, P.J.; Duenas-Decamp, M.J.; Sullivan, W.M.; Brown, R.P.; Ankghuambom, C.; Luzuriaga, K.; Robinson, J.; Burton, D.R.; Bell, J.; Simmonds, P.; et al. Variation in HIV-1 R5 macrophage-tropism correlates with sensitivity to reagents that block envelope: CD4 interactions but not with sensitivity to other entry inhibitors. Retrovirology 2008, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duenas-Decamp, M.J.; Clapham, P.R. HIV-1 gp120 Determinants Proximal to the CD4 Binding Site Shift Protective Glycans That Are Targeted by Monoclonal Antibody 2G12. J. Virol. 2010, 84, 9608–9612. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Gonzalez, J.F.; Salazar, M.G.; Keele, B.F.; Learn, G.H.; Giorgi, E.E.; Li, H.; Decker, J.M.; Wang, S.; Baalwa, J.; Kraus, M.H.; et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 2009, 206, 1273–1289. [Google Scholar] [CrossRef]
- Parrish, N.F.; Gao, F.; Li, H.; Giorgi, E.E.; Barbian, H.J.; Parrish, E.H.; Zajic, L.; Iyer, S.S.; Decker, J.M.; Kumar, A.; et al. Phenotypic properties of transmitted founder HIV-1. Proc. Natl. Acad. Sci. USA 2013, 110, 6626–6633. [Google Scholar] [CrossRef] [Green Version]
- Iyer, S.S.; Bibollet-Ruche, F.; Sherrill-Mix, S.; Learn, G.H.; Plenderleith, L.; Smith, A.G.; Barbian, H.J.; Russell, R.M.; Gondim, M.V.P.; Bahari, C.Y.; et al. Resistance to type 1 interferons is a major determinant of HIV-1 transmission fitness. Proc. Natl. Acad. Sci. USA 2017, 114, E590–E599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagar, M. HIV-1 Transmission Biology: Selection and Characteristics of Infecting Viruses. J. Infect. Dis. 2010, 202, S289–S296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnanakaran, S.; Bhattacharya, T.; Daniels, M.; Keele, B.F.; Hraber, P.T.; Lapedes, A.S.; Shen, T.; Gaschen, B.; Krishnamoorthy, M.; Li, H.; et al. Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections. PLoS Pathog. 2011, 7, e1002209. [Google Scholar] [CrossRef]
- Sagar, M.; Laeyendecker, O.; Lee, S.; Gamiel, J.; Wawer, M.J.; Gray, R.H.; Serwadda, D.; Sewankambo, N.K.; Shepherd, J.C.; Toma, J.; et al. Selection of HIV Variants with Signature Genotypic Characteristics during Heterosexual Transmission. J. Infect. Dis. 2009, 199, 580–589. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Rola, M.; West, J.T.; Tully, D.C.; Kubis, P.; He, J.; Kankasa, C.; Wood, C. Functional properties of the HIV-1 subtype C envelope glycoprotein associated with mother-to-child transmission. Virology 2010, 400, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Go, E.P.; Hewawasam, G.; Liao, H.-X.; Chen, H.; Ping, L.-H.; Anderson, J.A.; Hua, D.C.; Haynes, B.F.; Desaire, H. Characterization of Glycosylation Profiles of HIV-1 Transmitted/Founder Envelopes by Mass Spectrometry. J. Virol. 2011, 85, 8270–8284. [Google Scholar] [CrossRef] [Green Version]
- Derdeyn, C.A.; Decker, J.M.; Bibollet-Ruche, F.; Mokili, J.L.; Muldoon, M.; Denham, S.A.; Heil, M.L.; Kasolo, F.; Musonda, R.; Hahn, B.H.; et al. Envelope-Constrained Neutralization-Sensitive HIV-1 After Heterosexual Transmission. Science 2004, 303, 2019–2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chohan, B.; Lang, D.; Sagar, M.; Korber, B.; Lavreys, L.; Richardson, B.; Overbaugh, J. Selection for Human Immunodeficiency Virus Type 1 Envelope Glycosylation Variants with Shorter V1-V2 Loop Sequences Occurs during Transmission of Certain Genetic Subtypes and May Impact Viral RNA Levels. J. Virol. 2005, 79, 6528–6531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Parast, A.B.; Richardson, B.A.; Nduati, R.; John-Stewart, G.; Mbori-Ngacha, D.; Rainwater, S.M.J.; Overbaugh, J. Neutralization Escape Variants of Human Immunodeficiency Virus Type 1 Are Transmitted from Mother to Infant. J. Virol. 2006, 80, 835–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, A.Q.; Taylor, J.; Dong, W.; McCloskey, R.; Woods, C.; Danroth, R.; Hayashi, K.; Milloy, M.-J.; Poon, A.F.Y.; Brumme, Z.L. Differential evolution of a CXCR4-using HIV-1 strain in CCR5wt/wt and CCR5∆32/∆32 hosts revealed by longitudinal deep sequencing and phylogenetic reconstruction. Sci. Rep. 2015, 5, 17607. [Google Scholar] [CrossRef] [PubMed]
- AshokKumar, M.; Aralaguppe, S.G.; Tripathy, S.P.; Hanna, L.E.; Neogi, U. Unique Phenotypic Characteristics of Recently Transmitted HIV-1 Subtype C Envelope Glycoprotein gp120: Use of CXCR6 Coreceptor by Transmitted Founder Viruses. J. Virol. 2018, 92, e00063-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, H.; Unutmaz, D.; KewalRamani, V.N.; Littman, D.R. Expression Cloning of New Receptors Used by Simian and Human Immunodeficiency Viruses. Nature 1997, 388, 296–300. [Google Scholar] [CrossRef]
- Hodcroft, E.; Hadfield, J.D.; Fearnhill, E.; Phillips, A.; Dunn, D.; O’Shea, S.; Pillay, D.; Leigh Brown, A.J. The Contribution of Viral Genotype to Plasma Viral Set-Point in HIV Infection. PLoS Pathog. 2014, 10, e1004112. [Google Scholar] [CrossRef] [Green Version]
- Carlson, J.M.; Schaefer, M.; Monaco, D.C.; Batorsky, R.; Claiborne, D.T.; Prince, J.; Deymier, M.J.; Ende, Z.S.; Klatt, N.R.; DeZiel, C.E.; et al. Selection bias at the heterosexual HIV-1 transmission bottleneck. Science 2014, 345, 1254031. [Google Scholar] [CrossRef] [Green Version]
- Trifonova, R.T.; Lieberman, J.; Van Baarle, D. Distribution of Immune Cells in the Human Cervix and Implications for HIV Transmission. Am. J. Reprod. Immunol. 2014, 71, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Carias, A.M.; McCoombe, S.; McRaven, M.; Anderson, M.; Galloway, N.; Vandergrift, N.; Fought, A.J.; Lurain, J.; Duplantis, M.; Veazey, R.S.; et al. Defining the Interaction of HIV-1 with the Mucosal Barriers of the Female Reproductive Tract. J. Virol. 2013, 87, 11388–11400. [Google Scholar] [CrossRef] [Green Version]
- Fenton-May, A.E.; Dibben, O.; Emmerich, T.; Ding, H.; Pfafferott, K.; I Aasa-Chapman, M.M.; Pellegrino, P.; Williams, I.; Cohen, M.S.; Gao, F.; et al. Relative resistance of HIV-1 founder viruses to control by interferon-alpha. Retrovirology 2013, 10, 146. [Google Scholar] [CrossRef] [Green Version]
- Foster, T.L.; Wilson, H.; Iyer, S.S.; Coss, K.; Doores, K.; Smith, S.; Kellam, P.; Finzi, A.; Borrow, P.; Hahn, B.H.; et al. Resistance of Transmitted Founder HIV-1 to IFITM-Mediated Restriction. Cell Host Microbe 2016, 20, 429–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorosko, S.M.; Connor, R.I. Primary Human Mammary Epithelial Cells Endocytose HIV-1 and Facilitate Viral Infection of CD4+ T Lymphocytes. J. Virol. 2010, 84, 10533–10542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neidleman, J.A.; Chen, J.C.; Kohgadai, N.; Müller, J.A.; Laustsen, A.; Thavachelvam, K.; Jang, K.S.; Stürzel, C.M.; Jones, J.J.; Ochsenbauer, C.; et al. Mucosal stromal fibroblasts markedly enhance HIV infection of CD4+ T cells. PLoS Pathog. 2017, 13, e1006163. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-T.; Wang, T.; O’Dell, S.; Louder, M.K.; Schön, A.; Cheung, C.S.F.; Chuang, G.-Y.; Druz, A.; Lin, B.; McKee, K.; et al. Lattice engineering enables definition of molecular features allowing for potent small-molecule inhibition of HIV-1 entry. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Finzi, A.; Sodroski, J. The Conformational States of the HIV-1 Envelope Glycoproteins. Trends Microbiol. 2020, 28, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Chen, B. Molecular Mechanism of HIV-1 Entry. Trends Microbiol. 2019, 27, 878–891. [Google Scholar] [CrossRef]
- Salimi, H.; Johnson, J.; Flores, M.G.; Zhang, M.S.; O’Malley, Y.Q.; Houtman, J.C.; Schlievert, P.M.; Haim, H. The lipid membrane of HIV-1 stabilizes the viral envelope glycoproteins and modulates their sensitivity to antibody neutralization. J. Biol. Chem. 2020, 295, 348–362. [Google Scholar] [CrossRef]
- Ma, X.; Lu, M.; Gorman, J.; Terry, D.S.; Hong, X.; Zhou, Z.; Zhao, H.; Altman, R.B.; Arthos, J.; Blanchard, S.C.; et al. HIV-1 Env trimer opens through an asymmetric intermediate in which individual protomers adopt distinct conformations. eLife 2018, 7, e34271. [Google Scholar] [CrossRef]
- Dumas, F.; Preira, P.; Salomé, L. Membrane organization of virus and target cell plays a role in HIV entry. Biochimie 2014, 107 Pt A, 22–27. [Google Scholar] [CrossRef]
- Torralba, J.; De La Arada, I.; Carravilla, P.; Insausti, S.; Rujas, E.; Largo, E.; Eggeling, C.; Arrondo, J.L.R.; Apellaniz, B.; Nieva, J.L. Cholesterol Constrains the Antigenic Configuration of the Membrane-Proximal Neutralizing HIV-1 Epitope. ACS Infect. Dis. 2020, 6, 2155–2168. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.; Mandal, T.; Elkins, M.R.; Oh, Y.; Cui, Q.; Hong, M. Cholesterol Interaction with the Trimeric HIV Fusion Protein gp41 in Lipid Bilayers Investigated by Solid-State NMR Spectroscopy and Molecular Dynamics Simulations. J. Mol. Biol. 2020, 432, 4705–4721. [Google Scholar] [CrossRef] [PubMed]
- Chua, B.A.; Ngo, J.A.; Situ, K.; Morizono, K. Roles of phosphatidylserine exposed on the viral envelope and cell membrane in HIV-1 replication. Cell Commun. Signal. 2019, 17, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carravilla, P.; Nieva, J.L. HIV antivirals: Targeting the functional organization of the lipid envelope. Futur. Virol. 2018, 13, 129–140. [Google Scholar] [CrossRef]
