Genetic Diversity of Serine Protease Inhibitors in Myxozoan (Cnidaria, Myxozoa) Fish Parasites
Abstract
1. Introduction
2. Materials and Methods
2.1. Sequence Database Mining
2.2. PCR Verification of in Silico Identified Myxozoan Serpins
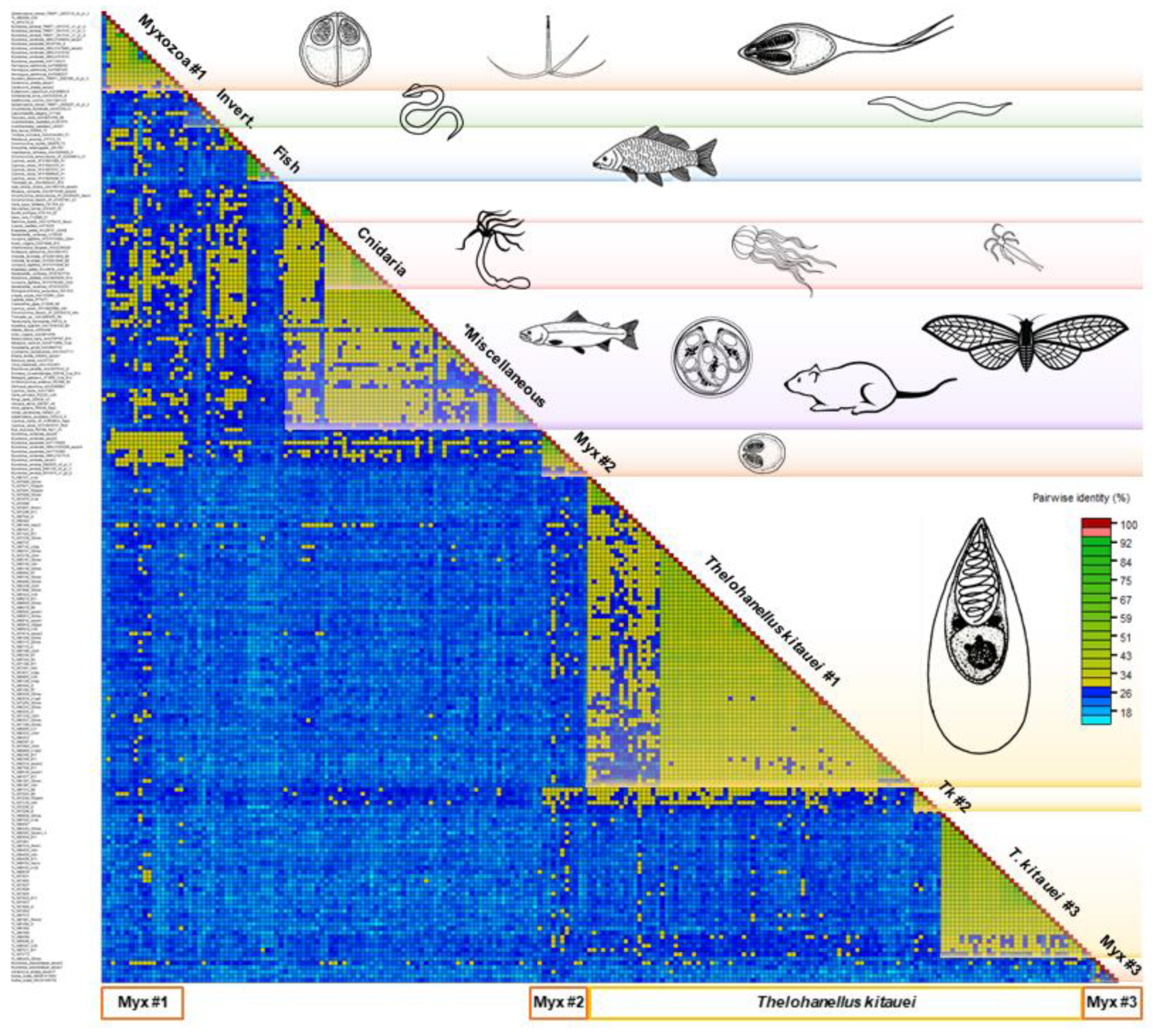
2.3. Sequence Alignment and Pairwise Sequence Identity Calculations
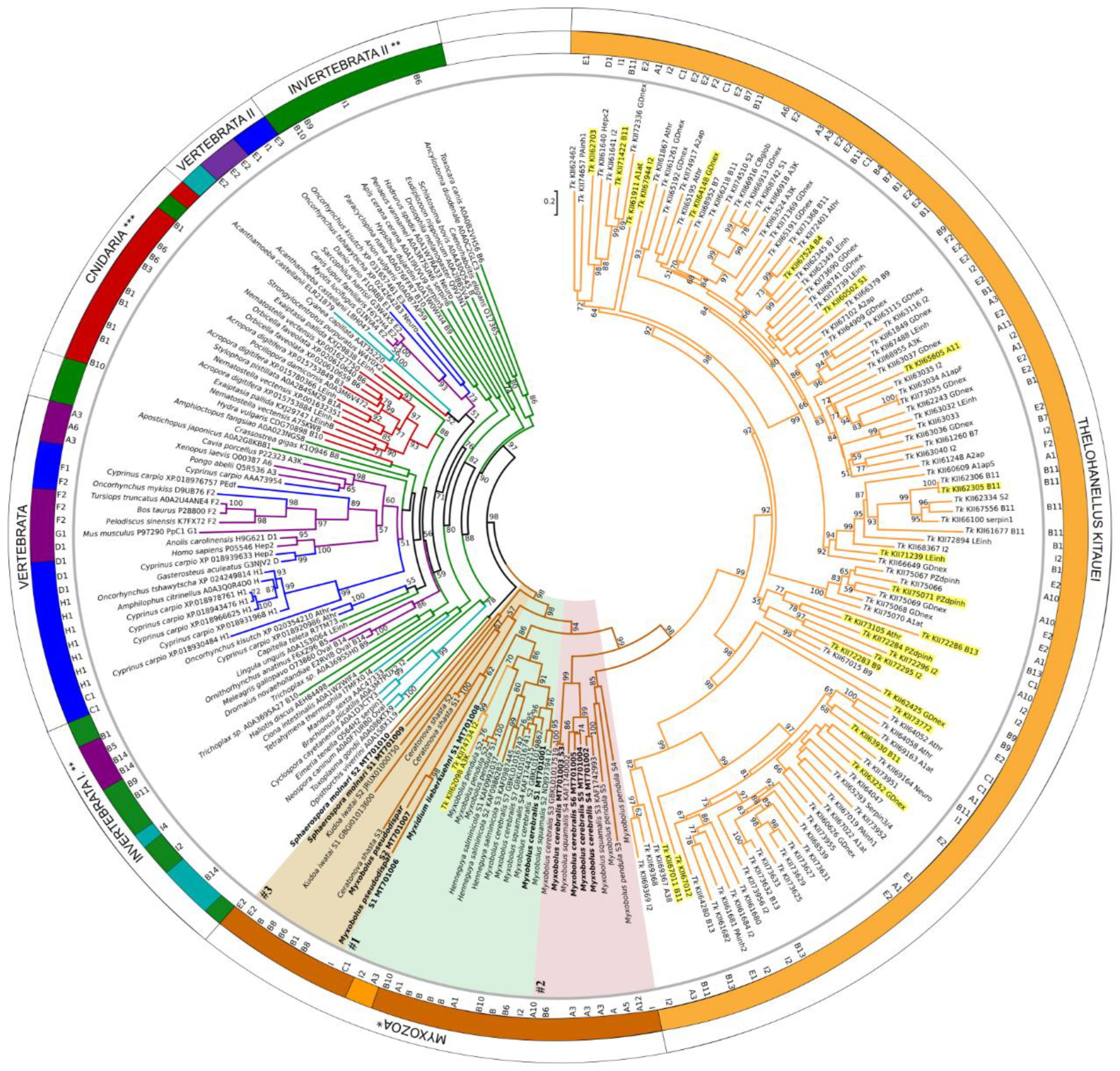
2.4. Phylogenetic Analysis
3. Results
3.1. Serpin Screening
3.2. Serpin Diversity
3.3. Conserved Domains
3.4. Serpin Phylogeny
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Okamura, B.; Gruhl, A.; Bartholomew, J.L. Myxozoan Evolution, Ecology and Development; Springer International Publishing: Cham, Switzerland, 2015; p. 441. [Google Scholar]
- Forró, B.; Eszterbauer, E. Correlation between host specificity and genetic diversity for the muscle-dwelling fish parasite Myxobolus pseudodispar: Examples of myxozoan host-shift? Folia Parasitol. 2016, 63, 2016.019. [Google Scholar] [CrossRef]
- Holzer, A.S.; Bartošová-Sojková, P.; Born-Torrijos, A.; Lövy, A.; Hartigan, A.; Fiala, I. The joint evolution of the Myxozoa and their alternate hosts: A cnidarian recipe for success and vast biodiversity. Mol. Ecol. 2018, 27, 1651–1666. [Google Scholar] [CrossRef]
- Lisnerová, M.; Fiala, I.; Cantatore, D.; Irigoitia, M.; Timi, J.; Pecková, H.; Bartošová-Sojková, P.; Sandoval, C.M.; Luer, C.; Morris, J.; et al. Mechanisms and Drivers for the Establishment of Life Cycle Complexity in Myxozoan Parasites. Biology 2020, 9, 10. [Google Scholar] [CrossRef]
- Eszterbauer, E.; Kallert, D.M.; Grabner, D.; El-Matbouli, M. Differentially expressed parasite genes involved in host recognition and invasion of the triactinomyxon stage of Myxobolus cerebralis (Myxozoa). Parasitology 2009, 136, 367–377. [Google Scholar] [CrossRef]
- Hartigan, A.; Estensoro, I.; Vancová, M.; Bílý, T.; Patra, S.; Eszterbauer, E.; Holzer, A.S. New cell motility model observed in parasitic cnidarian Sphaerospora molnari (Myxozoa:Myxosporea) blood stages in fish. Sci. Rep. 2016, 6, 39093. [Google Scholar] [CrossRef]
