Supplementation with Combined Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 Across Development Reveals Sex Differences in Physiological and Behavioural Effects of Western Diet in Long–Evans Rats
Abstract
:1. Introduction
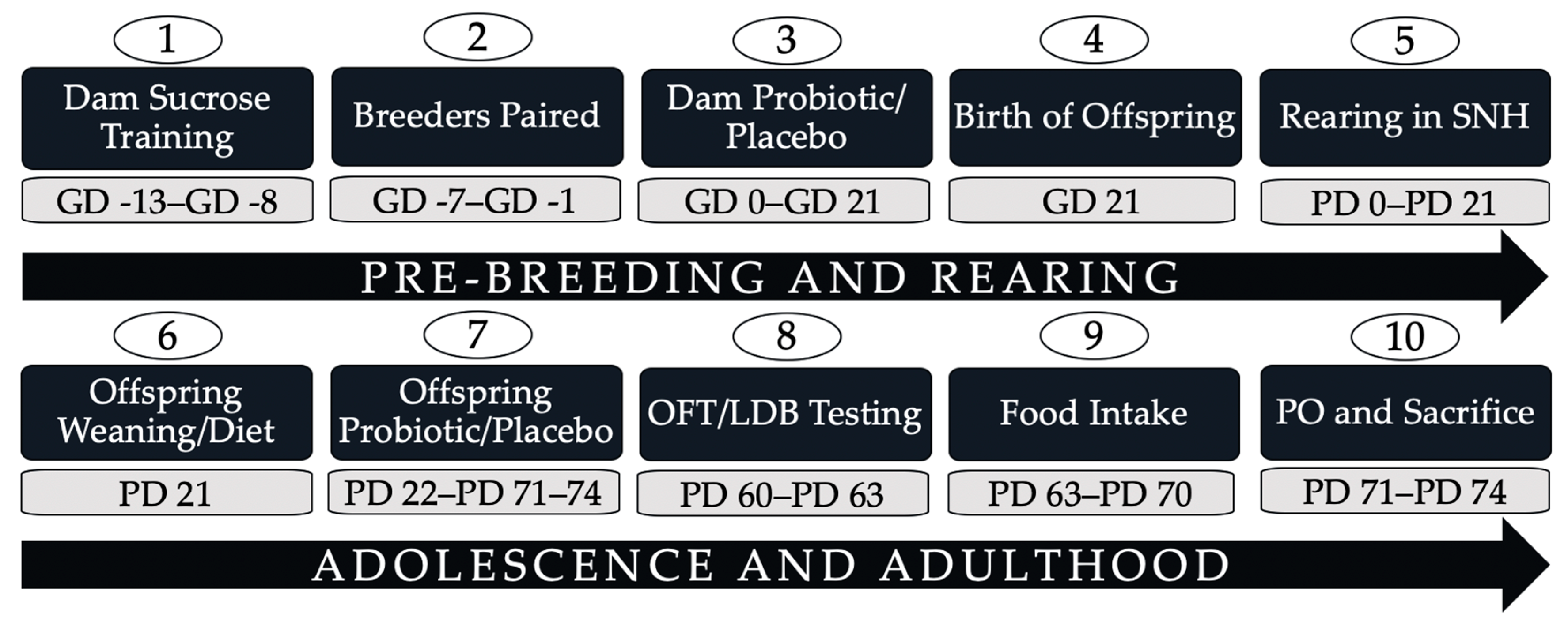
2. Materials and Methods
2.1. Animal and Breeding
2.2. Probiotic Administration