- Saphire, A.C.; Bobardt, M.D.; Zhang, Z.; David, G.; Gallay, P.A. Syndecans Serve as Attachment Receptors for Human Immunodeficiency Virus Type 1 on Macrophages. J. Virol. 2001, 75, 9187–9200. [Google Scholar] [CrossRef] [Green Version]
- Herrera, R.; Morris, M.; Rosbe, K.; Feng, Z.; Weinberg, A.; Tugizov, S. Human beta-defensins 2 and -3 cointernalize with human immunodeficiency virus via heparan sulfate proteoglycans and reduce infectivity of intracellular virions in tonsil epithelial cells. Virology 2016, 487, 172–187. [Google Scholar] [CrossRef] [Green Version]
- Fantini, J.; Hammache, D.; Delézay, O.; Yahi, N.; André-Barrès, C.; Rico-Lattes, I.; Lattes, A. Synthetic Soluble Analogs of Galactosylceramide (GalCer) Bind to the V3 Domain of HIV-1 gp120 and Inhibit HIV-1-induced Fusion and Entry. J. Biol. Chem. 1997, 272, 7245–7252. [Google Scholar] [CrossRef] [Green Version]
- Trujillo, J.R.; Rogers, R.; Molina, R.M.; Dangond, F.; McLane, M.F.; Essex, M.; Brain, J.D. Noninfectious entry of HIV-1 into peripheral and brain macrophages mediated by the mannose receptor. Proc. Natl. Acad. Sci. USA 2007, 104, 5097–5102. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.G.; Hildreth, J.E.K. Involvement of macrophage mannose receptor in the binding and transmission of HIV by macrophages. Eur. J. Immunol. 2003, 33, 483–493. [Google Scholar] [CrossRef]
- Arthos, J.; Cicala, C.; Nawaz, F.; Byrareddy, S.N.; Villinger, F.; Santangelo, P.J.; Ansari, A.A.; Fauci, A.S. The Role of Integrin α4β7 in HIV Pathogenesis and Treatment. Curr. HIV/AIDS Rep. 2018, 15, 127–135. [Google Scholar] [CrossRef] [Green Version]
- De Witte, L.; Nabatov, A.; Geijtenbeek, T.B.H. Distinct roles for DC-SIGN+-dendritic cells and Langerhans cells in HIV-1 transmission. Trends Mol. Med. 2008, 14, 12–19. [Google Scholar] [CrossRef] [PubMed]
- De Witte, L.; Nabatov, A.; Pion, M.; Fluitsma, D.; De Jong, M.A.W.P.; De Gruijl, T.; Piguet, V.; Van Kooyk, Y.; Geijtenbeek, T.B.H. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 2007, 13, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.; Upadhyay, C.; Hioe, C.E. HIV-1 Envelope Glycan Composition as a Key Determinant of Efficient Virus Transmission via DC-SIGN and Resistance to Inhibitory Lectins. iScience 2019, 21, 413–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolic, D.S.; Lehmann, M.; Felts, R.; Garcia, E.; Blanchet, F.P.; Subramaniam, S.; Piguet, V. HIV-1 activates Cdc42 and induces membrane extensions in immature dendritic cells to facilitate cell-to-cell virus propagation. Blood 2011, 118, 4841–4852. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.; Kwon, D.S.; Torensma, R.; Van Vliet, S.J.; Van Duijnhoven, G.C.; Middel, J.; Cornelissen, I.L.; Nottet, H.S.; KewalRamani, V.N.; Littman, D.R.; et al. DC-SIGN, a Dendritic Cell–Specific HIV-1-Binding Protein that Enhances trans-Infection of T Cells. Cell 2000, 100, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Garcia, E.; Pion, M.; Pelchen-Matthews, A.; Collinson, L.; Arrighi, J.-F.; Blot, G.; Leuba, F.; Escola, J.-M.; Demaurex, N.; Marsh, M.; et al. HIV-1 Trafficking to the Dendritic Cell-T-Cell Infectious Synapse Uses a Pathway of Tetraspanin Sorting to the Immunological Synapse. Traffic 2005, 6, 488–501. [Google Scholar] [CrossRef]
- Arthos, J.; Cicala, C.; Martinelli, E.; MacLeod, K.; Van Ryk, D.; Wei, D.; Xiao, Z.; Veenstra, T.D.; Conrad, T.P.; Lempicki, R.A.; et al. HIV-1 envelope protein binds to and signals through integrin α4β7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 2008, 9, 301–309. [Google Scholar] [CrossRef]
- Byrareddy, S.N.; Kallam, B.; Arthos, J.; Cicala, C.; Nawaz, F.; Hiatt, J.; Kersh, E.N.; McNicholl, J.M.; Hanson, D.L.; Reimann, K.A.; et al. Targeting α4β7 integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection. Nat. Med. 2014, 20, 1397–1400. [Google Scholar] [CrossRef]
- Girard, A.; Rallón, N.; Benito, J.M.; Jospin, F.; Rodriguez, C.; Chanut, B.; Benjelloun, F.; Del Romero, J.; Verrier, B.; Lucht, F.; et al. A high mucosal blocking score is associated with HIV protection. AIDS 2019, 33, 411–423. [Google Scholar] [CrossRef]
- Shen, R.; Raska, M.; Bimczok, D.; Novak, J.; Smith, P.D. HIV-1 Envelope Glycan Moieties Modulate HIV-1 Transmission. J. Virol. 2014, 88, 14258–14267. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Jin, W.; Hu, K.; Luo, S.; Du, T.; Griffin, G.E.; Shattock, R.J.; Hu, Q. Highly conserved HIV-1 gp120 glycans proximal to CD4-binding region affect viral infectivity and neutralizing antibody induction. Virology 2012, 423, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Auwerx, J.; François, K.O.; Covens, K.; Van Laethem, K.; Balzarini, J. Glycan deletions in the HIV-1 gp120 V1/V2 domain compromise viral infectivity, sensitize the mutant virus strains to carbohydrate-binding agents and represent a specific target for therapeutic intervention. Virology 2008, 382, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Wolk, T.; Schreiber, M. N-Glycans in the gp120 V1/V2 domain of the HIV-1 strain NL4-3 are indispensable for viral infectivity and resistance against antibody neutralization. Med. Microbiol. Immunol. 2006, 195, 165–172. [Google Scholar] [CrossRef]
- Wang, W.; Nie, J.; Prochnow, C.; Truong, C.; Jia, Z.; Wang, S.; Chen, X.S.; Wang, Y. A systematic study of the N-glycosylation sites of HIV-1 envelope protein on infectivity and antibody-mediated neutralization. Retrovirology 2013, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Mathys, L.; Balzarini, J. The role of N-glycans of HIV-1 gp41 in virus infectivity and susceptibility to the suppressive effects of carbohydrate-binding agents. Retrovirology 2014, 11, 107. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.-F.; Wang, S.-F.; Lin, Y.-T.; Ho, D.D.; Chen, Y.-M.A. Identification of the DC-SIGN-Interactive Domains on the Envelope Glycoprotein of HIV-1 CRF07_BC. AIDS Res. Hum. Retrovir. 2011, 27, 831–839. [Google Scholar] [CrossRef]
- Hong, P.W.-P.; Nguyen, S.; Young, S.; Su, S.V.; Lee, B. Identification of the Optimal DC-SIGN Binding Site on Human Immunodeficiency Virus Type 1 gp120. J. Virol. 2007, 81, 8325–8336. [Google Scholar] [CrossRef] [Green Version]
- Doria-Rose, N.A.; Landais, E. Coevolution of HIV-1 and broadly neutralizing antibodies. Curr. Opin. HIV AIDS 2019, 14, 286–293. [Google Scholar] [CrossRef]
- Yasen, A.; Herrera, R.; Rosbe, K.; Lien, K.; Tugizov, S.M. HIV internalization into oral and genital epithelial cells by endocytosis and macropinocytosis leads to viral sequestration in the vesicles. Virology 2018, 515, 92–107. [Google Scholar] [CrossRef]
- Daecke, J.; Fackler, O.T.; Dittmar, M.T.; Kräusslich, H.-G. Involvement of Clathrin-Mediated Endocytosis in Human Immunodeficiency Virus Type 1 Entry. J. Virol. 2005, 79, 1581–1594. [Google Scholar] [CrossRef] [Green Version]
- Yasen, A.; Herrera, R.; Rosbe, K.; Lien, K.; Tugizov, S.M. Release of HIV-1 sequestered in the vesicles of oral and genital mucosal epithelial cells by epithelial-lymphocyte interaction. PLoS Pathog. 2017, 13, e1006247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tugizov, S.M. Human immunodeficiency virus interaction with oral and genital mucosal epithelia may lead to epithelial–mesenchymal transition and sequestration of virions in the endosomal compartments. Oral Dis. 2020, 26 (Suppl. 1), 40–46. [Google Scholar] [CrossRef]
- Ni, C.; Huang, L.; Chen, Y.; He, M.; Hu, Y.; Liu, S.; Fang, X.; Li, J.; Sun, Q.; Wang, X. Implication of cell-in-cell structures in the transmission of HIV to epithelial cells. Cell Res. 2015, 25, 1265–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lien, K.; Mayer, W.; Herrera, R.; Rosbe, K.; Tugizov, S.M. HIV-1 proteins gp120 and tat induce the epithelial–mesenchymal transition in oral and genital mucosal epithelial cells. PLoS ONE 2019, 14, e0226343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tugizov, S. Human immunodeficiency virus-associated disruption of mucosal barriers and its role in HIV transmission and pathogenesis of HIV/AIDS disease. Tissue Barriers 2016, 4, e1159276. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.J.; Li, Q.; Abel, K.; Kim, E.-Y.; Ma, Z.-M.; Wietgrefe, S.; La Franco-Scheuch, L.; Compton, L.; Duan, L.; Shore, M.D.; et al. Propagation and Dissemination of Infection after Vaginal Transmission of Simian Immunodeficiency Virus. J. Virol. 2005, 79, 9217–9227. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, K.; Baeten, J.M.; Beksinska, M.; Bekker, L.-G.; Bukusi, E.A.; Donnell, D.; Gichangi, P.B.; Heller, K.B.; Hofmeyr, G.J.; Justman, J.; et al. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: A randomised, multicentre, open-label trial. Lancet 2019, 394, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C. DMPA suppresses response to HIV-1. Nat. Rev. Endocrinol. 2013, 9, 187. [Google Scholar] [CrossRef]
- McKinnon, L.R.; Kaul, R. Quality and Quantity: Mucosal CD4 + T Cells and HIV Susceptibility. Curr. Opin. HIV AIDS 2012, 7, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Bertram, K.M.; Botting, R.A.; Baharlou, H.; Rhodes, J.W.; Rana, H.; Graham, J.D.; Patrick, E.; Fletcher, J.; Plasto, T.M.; Truong, N.R.; et al. Identification of HIV transmitting CD11c+ human epidermal dendritic cells. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hertoghs, N.; Nijmeijer, B.M.; Van Teijlingen, N.H.; Fenton-May, A.E.; Kaptein, T.M.; Van Hamme, J.L.; Kappes, J.C.; Kootstra, N.A.; Hahn, B.H.; Borrow, P.; et al. Sexually transmitted founder HIV-1 viruses are relatively resistant to Langerhans cell-mediated restriction. PLoS ONE 2019, 14, e0226651. [Google Scholar] [CrossRef]