- Feist, S.W.; Morris, D.J.; Alama-Bermejo, G.; Holzer, A.S. Cellular Processes in Myxozoans. In Myxozoan Evolution, Ecology and Development; Springer International Publishing: Cham, Switzerland, 2015; pp. 139–154. [Google Scholar]
- Alama-Bermejo, G.; Holzer, A.S.; Bartholomew, J.L. Myxozoan Adhesion and Virulence: Ceratonova shasta on the Move. Microorganisms 2019, 7, 397. [Google Scholar] [CrossRef] [PubMed]
- Irving, J.A.; Pike, R.N.; Lesk, A.M.; Whisstock, J.C. Phylogeny of the Serpin Superfamily: Implications of Patterns of Amino Acid Conservation for Structure and Function. Genome Res. 2000, 10, 1845–1864. [Google Scholar] [CrossRef]
- Silverman, G.A.; Bird, P.I.; Carrell, R.W.; Church, F.C.; Coughlin, P.B.; Gettins, P.G.W.; Irving, J.A.; Lomas, D.A.; Luke, C.J.; Moyer, R.W.; et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J. Biol. Chem. 2001, 276, 33293–33296. [Google Scholar] [CrossRef]
- Mulenga, A.; Sugino, M.; Nakajima, M.; Sugimoto, C.; Onuma, M. Tick-encoded serine proteinase inhibitors (Serpins); Potential target antigens for tick vaccine development. J. Vet. Med. Sci. 2001, 63, 1063–1069. [Google Scholar] [CrossRef]
- Xu, T.; Lew-Tabor, A.; Rodriguez-Valle, M. Effective inhibition of thrombin by Rhipicephalus microplus serpin-15 (RmS-15) obtained in the yeast Pichia pastoris. Ticks Tick Borne Dis 2016, 7, 180–187. [Google Scholar] [CrossRef]
- Tirloni, L.; Reck, J.; Terra, R.M.S.; Martins, J.R.; Mulenga, A.; Sherman, N.E.; Fox, J.W.; Yates, J.R.; Termignoni, C.; Pinto, A.F.M.; et al. Proteomic Analysis of Cattle Tick Rhipicephalus (Boophilus) microplus Saliva: A Comparison between Partially and Fully Engorged Females. PLoS ONE 2014, 9, e94831. [Google Scholar] [CrossRef] [PubMed]
- Tirloni, L.; Kim, T.K.; Coutinho, M.L.; Ali, A.; Seixas, A.; Termignoni, C.; Mulenga, A.; da Silva Vaz, I. The putative role of Rhipicephalus microplus salivary serpins in the tick-host relationship. Insect Biochem. Mol. Biol. 2016, 71, 12–28. [Google Scholar] [CrossRef]