2.3. Diet Manipulation
2.4. Weight and Food Intake
2.5. Anxiety-Like Behaviours
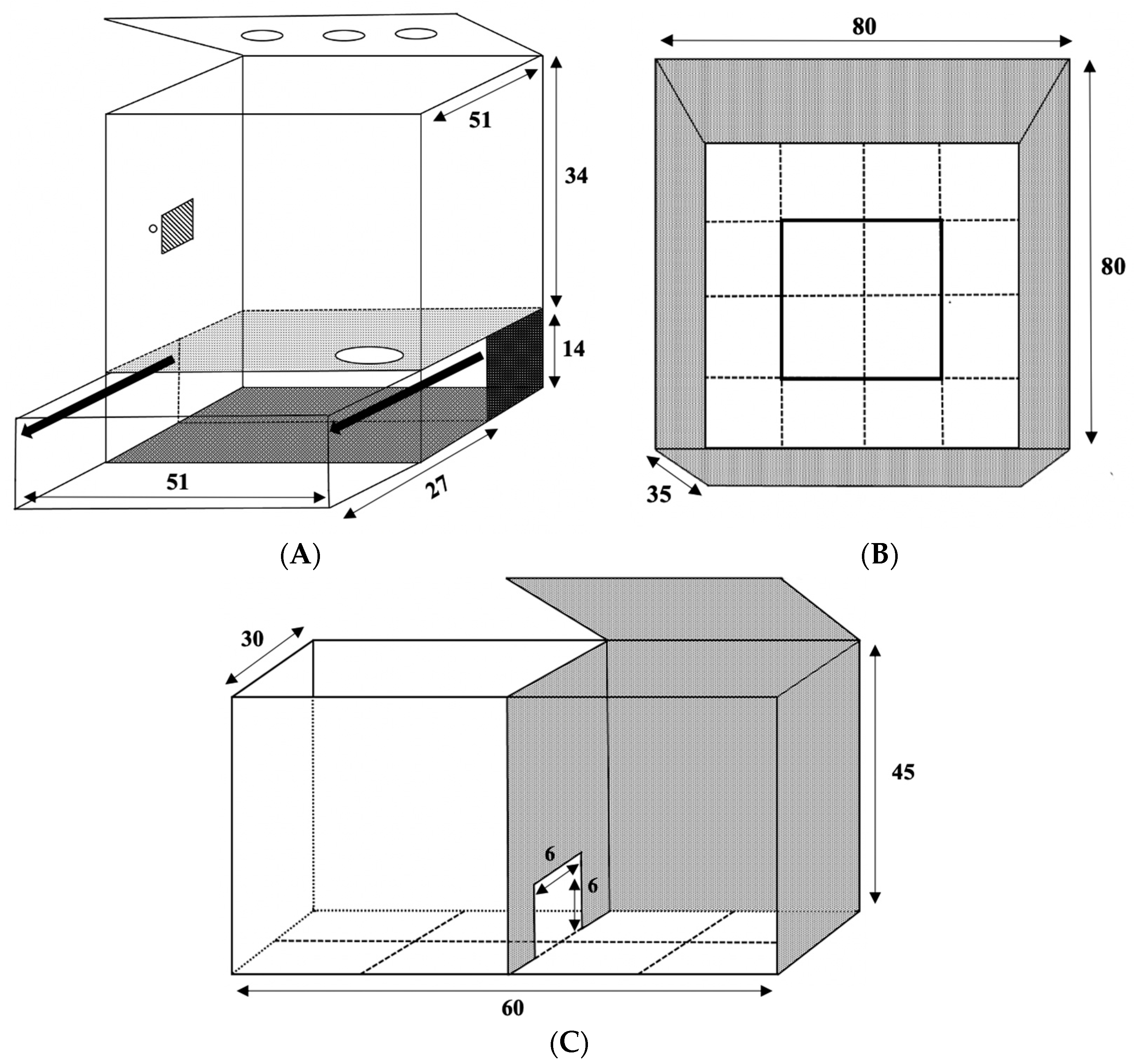
2.5.1. Open Field Test
2.5.2. Light–Dark Box