- Ganor, Y.; Zhou, Z.; Bodo, J.; Tudor, D.; Leibowitch, J.; Mathez, D.; Schmitt, A.; Vacher-Lavenu, M.-C.; Revol, M.; Bomsel, M. The adult penile urethra is a novel entry site for HIV-1 that preferentially targets resident urethral macrophages. Mucosal Immunol. 2013, 6, 776–786. [Google Scholar] [CrossRef]
- Shen, R.; Richter, H.E.; Smith, P.D. Interactions between HIV-1 and Mucosal Cells in the Female Reproductive Tract. Am. J. Reprod. Immunol. 2014, 71, 608–617. [Google Scholar] [CrossRef] [Green Version]
- Dupont, M.; Sattentau, Q.J. Macrophage Cell-Cell Interactions Promoting HIV-1 Infection. Viruses 2020, 12, 492. [Google Scholar] [CrossRef]
- Ribeiro, C.M.S.; Sarrami-Forooshani, R.; Setiawan, L.C.; Zijlstra-Willems, E.M.; Van Hamme, J.L.; Tigchelaar, W.; Van Der Wel, N.N.; Kootstra, N.A.; Gringhuis, S.I.; Geijtenbeek, T.B.H. Receptor usage dictates HIV-1 restriction by human TRIM5α in dendritic cell subsets. Nat. Cell Biol. 2016, 540, 448–452. [Google Scholar] [CrossRef]
- Nijmeijer, B.M.; Langedijk, C.J.M.; Geijtenbeek, T.B.H. Mucosal Dendritic Cell Subsets Control HIV-1’s Viral Fitness. Annu. Rev. Virol. 2020, 7, 385–402. [Google Scholar] [CrossRef]
- Martín-Moreno, A.; Muñoz-Fernández, M.A. Dendritic Cells, the Double Agent in the War against HIV-1. Front. Immunol. 2019, 10, 2485. [Google Scholar] [CrossRef] [Green Version]
- Shacklett, B.L.; Ferre, A.L.; Kiniry, B.E. Tissue issues: Mucosal T-Cell Responses in HIV-1 Infection. Curr. Opin. HIV AIDS 2019, 14, 100–107. [Google Scholar] [CrossRef]
- Tokarev, A.; McKinnon, L.R.; Pagliuzza, A.; Sivro, A.; Omole, T.E.; Kroon, E.; Chomchey, N.; Phanuphak, N.; Schuetz, A.; Robb, M.L.; et al. Preferential Infection of α4β7+ Memory CD4+ T Cells During Early Acute Human Immunodeficiency Virus Type 1 Infection. Clin. Infect. Dis. 2020, 71, e735–e743. [Google Scholar] [CrossRef]
- Parrish, N.F.; Wilen, C.B.; Banks, L.B.; Iyer, S.S.; Pfaff, J.M.; Salazar-Gonzalez, J.F.; Salazar, M.G.; Decker, J.M.; Parrish, E.H.; Berg, A.; et al. Transmitted/Founder and Chronic Subtype C HIV-1 Use CD4 and CCR5 Receptors with Equal Efficiency and Are Not Inhibited by Blocking the Integrin α4β7. PLoS Pathog. 2012, 8, e1002686. [Google Scholar] [CrossRef]
- Liu, Q.; Lusso, P. Integrin α4β7 in HIV-1 infection: A critical review. J. Leukoc. Biol. 2020, 108, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Mwatelah, R.; McKinnon, L.R.; Baxter, C.; Abdool Karim, Q.; Abdool Karim, S.S. Mechanisms of Sexually Transmitted Infection-Induced Inflammation in Women: Implications for HIV Risk. J. Int. AIDS Soc. 2019, 22, e25346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKinnon, L.R.; Liebenberg, L.J.; Yende-Zuma, N.; Archary, D.; Ngcapu, S.; Sivro, A.; Nagelkerke, N.; Lerma, J.G.G.; Kashuba, A.D.; Masson, L.; et al. Genital inflammation undermines the effectiveness of tenofovir gel in preventing HIV acquisition in women. Nat. Med. 2018, 24, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Liebenberg, L.J.P.; Masson, L.; Arnold, K.B.; McKinnon, L.R.; Werner, L.; Proctor, E.; Archary, D.; Mansoor, L.E.; Lauffenburger, D.A.; Karim, Q.A.; et al. Genital—Systemic Chemokine Gradients and the Risk of HIV Acquisition in Women. JAIDS J. Acquir. Immune Defic. Syndr. 2017, 74, 318–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lumngwena, E.N.; Metenou, S.; Masson, L.; Cicala, C.; Arthos, J.; Woodman, Z. HIV-1 subtype C transmitted founders modulate dendritic cell inflammatory responses. Retrovirology 2020, 17, 1–13. [Google Scholar] [CrossRef]
- Eastment, M.C.; McClelland, R.S. Vaginal microbiota and susceptibility to HIV. AIDS 2018, 32, 687–698. [Google Scholar] [CrossRef]
- McKinnon, L.R.; Achilles, S.L.; Bradshaw, C.S.; Burgener, A.; Crucitti, T.; Fredricks, D.N.; Jaspan, H.B.; Kaul, R.; Kaushic, C.; Klatt, N.; et al. The Evolving Facets of Bacterial Vaginosis: Implications for HIV Transmission. AIDS Res. Hum. Retrovir. 2019, 35, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Hoang, T.; Toler, E.; Delong, K.; Mafunda, N.A.; Bloom, S.M.; Zierden, H.C.; Moench, T.R.; Coleman, J.S.; Hanes, J.; Kwon, D.S.; et al. The cervicovaginal mucus barrier to HIV-1 is diminished in bacterial vaginosis. PLoS Pathog. 2020, 16, e1008236. [Google Scholar] [CrossRef] [Green Version]
- De Melo, M.G.; Sprinz, E.; Gorbach, P.M.; Santos, B.; Rocha, T.D.M.; Simon, M.; Almeida, M.; Lira, R.; Chaves, M.C.; Kerin, T.; et al. HIV-1 heterosexual transmission and association with sexually transmitted infections in the era of treatment as prevention. Int. J. Infect. Dis. 2019, 87, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Reda, S.; Gonçalves, F.A.; Mazepa, M.M.; De Carvalho, N.S. Women infected with HIV and the impact of associated sexually transmitted infections. Int. J. Gynecol. Obstet. 2018, 142, 143–147. [Google Scholar] [CrossRef]
- Mbuya, W.; Mcharo, R.; Mhizde, J.; Mnkai, J.; Mahenge, A.; Mwakatima, M.; Mwalongo, W.; Chiwerengo, N.; Hölscher, M.; Lennemann, T.; et al. Depletion and activation of mucosal CD4 T cells in HIV infected women with HPV-associated lesions of the cervix uteri. PLoS ONE 2020, 15, e0240154. [Google Scholar] [CrossRef] [PubMed]
- Chanzu, N.; Ondondo, B. Induction of Potent and Long-Lived Antibody and Cellular Immune Responses in the Genitorectal Mucosa Could be the Critical Determinant of HIV Vaccine Efficacy. Front. Immunol. 2014, 5, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zapata, W.; Aguilar-Jiménez, W.; Pineda-Trujillo, N.; Rojas, W.; Estrada, H.; Rugeles, M.T. Influence ofCCR5andCCR2Genetic Variants in the Resistance/Susceptibility to HIV in Serodiscordant Couples from Colombia. AIDS Res. Hum. Retrovir. 2013, 29, 1594–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopalco, L.; Barassi, C.; Paolucci, C.; Breda, D.; Brunelli, D.; Nguyen, M.; Nouhin, J.; Luong, T.T.; Truong, L.X.; Clerici, M.; et al. Predictive value of anti-cell and anti-human immunodeficiency virus (HIV) humoral responses in HIV-1-exposed seronegative cohorts of European and Asian origin. J. Gen. Virol. 2005, 86, 339–348. [Google Scholar] [CrossRef]
- Fourcade, L.; Poudrier, J.; Roger, M. Natural Immunity to HIV: A Template for Vaccine Strategies. Viruses 2018, 10, 215. [Google Scholar] [CrossRef] [Green Version]
- Shen, R.; Smith, P.D. Mucosal Correlates of Protection in HIV-1-Exposed Sero-negative Persons. Am. J. Reprod. Immunol. 2014, 72, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Lai, S.K.; Hida, K.; Shukair, S.; Wang, Y.-Y.; Figueiredo, A.; Cone, R.; Hope, T.J.; Hanes, J. Human Immunodeficiency Virus Type 1 Is Trapped by Acidic but Not by Neutralized Human Cervicovaginal Mucus. J. Virol. 2009, 83, 11196–11200. [Google Scholar] [CrossRef] [Green Version]
- Boukari, H.; Brichacek, B.; Stratton, P.; Mahoney, S.F.; Lifson, J.D.; Margolis, L.; Nossal, R. Movements of HIV-Virions in Human Cervical Mucus. Biomacromolecules 2009, 10, 2482–2488. [Google Scholar] [CrossRef] [Green Version]
- Chiodi, F.; Institutet, K.; Ferrari, G.; Hinkula, J.; Ruprecht, R.M.; Kulkarni, V. Mucosal IgA Responses: Damaged in Established Hiv Infection-Yet, Effective Weapon against Hiv Transmission. Front. Immunol. 2017, 8, 1581. [Google Scholar] [CrossRef] [Green Version]
- Fouda, G.G.; Permar, S.R. Immune-based interventions to prevent postnatal HIV-1 transmission. Trends Microbiol. 2014, 22, 425–427. [Google Scholar] [CrossRef]
- Tay, M.Z.; Kunz, E.L.; Deal, A.; Zhang, L.; Seaton, K.E.; Rountree, W.; Eudailey, J.A.; Heptinstall, J.; McRaven, M.D.; Matias, E.; et al. Rare Detection of Antiviral Functions of Polyclonal IgA Isolated from Plasma and Breast Milk Compartments in Women Chronically Infected with HIV-1. J. Virol. 2019, 93, e02084-18. [Google Scholar] [CrossRef] [Green Version]
- Pollara, J.; McGuire, E.; Fouda, G.G.; Rountree, W.; Eudailey, J.; Overman, R.G.; Seaton, K.E.; Deal, A.; Edwards, R.W.; Tegha, G.; et al. Association of HIV-1 Envelope-Specific Breast Milk IgA Responses with Reduced Risk of Postnatal Mother-to-Child Transmission of HIV-1. J. Virol. 2015, 89, 9952–9961. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, L.; Trabattoni, D.; Kankasa, C.; Semrau, K.; Kasonde, P.; Lissoni, F.; Sinkala, M.; Ghosh, M.; Vwalika, C.; Aldrovandi, G.M.; et al. Alpha-defensins in the prevention of HIV transmission among breastfed infants. JAIDS J. Acquir. Immune Defic. Syndr. 2005, 39, 138–142. [Google Scholar]
- Mansour, R.G.; Stamper, L.; Jaeger, F.; McGuire, E.; Fouda, G.; Amos, J.; Barbas, K.; Ohashi, T.; Alam, S.M.; Erickson, H.; et al. The Presence and Anti-HIV-1 Function of Tenascin C in Breast Milk and Genital Fluids. PLoS ONE 2016, 11, e0155261. [Google Scholar] [CrossRef]
- Shen, R.; Achenbach, J.; Shen, Y.; Palaia, J.; Rahkola, J.T.; Nick, H.J.; Smythies, L.E.; McConnell, M.; Fowler, M.G.; Smith, P.D.; et al. Mother-to-Child HIV-1 Transmission Events Are Differentially Impacted by Breast Milk and Its Components from HIV-1-Infected Women. PLoS ONE 2015, 10, e0145150. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L. Broadly neutralizing antibodies and vaccine design against HIV-1 infection. Front. Med. 2020, 14, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Ruprecht, R.M.; Marasini, B.; Thippeshappa, R. Mucosal Antibodies: Defending Epithelial Barriers against HIV-1 Invasion. Vaccines 2019, 7, 194. [Google Scholar] [CrossRef] [Green Version]