- Modica, M.V.; Sunagar, K.; Holford, M.; Dutertre, S. Editorial: Diversity and Evolution of Animal Venoms: Neglected Targets, Ecological Interactions, Future Perspectives. Front. Ecol. Evol. 2020, 8, 8. [Google Scholar] [CrossRef]
- Schick, C.; Pemberton, P.A.; Shi, G.P.; Kamachi, Y.; Çataltepe, S.; Bartuski, A.J.; Gornstein, E.R.; Brömme, D.; Chapman, H.A.; Silverman, G.A. Cross-class inhibition of the cysteine proteinases cathepsins K, L, and S by the serpin squamous cell carcinoma antigen 1: A kinetic analysis. Biochemistry 1998, 37, 5258–5266. [Google Scholar] [CrossRef] [PubMed]
- Huntington, J.A.; Read, R.J.; Carrell, R.W. Structure of a serpin-protease complex shows inhibition by deformation. Nature 2000, 407, 923–926. [Google Scholar] [CrossRef]
- Law, R.H.P.; Zhang, Q.; McGowan, S.; Buckle, A.M.; Silverman, G.A.; Wong, W.; Rosado, C.J.; Langendorf, C.G.; Pike, R.N.; Bird, P.I.; et al. An overview of the serpin superfamily. Genome Biol. 2006, 7, 1–11. [Google Scholar] [CrossRef][Green Version]
- Gettins, P.G.W. Serpin Structure, Mechanism, and Function. Chem. Rev. 2002, 102, 4751–4804. [Google Scholar] [CrossRef]
- Quezada, L.A.L.; McKerrow, J.H. Schistosome serine protease inhibitors: Parasite defense or homeostasis? An. Acad. Bras. Cienc. 2011, 83, 663–672. [Google Scholar] [CrossRef]
- Valdivieso, E.; Perteguer, M.J.; Hurtado, C.; Campioli, P.; Rodriguez, E.; Saborido, A.; Martinez-Sernandez, V.; Gomez-Puertas, P.; Ubeira, F.M.; Garate, T. ANISERP: a new serpin from the parasite Anisakis simplex. Parasit. Vectors 2015, 8, 399. [Google Scholar] [CrossRef]
- Maizels, R.M.; Gomez-Escobar, N.; Gregory, W.F.; Murray, J.; Zang, X.X. Immune evasion genes from filarial nematodes. Int. J. Parasitol. 2001, 31, 889–898. [Google Scholar] [CrossRef]
- Toubarro, D.; Lucena-Robles, M.; Nascimento, G.; Santos, R.; Montiel, R.; Veríssimo, P.; Pires, E.; Faro, C.; Coelho, A.V.; Simões, N. Serine protease-mediated host invasion by the parasitic nematode Steinernema carpocapsae. J. Biol. Chem. 2010, 285, 30666–30675. [Google Scholar] [CrossRef] [PubMed]