2.6. Predator Odour Exposure, Sacrifice, and Tissue Collection
2.7. Plasma Inflammatory Marker Multiplex Analysis
2.8. Plasma Leptin Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analyses
3. Results
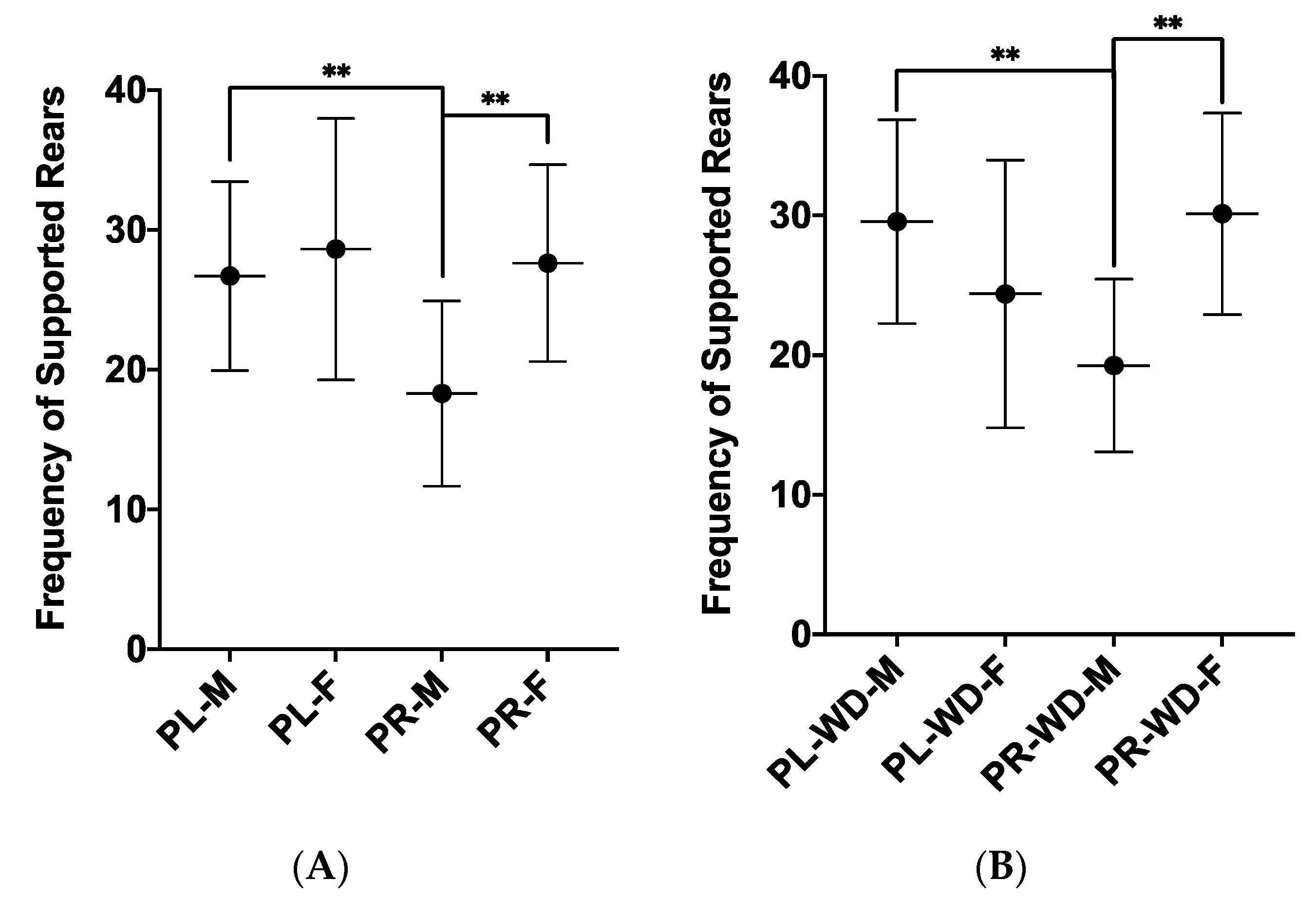
3.1. Anxiety-Like Behaviours
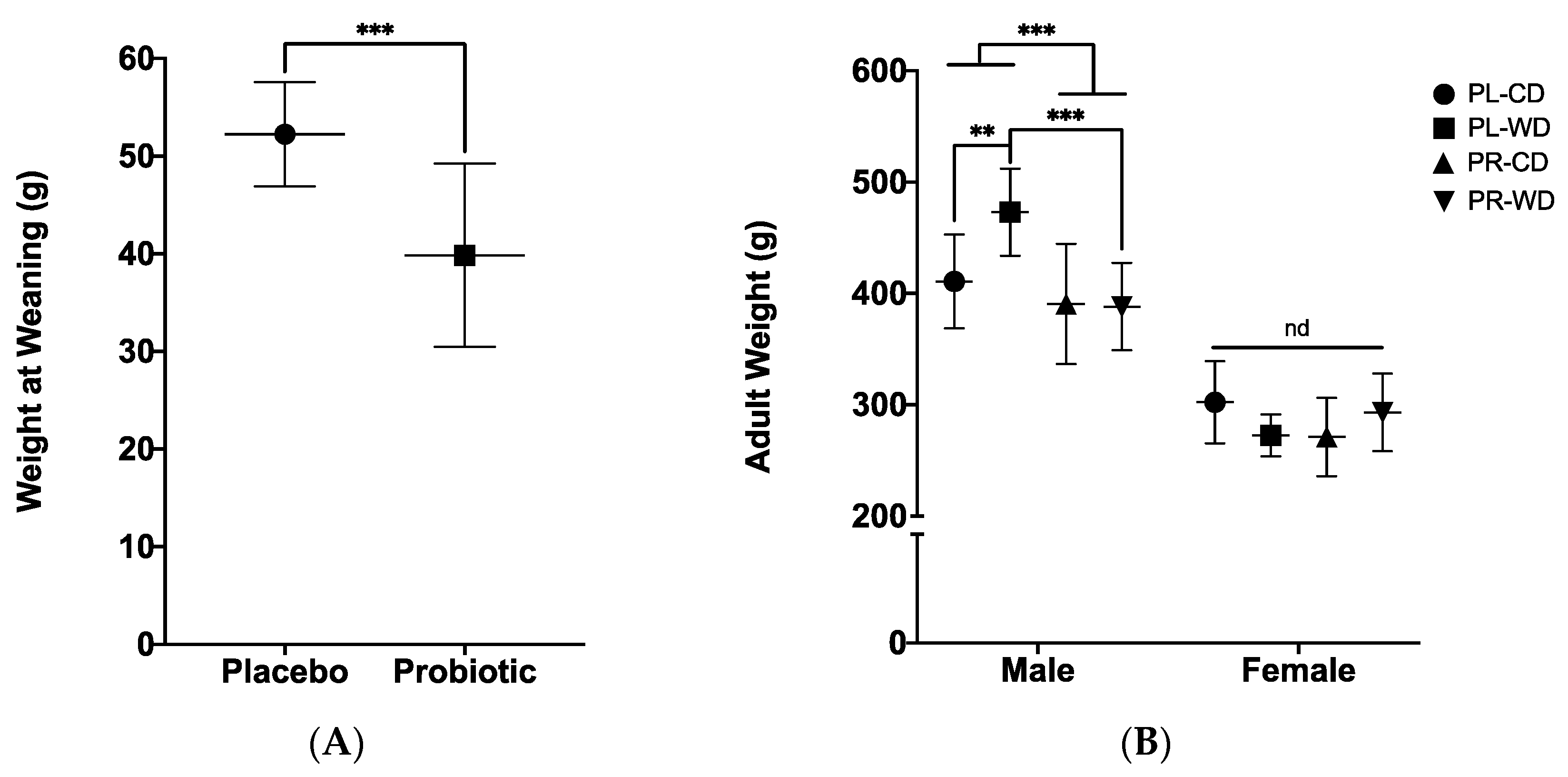
3.2. Metabolic Measures