- Julien, J.-P.; Cupo, A.; Sok, D.; Stanfield, R.L.; Lyumkis, D.; Deller, M.C.; Klasse, P.-J.; Burton, D.R.; Sanders, R.W.; Moore, J.P.; et al. Crystal Structure of a Soluble Cleaved HIV-1 Envelope Trimer. Science 2013, 342, 1477–1483. [Google Scholar] [CrossRef] [Green Version]
- Stewart-Jones, G.B.E.; Soto, C.; Lemmin, T.; Chuang, G.-Y.; Druz, A.; Kong, R.; Thomas, P.V.; Wagh, K.; Zhou, T.; Behrens, A.-J.; et al. Trimeric HIV-1-Env Structures Define Glycan Shields from Clades A, B, and G. Cell 2016, 165, 813–826. [Google Scholar] [CrossRef] [Green Version]
- Behrens, A.-J.; Vasiljevic, S.; Pritchard, L.K.; Harvey, D.J.; Andev, R.S.; Krumm, S.A.; Struwe, W.B.; Cupo, A.; Kumar, A.; Zitzmann, N.; et al. Composition and Antigenic Effects of Individual Glycan Sites of a Trimeric HIV-1 Envelope Glycoprotein. Cell Rep. 2016, 14, 2695–2706. [Google Scholar] [CrossRef] [Green Version]
- Struwe, W.B.; Chertova, E.; Allen, J.D.; Seabright, G.E.; Watanabe, Y.; Harvey, D.J.; Medina-Ramirez, M.; Roser, J.D.; Smith, R.; Westcott, D.; et al. Site-Specific Glycosylation of Virion-Derived HIV-1 Env Is Mimicked by a Soluble Trimeric Immunogen. Cell Rep. 2018, 24, 1958–1966.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gombos, R.B.; Kolodkin-Gal, D.; Eslamizar, L.; Owuor, J.O.; Mazzola, E.; Gonzalez, A.M.; Korioth-Schmitz, B.; Gelman, R.S.; Montefiori, D.C.; Haynes, B.F.; et al. Inhibitory Effect of Individual or Combinations of Broadly Neutralizing Antibodies and Antiviral Reagents against Cell-Free and Cell-to-Cell HIV-1 Transmission. J. Virol. 2015, 89, 7813–7828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Zony, C.; Chen, P.; Chen, B.K. Reduced Potency and Incomplete Neutralization of Broadly Neutralizing Antibodies against Cell-to-Cell Transmission of HIV-1 with Transmitted Founder Envs. J. Virol. 2017, 91, e02425-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reh, L.; Magnus, C.; Schanz, M.; Weber, J.; Uhr, T.; Rusert, P.; Trkola, A. Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent. PLoS Pathog. 2015, 11, e1004966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malbec, M.; Porrot, F.; Rua, R.; Horwitz, J.; Klein, F.; Halper-Stromberg, A.; Scheid, J.F.; Eden, C.; Mouquet, H.; Nussenzweig, M.C.; et al. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J. Exp. Med. 2013, 210, 2813–2821. [Google Scholar] [CrossRef] [Green Version]
- Cheeseman, H.M.; Olejniczak, N.J.; Rogers, P.M.; Evans, A.B.; King, D.F.L.; Ziprin, P.; Liao, H.-X.; Haynes, B.F.; Shattock, R.J. Broadly Neutralizing Antibodies Display Potential for Prevention of HIV-1 Infection of Mucosal Tissue Superior to That of Nonneutralizing Antibodies. J. Virol. 2016, 91, e01762-16. [Google Scholar] [CrossRef] [Green Version]
- Suphaphiphat, K.; Tolazzi, M.; Hua, S.; Desjardins, D.; Lorin, V.; Dereuddre-Bosquet, N.; Mouquet, H.; Scarlatti, G.; Grand, R.L.; Cavarelli, M. Broadly neutralizing antibodies potently inhibit cell-to-cell transmission of semen leukocyte-derived SHIV162P3. EBioMedicine 2020, 57, 102842. [Google Scholar] [CrossRef]
- Tomaras, G.D.; Ferrari, G.; Shen, X.; Alam, S.M.; Liao, H.-X.; Pollara, J.; Bonsignori, M.; Moody, M.A.; Fong, Y.; Chen, X.; et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc. Natl. Acad. Sci. USA 2013, 110, 9019–9024. [Google Scholar] [CrossRef] [Green Version]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; De Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef]
- Haynes, B.F.; Gilbert, P.B.; McElrath, M.J.; Zolla-Pazner, S.; Tomaras, G.D.; Alam, S.M.; Evans, D.T.; Montefiori, D.C.; Karnasuta, C.; Sutthent, R.; et al. Immune-Correlates Analysis of an HIV-1 Vaccine Efficacy Trial. N. Engl. J. Med. 2012, 366, 1275–1286. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, J.A.; Bar-On, Y.; Lu, C.-L.; Fera, D.; Lockhart, A.A.K.; Lorenzi, J.C.C.; Nogueira, L.; Golijanin, J.; Scheid, J.F.; Seaman, M.S.; et al. Non-Neutralizing Antibodies Alter the Course of HIV-1 Infection In Vivo. Cell 2017, 170, 637–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, M.S.; Chung, A.W.; Kent, S.J. Importance of Fc-mediated functions of anti-HIV-1 broadly neutralizing antibodies. Retrovirology 2018, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gajjar, M.R.; Yu, J.; Padte, N.N.; Gettie, A.; Blanchard, J.L.; Russell-Lodrigue, K.; Liao, L.E.; Perelson, A.S.; Huang, Y.; et al. Quantifying the contribution of Fc-mediated effector functions to the antiviral activity of anti–HIV-1 IgG1 antibodies in vivo. Proc. Natl. Acad. Sci. USA 2020, 117, 18002–18009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Irimia, A.; He, L.; Landais, E.; Rantalainen, K.; Leaman, D.P.; Vollbrecht, T.; Stano, A.; Sands, D.I.; Kim, A.S.; et al. An MPER antibody neutralizes HIV-1 using germline features shared among donors. Nat. Commun. 2019, 10, 5289. [Google Scholar] [CrossRef] [PubMed]
- Krebs, S.J.; Kwon, Y.D.; Schramm, C.A.; Law, W.H.; Donofrio, G.; Zhou, K.H.; Gift, S.; Dussupt, V.; Georgiev, I.S.; Schätzle, S.; et al. Longitudinal Analysis Reveals Early Development of Three MPER-Directed Neutralizing Antibody Lineages from an HIV-1-Infected Individual. Immunity 2019, 50, 677–691.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- I Richardson, S.; Lambson, B.E.; Crowley, A.R.; Bashirova, A.; Scheepers, C.; Garrett, N.; Karim, S.A.; Mkhize, N.N.; Carrington, M.; Ackerman, M.E.; et al. IgG3 enhances neutralization potency and Fc effector function of an HIV V2-specific broadly neutralizing antibody. PLoS Pathog. 2019, 15, e1008064. [Google Scholar] [CrossRef] [Green Version]
- Jia, M.; Liberatore, R.A.; Guo, Y.; Chan, K.-W.; Pan, R.; Lu, H.; Waltari, E.; Mittler, E.; Chandran, K.; Finzi, A.; et al. VSV-Displayed HIV-1 Envelope Identifies Broadly Neutralizing Antibodies Class-Switched to IgG and IgA. Cell Host Microbe 2020, 27, 963–975.e5. [Google Scholar] [CrossRef]
- Scheepers, C.; Bekker, V.; Anthony, C.; Richardson, S.I.; Oosthuysen, B.; Moyo, T.; Kgagudi, P.; Kitchin, D.; Nonyane, M.; York, T.; et al. Antibody Isotype Switching as a Mechanism to Counter HIV Neutralization Escape. Cell Rep. 2020, 33, 108430. [Google Scholar] [CrossRef]
- Astronomo, R.D.; Santra, S.; Ballweber-Fleming, L.; Westerberg, K.G.; Mach, L.; Hensley-McBain, T.; Sutherland, L.; Mildenberg, B.; Morton, G.; Yates, N.L.; et al. Neutralization Takes Precedence Over IgG or IgA Isotype-related Functions in Mucosal HIV-1 Antibody-mediated Protection. EBioMedicine 2016, 14, 97–111. [Google Scholar] [CrossRef] [Green Version]
- Fouda, G.G.; Eudailey, J.; Kunz, E.L.; Amos, J.D.; Liebl, B.E.; Himes, J.; Boakye-Agyeman, F.; Beck, K.; Michaels, A.J.; Cohen-Wolkowiez, M.; et al. Systemic administration of an HIV-1 broadly neutralizing dimeric IgA yields mucosal secretory IgA and virus neutralization. Mucosal Immunol. 2016, 10, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Gorlani, A.; Forthal, D.N. Antibody-Dependent Enhancement and the Risk of HIV Infection. Curr. HIV Res. 2013, 11, 421–426. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Ackers, M.L.; Berman, P.W.; Francis, D.P.; Popovic, V.; Hu, D.J.; Heyward, W.L.; Sinangil, F.; E Shepherd, B.E.; Gurwith, M. HIV-1 Virologic and Immunologic Progression and Initiation of Antiretroviral Therapy among HIV-1–Infected Subjects in a Trial of the Efficacy of Recombinant Glycoprotein 120 Vaccine. J. Infect. Dis. 2005, 192, 974–983. [Google Scholar] [CrossRef]
- Forthal, D.N.; Landucci, G.; Bream, J.; Jacobson, L.P.; Phan, T.B.; Montoya, B. FcγRIIa Genotype Predicts Progression of HIV Infection. J. Immunol. 2007, 179, 7916–7923. [Google Scholar] [CrossRef]
- Brouwer, K.C.; Lal, R.B.; Mirel, L.B.; Yang, C.; Van Eijk, A.M.; Ayisi, J.; Otieno, J.; Nahlen, B.L.; Steketee, R.; Lal, A.A.; et al. Polymorphism of Fc receptor IIa for IgG in infants is associated with susceptibility to perinatal HIV-1 infection. AIDS 2004, 18, 1187–1194. [Google Scholar] [CrossRef]
- Bricault, C.A.; Yusim, K.; Seaman, M.S.; Yoon, H.; Theiler, J.; Giorgi, E.E.; Wagh, K.; Theiler, M.; Hraber, P.; Macke, J.P.; et al. HIV-1 Neutralizing Antibody Signatures and Application to Epitope-Targeted Vaccine Design. Cell Host Microbe 2019, 25, 59–72.e8. [Google Scholar] [CrossRef] [Green Version]
- Hraber, P.T.; Rademeyer, C.; Williamson, C.; Seaman, M.S.; Gottardo, R.; Tang, H.; Greene, K.; Gao, H.; Labranche, C.; Mascola, J.R.; et al. Panels of HIV-1 Subtype C Env Reference Strains for Standardized Neutralization Assessments. J. Virol. 2017, 91, e00991-17. [Google Scholar] [CrossRef] [Green Version]
- Richman, D.D.; Wrin, T.; Little, S.J.; Petropoulos, C.J. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 2003, 100, 4144–4149. [Google Scholar] [CrossRef] [Green Version]
- Sagar, M.; Wu, X.; Lee, S.; Overbaugh, J. Human Immunodeficiency Virus Type 1 V1-V2 Envelope Loop Sequences Expand and Add Glycosylation Sites over the Course of Infection, and These Modifications Affect Antibody Neutralization Sensitivity. J. Virol. 2006, 80, 9586–9598. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Decker, J.M.; Wang, S.; Hui, H.; Kappes, J.C.; Wu, X.; Salazar-Gonzalez, J.F.; Salazar, M.G.; Kilby, J.M.; Saag, M.S.; et al. Antibody neutralization and escape by HIV-1. Nat. Cell Biol. 2003, 422, 307–312. [Google Scholar] [CrossRef]