- Doyle, P.S.; Zhou, Y.M.; Hsieh, I.; Greenbaum, D.C.; McKerrow, J.H.; Engel, J.C. The trypanosoma cruzi protease cruzain mediates immune evasion. PLoS Pathog. 2011, 7, e1002139. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.S.; Reis, F.C.; Azevedo-Pereira, R.L.; Morrison, L.S.; Mottram, J.C.; Lima, A.P. Leishmania inhibitor of serine peptidase 2 prevents TLR4 activation by neutrophil elastase promoting parasite survival in murine macrophages. J. Immunol. 2011, 186, 411–422. [Google Scholar] [CrossRef]
- Alam, A.; Bhatnagar, R.K.; Relan, U.; Mukherjee, P.; Chauhan, V.S. Proteolytic activity of Plasmodium falciparum subtilisin-like protease 3 on parasite profilin, a multifunctional protein. Mol. Biochem. Parasitol. 2013, 191, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiong, J.; Zhou, Z.; Huo, F.; Miao, W.; Ran, C.; Liu, Y.; Zhang, J.; Feng, J.; Wang, M.; et al. The genome of the myxosporean Thelohanellus kitauei shows adaptations to nutrient acquisition within its fish host. Genome Biol. Evol. 2014, 6, 3182–3198. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.S.; Neuhof, M.; Rubinstein, N.D.; Diamant, A.; Philippe, H.; Huchon, D.; Cartwright, P. Genomic insights into the evolutionary origin of Myxozoa within Cnidaria. Proc. Natl. Acad. Sci. USA 2015, 112, 14912–14917. [Google Scholar] [CrossRef] [PubMed]
- Yahalomi, D.; Atkinson, S.D.; Neuhof, M.; Sally Chang, E.; Philippe, H.; Cartwright, P.; Bartholomew, J.L.; Huchon, D. A cnidarian parasite of salmon (Myxozoa: Henneguya) lacks a mitochondrial genome. Proc. Natl. Acad. Sci. USA 2020, 117, 5358–5363. [Google Scholar] [CrossRef]
- Foox, J.; Ringuette, M.; Desser, S.S.; Siddall, M.E. In silico hybridization enables transcriptomic illumination of the nature and evolution of Myxozoa. BMC Genom. 2015, 16, 840. [Google Scholar] [CrossRef]
- Nesnidal, M.P.; Helmkampf, M.; Bruchhaus, I.; El-Matbouli, M.; Hausdorf, B. Agent of whirling disease meets orphan worm: phylogenomic analyses firmly place Myxozoa in Cnidaria. PLoS ONE 2013, 8, e54576. [Google Scholar] [CrossRef]
- Alama-Bermejo, G.; Meyer, E.; Atkinson, S.D.; Holzer, A.S.; Wiśniewska, M.M.; Kolísko, M.; Bartholomew, J.L. Transcriptome-wide comparisons and virulence gene polymorphisms of host-associated genotypes of the cnidarian parasite Ceratonova shasta in salmonids. Genome Biol. Evol. 2020, 12, 1258–1276. [Google Scholar] [CrossRef]
- Hartigan, A.; Kosakyan, A.; Pecková, H.; Eszterbauer, E.; Holzer, A.S. Transcriptome of Sphaerospora molnari (Cnidaria, Myxosporea) blood stages provides proteolytic arsenal as potential therapeutic targets against sphaerosporosis in common carp. BMC Genom. 2020, 21, 404. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.H.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef] [PubMed]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Bateman, A. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef]
- Sipos, D.; Ursu, K.; Dán, Á.; Herczeg, D.; Eszterbauer, E. Susceptibility-related differences in the quantity of developmental stages of Myxobolus spp. (Myxozoa) in fish blood. PLoS ONE 2018, 13, e0204437. [Google Scholar] [CrossRef]
- Eszterbauer, E.; Atkinson, S.; Diamant, A.; Morris, D.; El-Matbouli Mansour, M.; Hartikainen, H. Myxozoan life cycles: Practical approaches and insights. In Myxozoan Evolution, Ecology and Development; Springer International Publishing: Cham, Switzerland, 2015; pp. 175–198. ISBN 9783319147536. [Google Scholar]
- Korytář, T.; Wiegertjes, G.F.; Zusková, E.; Tomanová, A.; Lisnerová, M.; Patra, S.; Sieranski, V.; Šíma, R.; Born-Torrijos, A.; Wentzel, A.S.; et al. The kinetics of cellular and humoral immune responses of common carp to presporogonic development of the myxozoan Sphaerospora molnari. Parasit. Vectors 2019, 12, 208. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: a virus classification tool based on pairwise sequence alignment and identity calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: a new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evol. 2020, 37, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Le, S.Q.; Gascuel, O. An improved general amino acid replacement matrix. Mol. Biol. Evol. 2008, 25, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable, and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Kumar, A.; Ragg, H. Ancestry and evolution of a secretory pathway serpin. BMC Evol. Biol. 2008, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wei, W.Y.; Wang, K.Y.; Yang, Q.; Wang, E.L. Pathological and immunological analyses of Thelohanellus kitauei (Myxozoa:Myxosporea) infection in the scattered mirror carp, Cyprinus carpio. Sci. Rep. 2019, 9, 20014. [Google Scholar] [CrossRef]
- Jordan, R.E. Antithrombin in vertebrate species: Conservation of the heparin-dependent anticoagulant mechanism. Arch. Biochem. Biophys. 1983, 227, 587–595. [Google Scholar] [CrossRef]
- Han, X.; Fiehler, R.; Broze, G.J. Isolation of a protein Z-dependent plasma protease inhibitor. Proc. Natl. Acad. Sci. USA 1998, 95, 9250–9255. [Google Scholar] [CrossRef]
- Sitjà-Bobadilla, A.; Calduch-Giner, J.; Saera-Vila, A.; Palenzuela, O.; Álvarez-Pellitero, P.; Pérez-Sánchez, J. Chronic exposure to the parasite Enteromyxum leei (Myxozoa: Myxosporea) modulates the immune response and the expression of growth, redox and immune relevant genes in gilthead sea bream, Sparus aurata L. Fish Shellfish Immunol. 2008, 24, 610–619. [Google Scholar] [CrossRef]
- Sitjà-Bobadilla, A.; Schmidt-Posthaus, H.; Wahli, T.; Holland, J.W.; Secombes, C.J. Fish Immune Responses to Myxozoa. In Myxozoan Evolution, Ecology and Development; Springer International Publishing: Cham, Switzerland, 2015; pp. 253–280. ISBN1 978-3-319-14752-9. ISBN2 978-3-319-14753-6. [Google Scholar]