- Bouvin-Pley, M.; Beretta, M.; Moreau, A.; Roch, E.; Essat, A.; Goujard, C.; Chaix, M.-L.; Moiré, N.; Martin, L.; Meyer, L.; et al. Evolution of the Envelope Glycoprotein of HIV-1 Clade B toward Higher Infectious Properties over the Course of the Epidemic. J. Virol. 2019, 93, e01171-18. [Google Scholar] [CrossRef] [Green Version]
- Stefic, K.; Bouvin-Pley, M.; Essat, A.; Visdeloup, C.; Moreau, A.; Goujard, C.; Chaix, M.-L.; Braibant, M.; Meyer, L.; Barin, F. Sensitivity to Broadly Neutralizing Antibodies of Recently Transmitted HIV-1 Clade CRF02_AG Viruses with a Focus on Evolution over Time. J. Virol. 2019, 93, e01492-18. [Google Scholar] [CrossRef] [Green Version]
- Wilen, C.B.; Parrish, N.F.; Pfaff, J.M.; Decker, J.M.; Henning, E.A.; Haim, H.; Petersen, J.E.; Wojcechowskyj, J.A.; Sodroski, J.; Haynes, B.F.; et al. Phenotypic and Immunologic Comparison of Clade B Transmitted/Founder and Chronic HIV-1 Envelope Glycoproteins. J. Virol. 2011, 85, 8514–8527. [Google Scholar] [CrossRef] [Green Version]
- Hraber, P.; Korber, B.T.; Lapedes, A.S.; Bailer, R.T.; Seaman, M.S.; Gao, H.; Greene, K.M.; McCutchan, F.; Williamson, C.; Kim, J.H.; et al. Impact of Clade, Geography, and Age of the Epidemic on HIV-1 Neutralization by Antibodies. J. Virol. 2014, 88, 12623–12643. [Google Scholar] [CrossRef] [Green Version]
- Fouda, G.G.; Mahlokozera, T.; Salazar-Gonzalez, J.F.; Salazar, M.G.; Learn, G.; Kumar, S.B.; Dennison, S.M.; Russell, E.; Rizzolo, K.; Jaeger, F.; et al. Postnatally-transmitted HIV-1 Envelope variants have similar neutralization-sensitivity and function to that of nontransmitted breast milk variants. Retrovirology 2013, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- Rademeyer, C.; Korber, B.; Seaman, M.S.; Giorgi, E.E.; Thebus, R.; Robles, A.; Sheward, D.J.; Wagh, K.; Garrity, J.; Carey, B.R.; et al. Features of Recently Transmitted HIV-1 Clade C Viruses that Impact Antibody Recognition: Implications for Active and Passive Immunization. PLoS Pathog. 2016, 12, e1005742. [Google Scholar] [CrossRef]
- Angel, C.J.L.; Tomaras, G.D. Bringing the path toward an HIV-1 vaccine into focus. PLoS Pathog. 2020, 16, e1008663. [Google Scholar] [CrossRef]
- Karuna, S.T.; Corey, L. Broadly Neutralizing Antibodies for HIV Prevention. Annu. Rev. Med. 2020, 71, 329–346. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, E.J.; Cavacini, L.A.; Samore, M.H.; Posner, M.R.; Kozial, C.; Spino, C.; Trapnell, C.B.; Ketter, N.; Hammer, S.; Gambertoglio, J.G. Pharmacokinetics of F105, a human monoclonal antibody, in persons infected with human immunodeficiency virus type 1. Clin. Pharmacol. Ther. 1996, 59, 662–667. [Google Scholar] [CrossRef]
- Scheid, J.F.; Mouquet, H.; Ueberheide, B.; Diskin, R.; Klein, F.; Oliveira, T.Y.K.; Pietzsch, J.; Fenyo, D.; Abadir, A.; Velinzon, K.; et al. Sequence and Structural Convergence of Broad and Potent HIV Antibodies That Mimic CD4 Binding. Science 2011, 333, 1633–1637. [Google Scholar] [CrossRef] [Green Version]
- Scheid, J.F.; Horwitz, J.A.; Bar-On, Y.; Kreider, E.F.; Lu, C.L.; Lorenzi, J.C.C.; Feldmann, A.; Braunschweig, M.; Nogueira, L.; Oliveira, T.; et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nat. Cell Biol. 2016, 535, 556–560. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.J.; Bai, J.; Liu, F.L.; Zhang, X.Y.; Wang, J.Z. Focus on the therapeutic efficacy of 3BNC117 against HIV-1: In vitro studies, in vivo studies, clinical trials and challenges. Int. Immunopharmacol. 2017, 52, 44–50. [Google Scholar] [CrossRef]
- Cohen, Y.Z.; Butler, A.L.; Millard, K.; Witmer-Pack, M.; Levin, R.; Unson-O’Brien, C.; Patel, R.; Shimeliovich, I.; Lorenzi, J.C.C.; Horowitz, J.; et al. Safety, pharmacokinetics, and immunogenicity of the combination of the broadly neutralizing anti-HIV-1 antibodies 3BNC117 and 10-1074 in healthy adults: A randomized, phase 1 study. PLoS ONE 2019, 14, e0219142. [Google Scholar] [CrossRef] [Green Version]
- Gautam, R.; Nishimura, Y.; Gaughan, N.; Gazumyan, A.; Schoofs, T.; Buckler-White, A.; Seaman, M.S.; Swihart, B.J.; Follmann, D.A.; Nussenzweig, M.C.; et al. A single injection of crystallizable fragment domain–modified antibodies elicits durable protection from SHIV infection. Nat. Med. 2018, 24, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Ledgerwood, J.E.; Coates, E.E.; Yamshchikov, G.; Saunders, J.G.; Holman, L.; Enama, M.E.; DeZure, A.; Lynch, R.M.; Gordon, I.; Plummer, S.; et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin. Exp. Immunol. 2015, 182, 289–301. [Google Scholar] [CrossRef] [Green Version]
- Gaudinski, M.R.; Coates, E.E.; Houser, K.V.; Chen, G.L.; Yamshchikov, G.; Saunders, J.G.; Holman, L.S.A.; Gordon, I.; Plummer, S.; Hendel, C.S.; et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults. PLoS Med. 2018, 15, e1002493. [Google Scholar] [CrossRef]
- Sok, D.; Le, K.M.; Vadnais, M.; Saye-Francisco, K.L.; Jardine, J.G.; Torres, J.L.; Berndsen, Z.T.; Kong, L.; Stanfield, R.; Ruiz, J.; et al. Rapid elicitation of broadly neutralizing antibodies to HIV by immunization in cows. Nat. Cell Biol. 2017, 548, 108–111. [Google Scholar] [CrossRef] [Green Version]
- Alfsen, A.; Iniguez, P.; Bouguyon, E.; Bomsel, M. Secretory IgA Specific for a Conserved Epitope on gp41 Envelope Glycoprotein Inhibits Epithelial Transcytosis of HIV-1. J. Immunol. 2001, 166, 6257–6265. [Google Scholar] [CrossRef] [Green Version]
- Bomsel, M.; Heyman, M.; Hocini, H.; Lagaye, S.; Belec, L.; Dupont, C.; Desgranges, C. Intracellular Neutralization of HIV Transcytosis across Tight Epithelial Barriers by Anti-HIV Envelope Protein dIgA or IgM. Immunity 1998, 9, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Veazey, R.S.; Shattock, R.J.; Pope, M.; Kirijan, J.C.; Jones, J.; Hu, Q.; Ketas, T.; Marx, P.A.; Klasse, P.J.; Burton, D.R.; et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 2003, 9, 343–346. [Google Scholar] [CrossRef]
- Watkins, J.D.; Sholukh, A.M.; Mukhtar, M.M.; Siddappa, N.B.; Lakhashe, S.K.; Kim, M.; Reinherz, E.L.; Gupta, S.; Forthal, D.N.; Sattentau, Q.J.; et al. Anti-HIV IgA isotypes: Differential Virion Capture and Inhibition of Transcytosis Are Linked to Prevention of Mucosal R5 SHIV Transmission. AIDS 2013, 27, F13–F20. [Google Scholar] [CrossRef] [Green Version]
- Sholukh, A.M.; Watkins, J.D.; Vyas, H.K.; Gupta, S.; Lakhashe, S.K.; Thorat, S.; Zhou, M.; Hemashettar, G.; Bachler, B.C.; Forthal, D.N.; et al. Defense-in-depth by mucosally administered anti-HIV dimeric IgA2 and systemic IgG1 mAbs: Complete protection of rhesus monkeys from mucosal SHIV challenge. Vaccine 2015, 33, 2086–2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, T.; Thebus, E.; Bekker, L.-G.; McIntyre, J.; Abrams, E.J.; Myer, L. Disengagement of HIV-positive pregnant and postpartum women from antiretroviral therapy services: A cohort study. J. Int. AIDS Soc. 2014, 17, 19242. [Google Scholar] [CrossRef] [PubMed]
- Himes, J.E.; Goswami, R.; Mangan, R.J.; Kumar, A.; Jeffries, T.L.; Eudailey, J.A.; Heimsath, H.; Nguyen, Q.N.; Pollara, J.; Labranche, C.; et al. Polyclonal HIV envelope-specific breast milk antibodies limit founder SHIV acquisition and cell-associated virus loads in infant rhesus monkeys. Mucosal Immunol. 2018, 11, 1716–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bomsel, M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 1997, 3, 42–47. [Google Scholar] [CrossRef]
- Alfsen, A.; Yu, H.; Magerus-Chatinet, A.; Schmitt, A.; Bomsel, M. HIV-1-infected Blood Mononuclear Cells Form an Integrin- and Agrin-dependent Viral Synapse to Induce Efficient HIV-1 Transcytosis across Epithelial Cell Monolayer. Mol. Biol. Cell 2005, 16, 4267–4279. [Google Scholar] [CrossRef] [Green Version]
- Ko, S.-Y.; Pegu, A.; Rudicell, R.S.; Yang, Z.; Joyce, M.G.; Chen, X.; Wang, K.; Bao, S.; Kraemer, T.D.; Rath, T.; et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nat. Cell Biol. 2014, 514, 642–645. [Google Scholar] [CrossRef] [Green Version]
- Zalevsky, J.; Chamberlain, A.K.; Horton, H.M.; Karki, S.; Leung, I.W.L.; Sproule, T.J.; Lazar, G.A.; Roopenian, D.C.; DesJarlais, J.R. Enhanced antibody half-life improves in vivo activity. Nat. Biotechnol. 2010, 28, 157–159. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zeng, M.; Duan, L.; Voss, J.E.; Smith, A.J.; Pambuccian, S.; Shang, L.; Wietgrefe, S.; Southern, P.J.; Reilly, C.S.; et al. Live Simian Immunodeficiency Virus Vaccine Correlate of Protection: Local Antibody Production and Concentration on the Path of Virus Entry. J. Immunol. 2014, 193, 3113–3125. [Google Scholar] [CrossRef] [Green Version]