- Fiala, I.; Bartosova, P. History of myxozoan character evolution on the basis of rDNA and EF-2 data. BMC Evol. Biol. 2010, 10, 228. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Lomas, D.A. Intracellular serpins, firewalls and tissue necrosis. Trends Cell Biol. 2008, 18, 45–47. [Google Scholar] [CrossRef] [PubMed]
- AmbuAli, A.; Monaghan, S.J.; McLean, K.; Inglis, N.F.; Bekaert, M.; Wehner, S.; Bron, J.E. Identification of proteins from the secretory/excretory products (SEPs) of the branchiuran ectoparasite Argulus foliaceus (Linnaeus, 1758) reveals unique secreted proteins amongst haematophagous ecdysozoa. Parasit Vectors 2020, 13, 88. [Google Scholar] [CrossRef]
- Putnam, N.H.; Srivastava, M.; Hellsten, U.; Dirks, B.; Chapman, J.; Salamov, A.; Terry, A.; Shapiro, H.; Lindquist, E.; Kapitonov, V.V.; et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 2007, 317, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.B.; Miller, D.; Rometo, D.; Greenberg, R.M.; Brömme, D.; Çataltepe, S.; Pak, S.C.; Mills, D.R.; Silverman, G.A.; Luke, C.J. Identification and activity of a lower eukaryotic serine proteinase inhibitor (serpin) from Cyanea capillata: Analysis of a jellyfish serpin, jellypin. Biochemistry 2004, 43, 11750–11759. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.H.; Hejgaard, J.; Saunders, N.F.W.; Cavicchioli, R.; Curmi, P.M.G. Serpins in unicellular Eukarya, Archaea, and Bacteria: Sequence analysis and evolution. J. Mol. Evol. 2004, 59, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Maizels, R.M. Serine proteinase inhibitors from nematodes and the arms race between host and pathogen. Trends Biochem. Sci. 2001, 26, 191–197. [Google Scholar] [CrossRef]

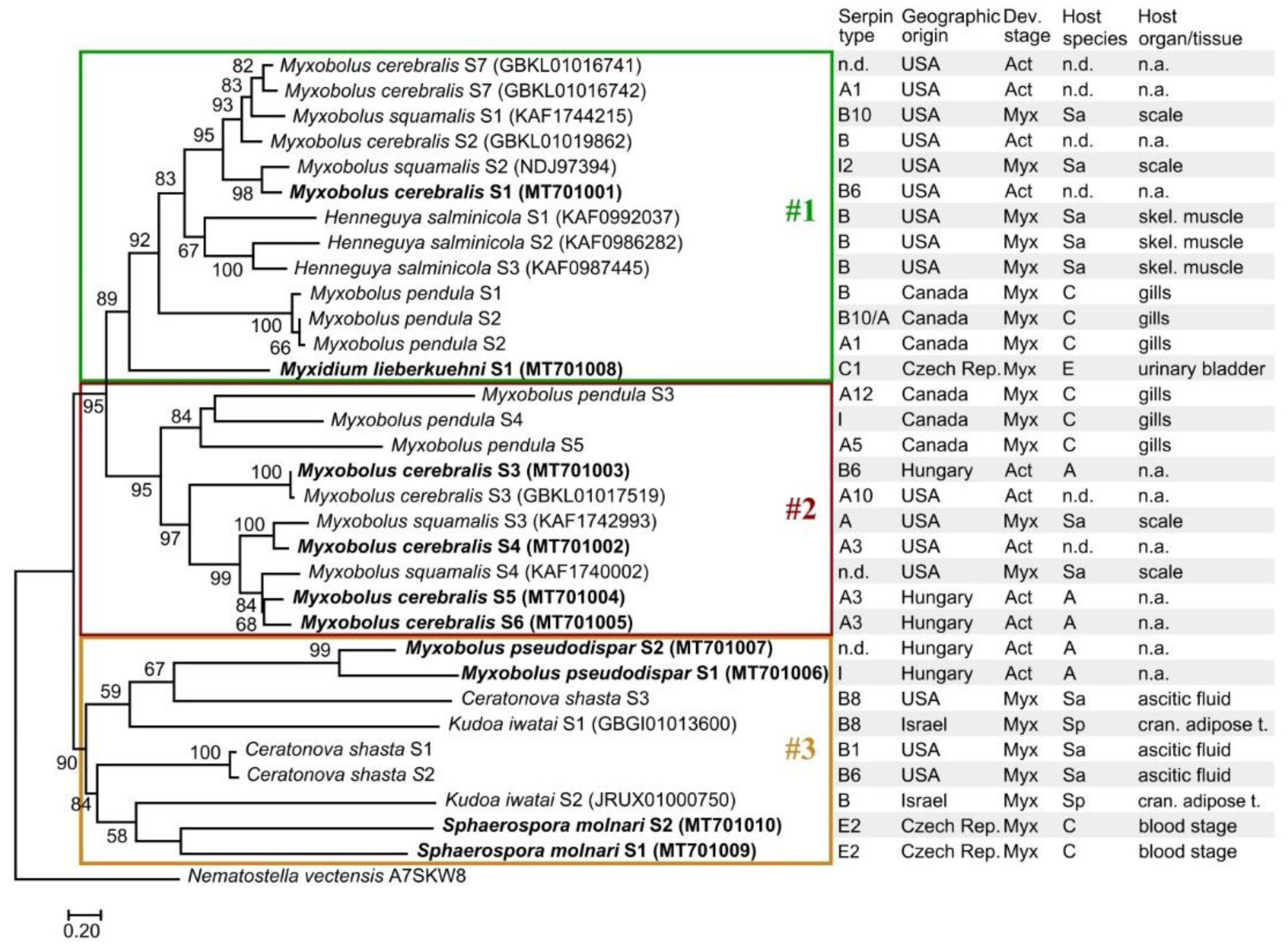
| Clade Identifier | Clade Name | Predicted Representatives among Myxozoans |
|---|---|---|
| A | alpha1-proteinase inhibitor (antitrypsin-like, extracellular) | Mc-S4 (MT701002); Mc-S5 (MT701004); Mc-S6 (MT701005); Mc-S7 (GBKL01016742); Mpe-S2 (SRX1269149); Mpe-S3 (SRX1269149); Mpe-S5 (SRX1269149); Ms-S3 (KAF1742993); Tk (various; see in Table S1) |
| B | intracellular; ov-serpins | Mc-S1 (MT701001); Mc-S2 (GBKL01019862); Mc-S3 (MT701003); Mpe-S1 (SRX1269149); Ms-S1 (KAF1744215); Cs-S1 (SRX3741971); Cs-S2 (SRX3741971); Cs-S3 (SRX3741971); Hs-S1 (KAF0992037); Hs-S2 (KAF0986282); Hs-S3 (KAF0987445); Ki-S1 (GBGI01013600); Ki-S2 (JRUX01000750); Tk (various; see in Table S1) |
| C | antithrombin | Ml-S1 (MT701008); Tk-(KII73105); Tk-(KII72401); Tk-(KII65195); Tk-(KII64058); Tk-(KII64052); Tk-(KII61867) |
| D | heparin cofactor 2 | Tk-(KII61640) |
| E | proteinase nexin | Sm-S1 (MT701009); Sm-S2 (MT701010); Tk (various) |
| F | alpha2-antiplasmin | Tk-(KII74917); Tk-(KII67102); Tk-(KII63034); Tk-(KII61248) |
| I | neuroserpin | Mps-S1 (MT701006); Ms-S2 (NDJ97394); Mpe-S4 (SRX1269149); Tk (various; see in Table S1) |
| - | unknown | Mps-S2 (MT701007); Ms-S4 (KAF1740002) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eszterbauer, E.; Sipos, D.; Kaján, G.L.; Szegő, D.; Fiala, I.; Holzer, A.S.; Bartošová-Sojková, P. Genetic Diversity of Serine Protease Inhibitors in Myxozoan (Cnidaria, Myxozoa) Fish Parasites. Microorganisms 2020, 8, 1502. https://doi.org/10.3390/microorganisms8101502
Eszterbauer E, Sipos D, Kaján GL, Szegő D, Fiala I, Holzer AS, Bartošová-Sojková P. Genetic Diversity of Serine Protease Inhibitors in Myxozoan (Cnidaria, Myxozoa) Fish Parasites. Microorganisms. 2020; 8(10):1502. https://doi.org/10.3390/microorganisms8101502
Chicago/Turabian StyleEszterbauer, Edit, Dóra Sipos, Győző L. Kaján, Dóra Szegő, Ivan Fiala, Astrid S. Holzer, and Pavla Bartošová-Sojková. 2020. "Genetic Diversity of Serine Protease Inhibitors in Myxozoan (Cnidaria, Myxozoa) Fish Parasites" Microorganisms 8, no. 10: 1502. https://doi.org/10.3390/microorganisms8101502
APA StyleEszterbauer, E., Sipos, D., Kaján, G. L., Szegő, D., Fiala, I., Holzer, A. S., & Bartošová-Sojková, P. (2020). Genetic Diversity of Serine Protease Inhibitors in Myxozoan (Cnidaria, Myxozoa) Fish Parasites. Microorganisms, 8(10), 1502. https://doi.org/10.3390/microorganisms8101502