- Barouch, D.H.; Liu, J.; Li, H.; Maxfield, L.F.; Abbink, P.; Lynch, D.M.; Iampietro, M.J.; SanMiguel, A.; Seaman, M.S.; Ferrari, G.; et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nat. Cell Biol. 2012, 482, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Bomsel, M.; Tudor, D.; Drillet, A.-S.; Alfsen, A.; Ganor, Y.; Roger, M.-G.; Mouz, N.; Amacker, M.; Chalifour, A.; Diomede, L.; et al. Immunization with HIV-1 gp41 Subunit Virosomes Induces Mucosal Antibodies Protecting Nonhuman Primates against Vaginal SHIV Challenges. Immunity 2011, 34, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.T.; Shen, X.; Walter, K.L.; Labranche, C.C.; Wyatt, L.S.; Tomaras, G.D.; Montefiori, D.C.; Moss, B.; Barouch, D.H.; Clements, J.D.; et al. HIV-1 vaccination by needle-free oral injection induces strong mucosal immunity and protects against SHIV challenge. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, W.; Wang, S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev. Vaccines 2015, 14, 1509–1523. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Patel, G.B.; Hu, S.; Chen, W. Induction of mucosal immunity through systemic immunization: Phantom or reality? Hum. Vaccines Immunother. 2016, 12, 1070–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matchett, W.E.; Anguiano-Zarate, S.S.; Nehete, P.N.; Shelton, K.; Nehete, B.P.; Yang, G.; Dorta-Estremera, S.; Barnette, P.; Xiao, P.; Byrareddy, S.N.; et al. Divergent HIV-1-Directed Immune Responses Generated by Systemic and Mucosal Immunization with Replicating Single-Cycle Adenoviruses in Rhesus Macaques. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [Green Version]
- Rao, D.S. Chapter 19—Overview and Compartmentalization of the Immune System. In Hematology, 7th ed.; Hoffman, R., Benz, E.J., Silberstein, L.E., Heslop, H.E., Weitz, J.I., Anastasi, J., Salama, M.E., Abutalib, S.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 199–209. ISBN 978-0-323-35762-3. [Google Scholar]
- Fisher, B.S.; Dambrauskas, N.; Trakhimets, O.; Andrade, D.V.; Smedley, J.; Sodora, D.L.; Sather, D.N. Oral Immunization with HIV-1 Envelope SOSIP trimers elicits systemic immune responses and cross-reactive anti-V1V2 antibodies in non-human primates. PLoS ONE 2020, 15, e0233577. [Google Scholar] [CrossRef]
- Carbonetti, S.; Oliver, B.G.; Glenn, J.; Stamatatos, L.; Sather, D.N. Soluble HIV-1 Envelope Immunogens Derived from an Elite Neutralizer Elicit Cross-Reactive V1V2 Antibodies and Low Potency Neutralizing Antibodies. PLoS ONE 2014, 9, e86905. [Google Scholar] [CrossRef]
- Eudailey, J.A.; Dennis, M.L.; Parker, M.E.; Phillips, B.L.; Huffman, T.N.; Bay, C.P.; Hudgens, M.G.; Wiseman, R.W.; Pollara, J.J.; Fouda, G.G.; et al. Maternal HIV-1 Env Vaccination for Systemic and Breast Milk Immunity to Prevent Oral SHIV Acquisition in Infant Macaques. mSphere 2018, 3, e00505-17. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Wang, Y.; Sun, Y.; Cui, H.; Zhu, S.J.; Qiu, H.-J. Mucosal vaccines: Strategies and challenges. Immunol. Lett. 2020, 217, 116–125. [Google Scholar] [CrossRef]
- Planchais, C.; Kök, A.; Kanyavuz, A.; Lorin, V.; Bruel, T.; Guivel-Benhassine, F.; Rollenske, T.; Prigent, J.; Hieu, T.; Prazuck, T.; et al. HIV-1 Envelope Recognition by Polyreactive and Cross-Reactive Intestinal B Cells. Cell Rep. 2019, 27, 572–585.e7. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Clayton, K.; Gao, W.; Li, Y.; Zealey, C.; Budylowski, P.; Schwartz, J.; Yue, F.Y.; Bie, Y.; Rini, J.; et al. Trimeric HIV-1 gp140 fused with APRIL, BAFF, and CD40L on the mucosal gp140-specific antibody responses in mice. Vaccine 2020, 38, 2149–2159. [Google Scholar] [CrossRef]
- Isik, G.; Sliepen, K.; Van Montfort, T.; Sanders, R.W. Enhanced Immunogenicity of HIV-1 Envelope gp140 Proteins Fused to APRIL. PLoS ONE 2014, 9, e107683. [Google Scholar] [CrossRef] [PubMed]
- Lycke, N. Chapter 7—Induction and Regulation of Mucosal Memory B Cell Responses. In Mucosal Vaccines, 2nd ed.; Kiyono, H., Pascual, D.W., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 117–131. ISBN 978-0-12-811924-2. [Google Scholar]
- McHeyzer-Williams, L.J.; Milpied, P.J.; Okitsu, S.L.; McHeyzer-Williams, M.G. Class-switched memory B cells remodel BCRs within secondary germinal centers. Nat. Immunol. 2015, 16, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Victora, G.D.; Mouquet, H. What Are the Primary Limitations in B-Cell Affinity Maturation, and How Much Affinity Maturation Can We Drive with Vaccination? Cold Spring Harb. Perspect. Biol. 2017, 10, a029389. [Google Scholar] [CrossRef]
- Abbott, R.K.; Lee, J.H.; Menis, S.; Skog, P.; Rossi, M.; Ota, T.; Kulp, D.W.; Bhullar, D.; Kalyuzhniy, O.; Havenar-Daughton, C.; et al. Precursor Frequency and Affinity Determine B Cell Competitive Fitness in Germinal Centers, Tested with Germline-Targeting HIV Vaccine Immunogens. Immunity. 2018, 48, 133–146.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bemark, M.; Hazanov, H.; Strömberg, A.; Komban, R.; Holmqvist, J.; Köster, S.; Mattsson, J.; Sikora, P.; Mehr, R.; Lycke, N.Y. Limited clonal relatedness between gut IgA plasma cells and memory B cells after oral immunization. Nat. Commun. 2016, 7, 12698. [Google Scholar] [CrossRef] [PubMed]
- Pirrone, V.; Thakkar, N.; Jacobson, J.M.; Wigdahl, B.; Krebs, F.C. Combinatorial Approaches to the Prevention and Treatment of HIV-1 Infection. Antimicrob. Agents Chemother. 2011, 55, 1831–1842. [Google Scholar] [CrossRef] [Green Version]
- Günthard, H.F.; Saag, M.S.; Benson, C.A.; Del Rio, C.; Eron, J.J.; Gallant, J.E.; Hoy, J.F.; Mugavero, M.J.; Sax, P.E.; Thompson, M.A.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2016 Recommendations of the International Antiviral Society-USA Panel. JAMA 2016, 316, 191–210. [Google Scholar] [CrossRef] [Green Version]
- Kiertiburanakul, S.; Sungkanuparph, S. Emerging of HIV drug resistance: Epidemiology, diagnosis, treatment and prevention. Curr. HIV Res. 2009, 7, 273–278. [Google Scholar] [CrossRef]
- Su, X.; Wang, Q.; Wen, Y.; Jiang, S.; Lu, L. Protein- and Peptide-Based Virus Inactivators: Inactivating Viruses Before Their Entry into Cells. Front. Microbiol. 2020, 11, 1063. [Google Scholar] [CrossRef]
- Haqqani, A.A.; Tilton, J.C. Entry inhibitors and their use in the treatment of HIV-1 infection. Antivir. Res. 2013, 98, 158–170. [Google Scholar] [CrossRef]
- Falkenhagen, A.; Joshi, S. HIV Entry and Its Inhibition by Bifunctional Antiviral Proteins. Mol. Ther. Nucleic Acids 2018, 13, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Lu, L.; Li, W.; Jiang, S. Small-molecule HIV-1 entry inhibitors targeting gp120 and gp41: A patent review (2010–2015). Expert Opin. Ther. Patents 2017, 27, 707–719. [Google Scholar] [CrossRef]
- Pu, J.; Wang, Q.; Xu, W.; Lu, L.; Jiang, S. Development of Protein- and Peptide-Based HIV Entry Inhibitors Targeting gp120 or gp41. Viruses 2019, 11, 705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Li, W.; Jiang, S. Peptide fusion inhibitors targeting the HIV-1 gp41: A patent review (2009–2014). Expert Opin. Ther. Patents 2014, 25, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Chauthe, S.K. Small molecule HIV entry inhibitors: Part I. Chemokine receptor antagonists: 2004–2010. Expert Opin. Ther. Patents 2011, 21, 227–269. [Google Scholar] [CrossRef]
- Baranova, E.O.; Shastina, N.S.; Shvets, V.I. Polyanionic inhibitors of HIV adsorption. Russ. J. Bioorg. Chem. 2011, 37, 527–542. [Google Scholar] [CrossRef]
- Larijani, M.S.; Sadat, S.M.; Bolhassani, A.; Ramezani, A. A Shot at Dendritic Cell-Based Vaccine Strategy against HIV-1. J. Med. Microbiol. Infect. Dis. 2019, 7, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Abrams, D.I.; Kuno, S.; Wong, R.; Jeffords, K.; Nash, M.; Molaghan, J.B.; Gorter, R.; Ueno, R. Oral Dextran Sulfate (UAOO1) in the Treatment of the Acquired Immunodeficiency Syndrome (AIDS) and AIDS-Related Complex. Ann. Intern. Med. 1989, 110, 183. [Google Scholar] [CrossRef]
- Gordon, M.; Guralnik, M.; Kaneko, Y.; Mimura, T.; Baker, M.; Lang, W. A phase I study of curdlan sulfate—An HIV inhibitor. Tolerance, pharmacokinetics and effects on coagulation and on CD4 lymphocytes. J. Med. 1994, 25, 163–180. [Google Scholar]
- Rusconi, S.; Moonis, M.; Merrill, D.P.; Pallai, P.V.; Neidhardt, E.A.; Singh, S.K.; Willis, K.J.; Osburne, M.S.; Profy, A.T.; Jenson, J.C.; et al. Naphthalene sulfonate polymers with CD4-blocking and anti-human immunodeficiency virus type 1 activities. Antimicrob. Agents Chemother. 1996, 40, 234–236. [Google Scholar] [CrossRef] [Green Version]
- Kozal, M.; Aberg, J.; Pialoux, G.; Cahn, P.; Thompson, M.; Molina, J.M.; Grinsztejn, B.; Diaz, R.; Castagna, A.; Kumar, P.; et al. Fostemsavir in Adults with Multidrug-Resistant HIV-1 Infection. N. Engl. J. Med. 2020, 382, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Griffithsin, A Highly Potent Broad-Spectrum Antiviral Lectin from Red Algae: From Discovery to Clinical Application. Mar. Drugs 2019, 17, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, J.M.; Israel, R.J.; Lowy, I.; Ostrow, N.A.; Vassilatos, L.S.; Barish, M.; Tran, D.N.H.; Sullivan, B.M.; Ketas, T.J.; O’Neill, T.J.; et al. Treatment of Advanced Human Immunodeficiency Virus Type 1 Disease with the Viral Entry Inhibitor PRO 542. Antimicrob. Agents Chemother. 2004, 48, 423–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayana, S.; Khanlou, H. Maraviroc: A new CCR5 antagonist. Expert Rev. Anti-Infect. Ther. 2009, 7, 9–19. [Google Scholar] [CrossRef]
- Xue, C.-B.; Chen, L.; Cao, G.; Zhang, K.; Wang, A.; Meloni, D.; Glenn, J.; Anand, R.; Xia, M.; Kong, L.; et al. Discovery of INCB9471, a Potent, Selective, and Orally Bioavailable CCR5 Antagonist with Potent Anti-HIV-1 Activity. ACS Med. Chem. Lett. 2010, 1, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Stupple, P.A.; Batchelor, D.V.; Corless, M.; Dorr, P.K.; Ellis, D.; Fenwick, D.R.; Galan, S.R.G.; Jones, R.M.; Mason, H.J.; Middleton, D.S.; et al. An Imidazopiperidine Series of CCR5 Antagonists for the Treatment of HIV: The Discovery ofN-{(1S)-1-(3-Fluorophenyl)-3-[(3-endo)-3-(5-isobutyryl-2-methyl-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridin-1-yl)-8-azabicyclo[3.2.1]oct-8-yl]propyl}acetamide (PF-232798). J. Med. Chem. 2011, 54, 67–77. [Google Scholar] [CrossRef]
- Baba, M.; Takashima, K.; Miyake, H.; Kanzaki, N.; Teshima, K.; Wang, X.; Shiraishi, M.; Iizawa, Y. TAK-652 Inhibits CCR5-Mediated Human Immunodeficiency Virus Type 1 Infection In Vitro and Has Favorable Pharmacokinetics in Humans. Antimicrob. Agents Chemother. 2005, 49, 4584–4591. [Google Scholar] [CrossRef] [Green Version]
- Caseiro, M.M.; Nelson, M.; Diaz, R.S.; Gathe, J.; Neto, J.L.D.A.; Slim, J.; Solano, A.; Netto, E.M.; Mak, C.; Shen, J.; et al. Vicriviroc plus optimized background therapy for treatment-experienced subjects with CCR5 HIV-1 infection: Final results of two randomized phase III trials. J. Infect. 2012, 65, 326–335. [Google Scholar] [CrossRef]
- Nichols, W.G.; Steel, H.M.; Bonny, T.; Adkison, K.; Curtis, L.; Millard, J.; Kabeya, K.; Clumeck, N. Hepatotoxicity Observed in Clinical Trials of Aplaviroc (GW873140). Antimicrob. Agents Chemother. 2007, 52, 858–865. [Google Scholar] [CrossRef] [Green Version]
- Lalezari, J.; Yadavalli, G.K.; Para, M.; Richmond, G.; DeJesus, E.; Brown, S.J.; Cai, W.; Chen, C.; Zhong, J.; Novello, L.A.; et al. Safety, Pharmacokinetics, and Antiviral Activity of HGS004, a Novel Fully Human IgG4 Monoclonal Antibody against CCR5, in HIV-1–Infected Patients. J. Infect. Dis. 2008, 197, 721–727. [Google Scholar] [CrossRef] [Green Version]
- Dhody, K.; Pourhassan, N.; Kazempour, K.; Green, D.; Badri, S.; Mekonnen, H.; Burger, D.; Maddon, P.J. PRO 140, a monoclonal antibody targeting CCR5, as a long-acting, single-agent maintenance therapy for HIV-1 infection. HIV Clin. Trials 2018, 19, 85–93. [Google Scholar] [CrossRef]
- Johnson, V.A.; Cramer, Y.S.; Rosenkranz, S.L.; Becker, S.; Klingman, K.L.; Kallungal, B.; Coakley, E.; Acosta, E.P.; Calandra, G.; Saag, M.S.; et al. Antiretroviral Activity of AMD11070 (An Orally Administered CXCR4 Entry Inhibitor): Results of NIH/NIAID AIDS Clinical Trials Group Protocol A5210. AIDS Res. Hum. Retrovir. 2019, 35, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Joly, V.; Jidar, K.; Tatay, M.; Yeni, P. Enfuvirtide: From basic investigations to current clinical use. Expert Opin. Pharmacother. 2010, 11, 2701–2713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jin, R.; Yao, C.; Zhang, T.; Wang, M.; Xia, W.; Peng, H.; Wang, X.; Lu, R.; Wang, C.; et al. Combination of long-acting HIV fusion inhibitor albuvirtide and LPV/r showed potent efficacy in HIV-1 patients. AIDS Res. Ther. 2016, 13, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leslie, G.J.; Wang, J.; Richardson, M.W.; Haggarty, B.S.; Hua, K.L.; Duong, J.; Secreto, A.J.; Jordon, A.P.O.; Romano, J.; Kumar, K.E.; et al. Potent and Broad Inhibition of HIV-1 by a Peptide from the gp41 Heptad Repeat-2 Domain Conjugated to the CXCR4 Amino Terminus. PLOS Pathog. 2016, 12, e1005983. [Google Scholar] [CrossRef]
- Armbruster, C.; Stiegler, G.M.; Vcelar, B.A.; Jäger, W.; Köller, U.; Jilch, R.; Ammann, C.G.; Pruenster, M.; Stoiber, H.; Katinger, H.W.D. Passive immunization with the anti-HIV-1 human monoclonal antibody (hMAb) 4E10 and the hMAb combination 4E10/2F5/2G12. J. Antimicrob. Chemother. 2004, 54, 915–920. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Yu, J.; Lanzi, A.; Yao, X.; Andrews, C.D.; Tsai, L.; Gajjar, M.R.; Sun, M.; Seaman, M.S.; Padte, N.N.; et al. Engineered Bispecific Antibodies with Exquisite HIV-1-Neutralizing Activity. Cell 2016, 165, 1621–1631. [Google Scholar] [CrossRef] [Green Version]
- Beccari, M.V.; Mogle, B.T.; Sidman, E.F.; Mastro, K.A.; Asiago-Reddy, E.; Kufel, W.D. Ibalizumab, a Novel Monoclonal Antibody for the Management of Multidrug-Resistant HIV-1 Infection. Antimicrob. Agents Chemother. 2019, 63, e00110-19. [Google Scholar] [CrossRef] [Green Version]
- Bobardt, M.D.; Armand-Ugón, M.; Clotet, I.; Zhang, Z.; David, G.; Esté, J.A.; Gallay, P.A. Effect of polyanion-resistance on HIV-1 infection. Virol. 2004, 325, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Ito, M.; Baba, M.; Sato, A.; Pauwels, R.; De Clercq, E.; Shigeta, S. Mechanism of Inhibitory Effect of Dextran Sulfate and Heparin on Replication of Human Immunodeficiency Virus in Vitro. Antivir. Res. 1987, 7, 361–367. [Google Scholar] [CrossRef]
- Jagodziński, P.P.; Wiaderkiewicz, R.; Kurzawski, G.; Kloczewiak, M.; Nakashima, H.; Hyjek, E.; Yamamoto, N.; Uryu, T.; Kaneko, Y.; Posner, M.R.; et al. Mechanism of the Inhibitory Effect of Curdlan Sulfate on HIV-1 Infection in Vitro. Virology 1994, 202, 735–745. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.; Ramjee, G.; Kamali, A.; Rees, H.; Crook, A.M.; Gafos, M.; Jentsch, U.; Pool, R.; Chisembele, M.; Kapiga, S.; et al. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): A phase 3, randomised, double-blind, parallel-group trial. Lancet 2010, 376, 1329–1337. [Google Scholar] [CrossRef] [Green Version]
- Cahn, P.; Fink, V.; Patterson, P. Fostemsavir: A New CD4 Attachment Inhibitor. Curr. Opin. HIV AIDS 2018, 13, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Nowicka-Sans, B.; Gong, Y.-F.; McAuliffe, B.; Dicker, I.; Ho, H.-T.; Zhou, N.; Eggers, B.; Lin, P.-F.; Ray, N.; Wind-Rotolo, M.; et al. In VitroAntiviral Characteristics of HIV-1 Attachment Inhibitor BMS-626529, the Active Component of the Prodrug BMS-663068. Antimicrob. Agents Chemother. 2012, 56, 3498–3507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhou, N.; Sun, Y.; Ray, N.; Lataillade, M.; Hanna, G.J.; Krystal, M. Activity of the HIV-1 Attachment Inhibitor BMS-626529, the Active Component of the Prodrug BMS-663068, against CD4-Independent Viruses and HIV-1 Envelopes Resistant to Other Entry Inhibitors. Antimicrob. Agents Chemother. 2013, 57, 4172–4180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karadsheh, R.; Meuser, M.E.; Cocklin, S. Composition and Orientation of the Core Region of Novel HIV-1 Entry Inhibitors Influences Metabolic Stability. Molecules 2020, 25, 1430. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C.; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W.; McMahon, J.B.; et al. Isolation and Characterization of Griffithsin, a Novel HIV-inactivating Protein, from the Red Alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef] [Green Version]
- Lusvarghi, S.; Lohith, K.; Morin-Leisk, J.; Ghirlando, R.; Hinshaw, J.E.; Bewley, C.A. Binding Site Geometry and Subdomain Valency Control Effects of Neutralizing Lectins on HIV-1 Viral Particles. ACS Infect. Dis. 2016, 2, 882–891. [Google Scholar] [CrossRef]
- Alexandre, K.B.; Moore, P.L.; Nonyane, M.; Gray, E.S.; Ranchobe, N.; Chakauya, E.; McMahon, J.B.; O’Keefe, B.R.; Chikwamba, R.; Morris, L. Mechanisms of HIV-1 subtype C resistance to GRFT, CV-N and SVN. Virology 2013, 446, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Allaway, G.P.; Davis-Bruno, K.L.; Beaudry, G.A.; Garcia, E.B.; Wong, E.L.; Ryder, A.M.; Hasel, K.W.; Gauduin, M.-C.; Koup, R.A.; McDougal, J.S.; et al. Expression and Characterization of CD4-IgG2, a Novel Heterotetramer That Neutralizes Primary HIV Type 1 Isolates. AIDS Res. Hum. Retrovir. 1995, 11, 533–539. [Google Scholar] [CrossRef]
- Shearer, W.T.; Israel, R.J.; Starr, S.; Fletcher, C.V.; Wara, D.; Rathore, M.; Church, J.; Deville, J.; Fenton, T.; Graham, B.; et al. Recombinant CD4-IgG2 in Human Immunodeficiency Virus Type 1–Infected Children: Phase 1/2 Study. J. Infect. Dis. 2000, 182, 1774–1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggink, D.; Berkhout, B.; Sanders, R.W. Inhibition of HIV-1 by Fusion Inhibitors. Curr. Pharm. Des. 2010, 16, 3716–3728. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.; Oas, T.; McDanal, C.; Bolognesi, D.; Matthews, T. A synthetic peptide inhibitor of human immunodeficiency virus replication: Correlation between solution structure and viral inhibition. Proc. Natl. Acad. Sci. USA 1992, 89, 10537–10541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Cheng, Y.; Li, W.; Wenjie, M.; Jiahong, X.; Jiahai, X.; Mingxian, H.; Bo, C.; Bin, L.; Xiaolin, L.; et al. An Albumin-Conjugated Peptide Exhibits Potent Anti-HIV Activity and Long in vivo Half-Life. Antimicrob. Agents Chemother. 2009, 54, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Chong, H.; Yao, X.; Zhang, C.; Cai, L.; Cui, S.; Wang, Y.; He, Y. Biophysical Property and Broad Anti-HIV Activity of Albuvirtide, a 3-Maleimimidopropionic Acid-Modified Peptide Fusion Inhibitor. PLoS ONE 2012, 7, e32599. [Google Scholar] [CrossRef] [Green Version]
- Ofek, G.; Tang, M.; Sambor, A.; Katinger, H.; Mascola, J.R.; Wyatt, R.; Kwong, P.D. Structure and Mechanistic Analysis of the Anti-Human Immunodeficiency Virus Type 1 Antibody 2F5 in Complex with Its gp41 Epitope. J. Virol. 2004, 78, 10724–10737. [Google Scholar] [CrossRef] [Green Version]
- Mascola, J.R.; Louder, M.K.; VanCott, T.C.; Sapan, C.V.; Lambert, J.S.; Muenz, L.R.; Bunow, B.; Birx, D.L.; Robb, M.L. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J. Virol. 1997, 71, 7198–7206. [Google Scholar] [CrossRef] [Green Version]
- Rujas, E.; Gulzar, N.; Morante, K.; Tsumoto, K.; Scott, J.K.; Nieva, J.L.; Caaveiro, J.M. Structural and Thermodynamic Basis of Epitope Binding by Neutralizing and Nonneutralizing Forms of the Anti-HIV-1 Antibody 4E10. J. Virol. 2015, 89, 11975–11989. [Google Scholar] [CrossRef] [Green Version]
- Barbian, H.J.; Decker, J.M.; Bibollet-Ruche, F.; Galimidi, R.P.; West, A.P., Jr.; Learn, G.H.; Parrish, N.F.; Iyer, S.S.; Li, Y.; Pace, C.S.; et al. Neutralization Properties of Simian Immunodeficiency Viruses Infecting Chimpanzees and Gorillas. mBio 2015, 6, e00296-15. [Google Scholar] [CrossRef] [Green Version]
- Xiao, T.; Frey, G.; Fu, Q.; LaVine, C.L.; Scott, D.A.; Seaman, M.S.; Chou, J.J.; Chen, B. HIV-1 fusion inhibitors targeting the membrane-proximal external region of Env spikes. Nat. Chem. Biol. 2020, 16, 529–537. [Google Scholar] [CrossRef]
- Blair, H.A. Ibalizumab: A Review in Multidrug-Resistant HIV-1 Infection. Drugs 2020, 80, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.M.; Kuritzkes, D.R.; Godofsky, E.; DeJesus, E.; Larson, J.A.; Weinheimer, S.P.; Lewis, S.T. Safety, Pharmacokinetics, and Antiretroviral Activity of Multiple Doses of Ibalizumab (formerly TNX-355), an Anti-CD4 Monoclonal Antibody, in Human Immunodeficiency Virus Type 1-Infected Adults. Antimicrob. Agents Chemother. 2008, 53, 450–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connell, B.J.; Hermans, L.E.; Wensing, A.M.J.; Schellens, I.; Schipper, P.J.; Van Ham, P.M.; De Jong, D.T.C.M.; Otto, S.; Mathe, T.; Moraba, R.; et al. Immune activation correlates with and predicts CXCR4 co-receptor tropism switch in HIV-1 infection. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.; Armour, D. The Discovery of the CCR5 Receptor Antagonist, UK-427,857, A New Agent for the Treatment of HIV Infection and AIDS. Prog. Med. Chem. 2005, 43, 239–271. [Google Scholar] [CrossRef]
- Tan, Q.; Zhu, Y.; Li, J.; Chen, Z.; Han, G.W.; Kufareva, I.; Li, T.; Ma, L.; Fenalti, G.; Zhang, W.; et al. Structure of the CCR5 Chemokine Receptor-HIV Entry Inhibitor Maraviroc Complex. Science 2013, 341, 1387–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, N.; Solomon, K.; Zhou, N.; Wang, K.H.; Garlapati, V.; Thomas, B.; Li, Y.; Covington, M.; Baribaud, F.; Erickson-Viitanen, S.; et al. Identification and Characterization of INCB9471, an Allosteric Noncompetitive Small-Molecule Antagonist of C-C Chemokine Receptor 5 with Potent Inhibitory Activity against Monocyte Migration and HIV-1 Infection. J. Pharmacol. Exp. Ther. 2011, 338, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, Y.-L.; Li, J.; Liu, H.; Zhao, Q.; Wu, B.; Yang, Z. Molecular binding mode of PF-232798, a clinical anti-HIV candidate, at chemokine receptor CCR5. Acta Pharmacol. Sin. 2018, 40, 563–568. [Google Scholar] [CrossRef]
- Lalezari, J.; Gathe, J.; Brinson, C.; Thompson, M.; Cohen, C.; DeJesus, E.; Galindez, J.; Ernst, J.A.; Martin, D.E.; Palleja, S.M. Safety, Efficacy, and Pharmacokinetics of TBR-652, a CCR5/CCR2 Antagonist, in HIV-1–Infected, Treatment-Experienced, CCR5 Antagonist–Naive Subjects. JAIDS J. Acquir. Immune Defic. Syndr. 2011, 57, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Strizki, J.M.; Tremblay, C.; Xu, S.; Wojcik, L.; Wagner, N.; Gonsiorek, W.; Hipkin, R.W.; Chou, C.-C.; Pugliese-Sivo, C.; Xiao, Y.; et al. Discovery and Characterization of Vicriviroc (SCH 417690), a CCR5 Antagonist with Potent Activity against Human Immunodeficiency Virus Type 1. Antimicrob. Agents Chemother. 2005, 49, 4911–4919. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.-T.; Yang, Y.; Xu, Y.; An, J. Targeting Chemokine Receptor CXCR4 for Treatment of HIV-1 Infection, Tumor Progression, and Metastasis. Curr. Top. Med. Chem. 2014, 14, 1574–1589. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.S.Y.; Bodart, V.; Metz, M.; Labrecque, J.; Bridger, G.; Fricker, S.P. Comparison of the Potential Multiple Binding Modes of Bicyclam, Monocylam, and Noncyclam Small-Molecule CXC Chemokine Receptor 4 Inhibitors. Mol. Pharmacol. 2008, 74, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Donzella, G.A.; Schols, D.; Lin, S.W.; Esté, J.A.; Nagashima, K.A.; Maddon, P.J.; Allaway, G.P.; Sakmar, T.P.; Henson, G.; Declercq, E.; et al. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med. 1998, 4, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, C.W.; Flexner, C.; MacFarland, R.T.; Giandomenico, C.; Fuchs, E.J.; Redpath, E.; Bridger, G.; Henson, G.W. Pharmacokinetics and Safety of AMD-3100, a Novel Antagonist of the CXCR-4 Chemokine Receptor, in Human Volunteers. Antimicrob. Agents Chemother. 2000, 44, 1667–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, C.; Daugvilaite, V.; Steen, A.; Jørgensen, A.S.; Våbenø, J.; Rosenkilde, M.M. Inhibition of HIV Fusion by Small Molecule Agonists through Efficacy-Engineering of CXCR4. ACS Chem. Biol. 2018, 13, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Showa, S.P.; Nyabadza, F.; Hove-Musekwa, S.D. On the efficiency of HIV transmission: Insights through discrete time HIV models. PLoS ONE 2019, 14, e0222574. [Google Scholar] [CrossRef]
- Parsons, M.S.; Le Grand, R.; Kent, S.J. Neutralizing Antibody-Based Prevention of Cell-Associated HIV-1 Infection. Viruses 2018, 10, 333. [Google Scholar] [CrossRef] [Green Version]
- Rawat, P.; Hon, S.; Teodorof-Diedrich, C.; Spector, S.A. Trehalose Inhibits Human Immunodeficiency Virus Type 1 Infection in Primary Human Macrophages and CD4+ T Lymphocytes through Two Distinct Mechanisms. J. Virol. 2020, 94, 94. [Google Scholar] [CrossRef]
- Welch, J.L.; Xiang, J.; Okeoma, C.M.; Schlievert, P.M.; Stapleton, J.T. Glycerol Monolaurate, an Analogue to a Factor Secreted by Lactobacillus, Is Virucidal against Enveloped Viruses, Including HIV-1. mBio 2020, 11, e00686-20. [Google Scholar] [CrossRef]
- Li, Q.; Estes, J.D.; Schlievert, P.M.; Duan, L.; Brosnahan, A.J.; Southern, P.J.; Reilly, C.S.; Peterson, M.L.; Schultz-Darken, N.; Brunner, K.G.; et al. Glycerol monolaurate prevents mucosal SIV transmission. Nat. Cell Biol. 2009, 458, 1034–1038. [Google Scholar] [CrossRef]
- Gardner, M.R. Promise and Progress of an HIV-1 Cure by Adeno-Associated Virus Vector Delivery of Anti-HIV-1 Biologics. Front. Cell. Infect. Microbiol. 2020, 10, 176. [Google Scholar] [CrossRef]
- Priddy, F.H.; Lewis, D.J.M.; Gelderblom, H.C.; Hassanin, H.; Streatfield, C.; Labranche, C.; Hare, J.; Cox, J.H.; Dally, L.; Bendel, D.; et al. Adeno-associated virus vectored immunoprophylaxis to prevent HIV in healthy adults: A phase 1 randomised controlled trial. Lancet HIV 2019, 6, e230–e239. [Google Scholar] [CrossRef] [Green Version]


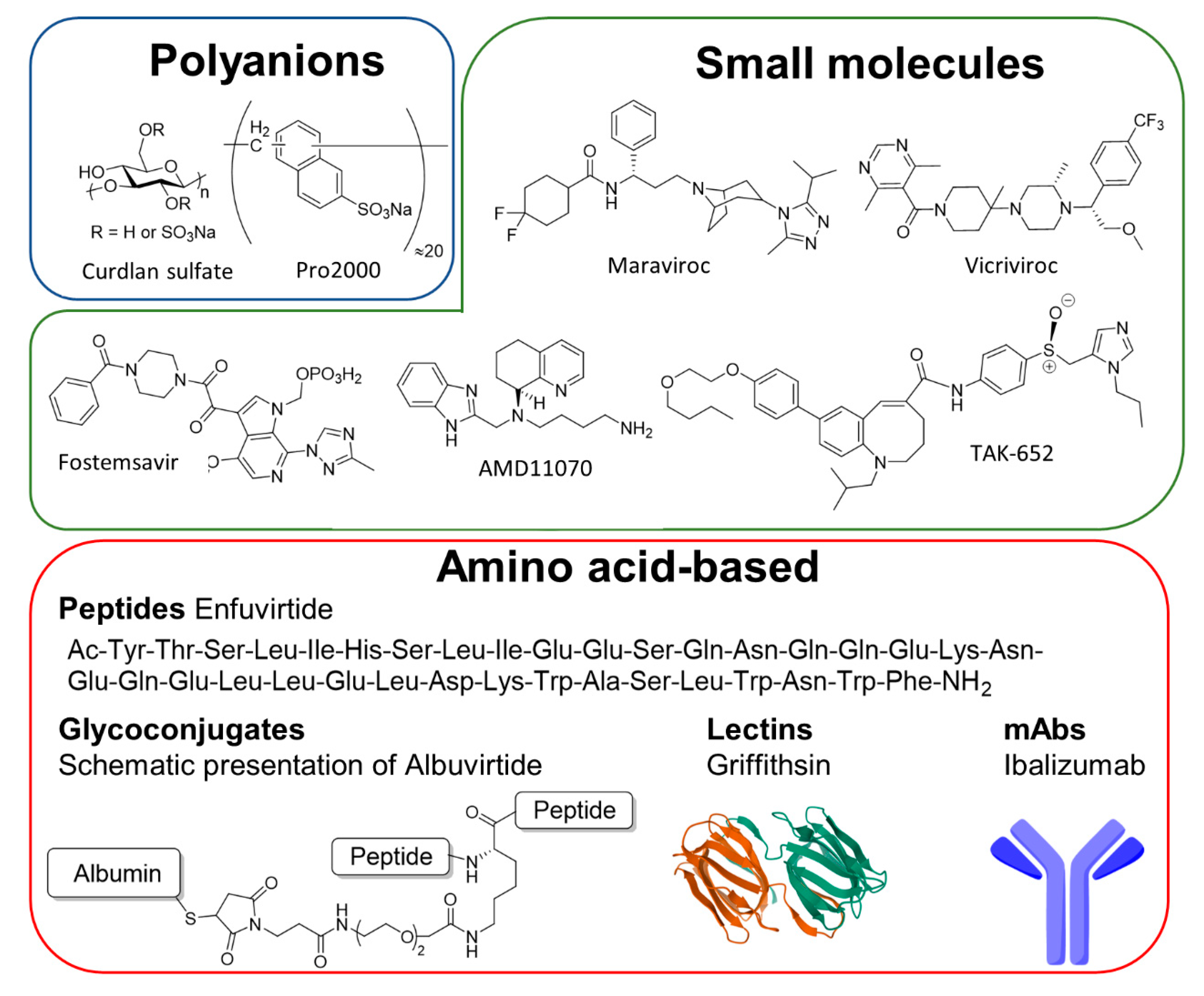
| Site of Interaction | Name of Inhibitor | Generic Name | Type of Molecule | Current Clinical Status | Reference |
|---|---|---|---|---|---|
| GP120 | Dextrane sulfate (UA001) | - | polyanion | Phase I, NCT00001009 | [230] |
| Curdlan sulfate | - | polyanion | Phase I, NCT00002100 | [231] | |
| Pro2000 | - | polyanion | Phase III, NCT00262106 | [232] | |
| Fostemsavir | Rukobia | small molecule | Approved | [233] | |
| Griffithsin gel | - | lectin | Phase I, NCT02875119 | [234] | |
| F105 | - | mAb | Phase I, NCT00001105 | [178] | |
| 3BNC117 | - | mAb | Phase I+II, NCT02588586 | [180,181] | |
| 10-1074-LS | - | mAb | Phase I, NCT03554408 | [182] | |
| VRC01 | - | mAb | Phase I+II, NCT02664415 | [184] | |
| VRC01LS | - | mAb | Phase I+II, NCT02797171 | [185] | |
| CD4-Ig2 (PRO 542) | - | bAVP | Phase I/II, NCT00055185 | [235] | |
| CCR5 | Maraviroc | Celsentri | small molecule | Approved | [236] |
| INCB009471 | - | small molecule | Phase II, NCT00393120 | [237] | |
| PF-232798 | - | small molecule | Phase II, NCT00495677 | [238] | |
| TAK-652 | Cenicriviroc | small molecule | Phase II, NCT01092104 | [239] | |
| MK-4176, SCH 417690 | Viciviroc | small molecule | Phase III, NCT00474370 | [240] | |
| GW873140, AK602 | Aplaviroc | small molecule | Phase II+III terminated, NCT00197145 | [241] | |
| HGS004 | - | mAb | Phase I, NCT00114699 | [242] | |
| PRO 140 | Leronlimab | mAb | Phase II/III, NCT03902522 | [243] | |
| CXCR4 | AMD11070 | - | small molecule | Phase I/II, NCT00089466 | [244] |
| GP41 | Enfuvirtide | Fuzeon | peptide | Approved | [245] |
| Albuvirtide | - | protein-conjugate | Phase II+III, NCT04560569 | [246] | |
| C34-CXCR4 | - | cell | Phase I, NCT03020524 | [247] | |
| 2F5/4E10 | - | mAb | Phase I/II, NCT00219986 | [248] | |
| 10E8.4/iMab | - | bAVP | Phase I, NCT03875209 | [249] | |
| CD4 | Ibalizumab (TNX-355) | Trogarzo | mAb | Approved | [250] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruxelle, J.-F.; Trattnig, N.; Mureithi, M.W.; Landais, E.; Pantophlet, R. HIV-1 Entry and Prospects for Protecting against Infection. Microorganisms 2021, 9, 228. https://doi.org/10.3390/microorganisms9020228
Bruxelle J-F, Trattnig N, Mureithi MW, Landais E, Pantophlet R. HIV-1 Entry and Prospects for Protecting against Infection. Microorganisms. 2021; 9(2):228. https://doi.org/10.3390/microorganisms9020228
Chicago/Turabian StyleBruxelle, Jean-François, Nino Trattnig, Marianne W. Mureithi, Elise Landais, and Ralph Pantophlet. 2021. "HIV-1 Entry and Prospects for Protecting against Infection" Microorganisms 9, no. 2: 228. https://doi.org/10.3390/microorganisms9020228
APA StyleBruxelle, J.-F., Trattnig, N., Mureithi, M. W., Landais, E., & Pantophlet, R. (2021). HIV-1 Entry and Prospects for Protecting against Infection. Microorganisms, 9(2), 228. https://doi.org/10.3390/microorganisms9020228