3.2.1. Wean and Adult Weights
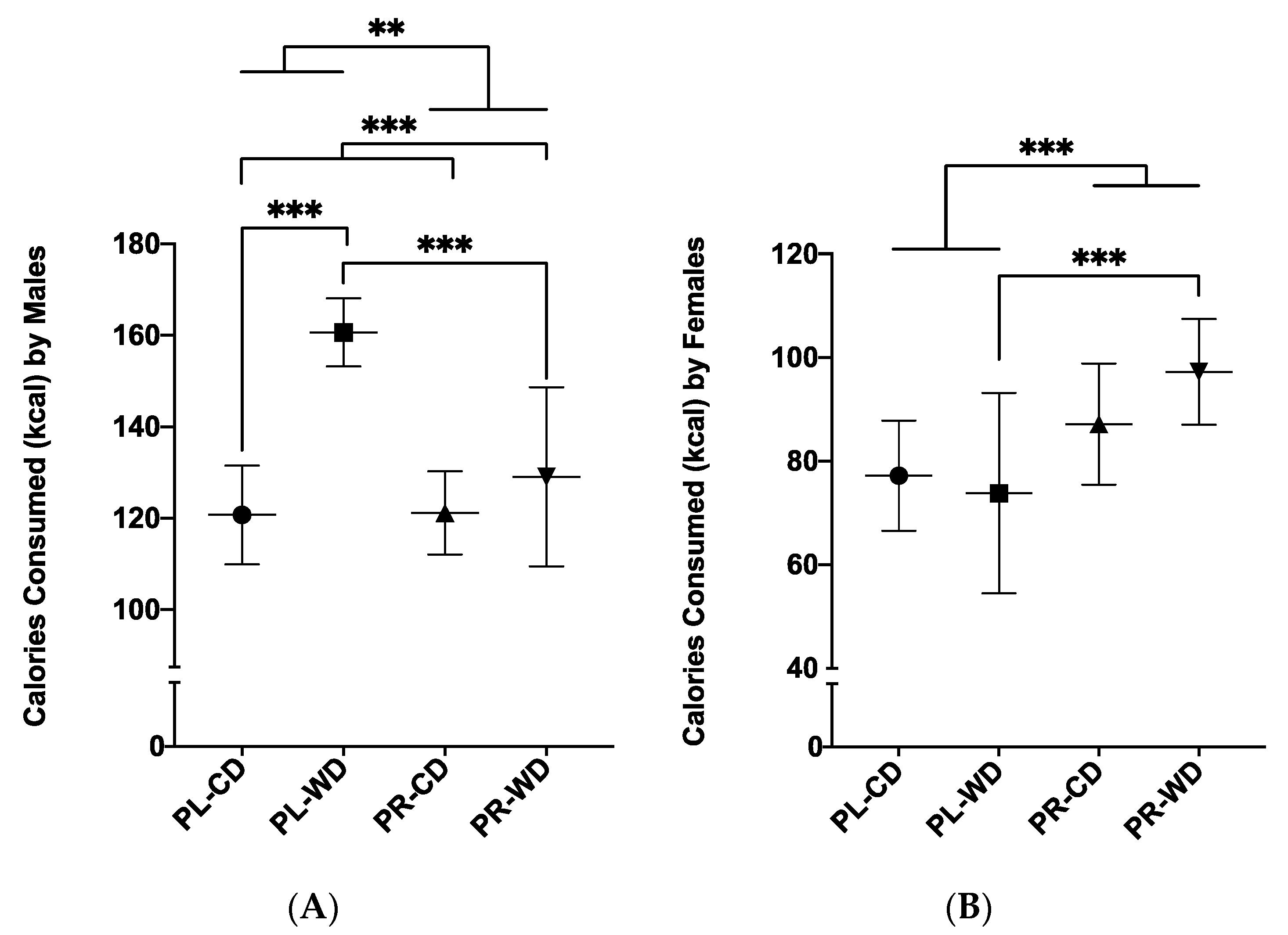
3.2.2. Average Daily Calorie Intake
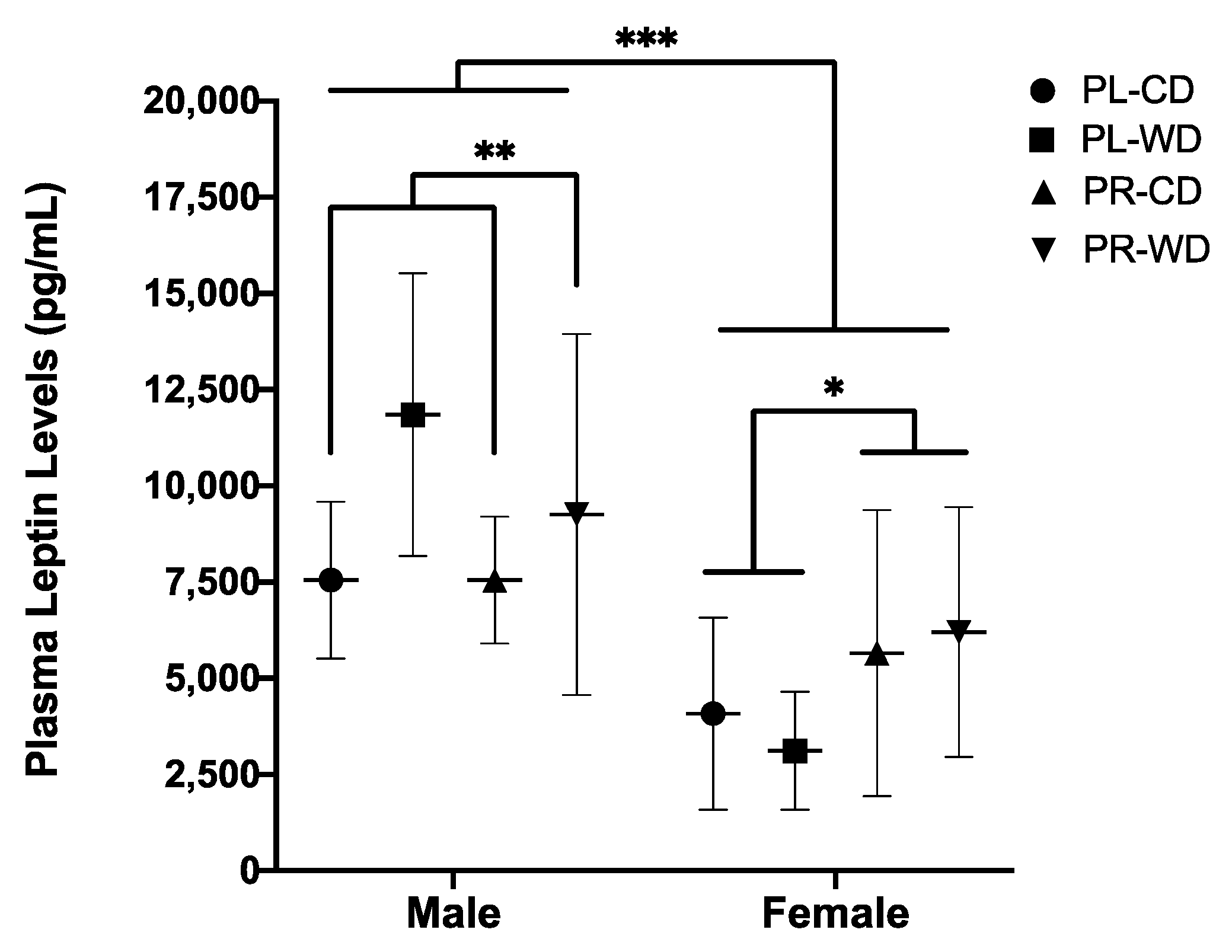
3.2.3. Plasma Leptin
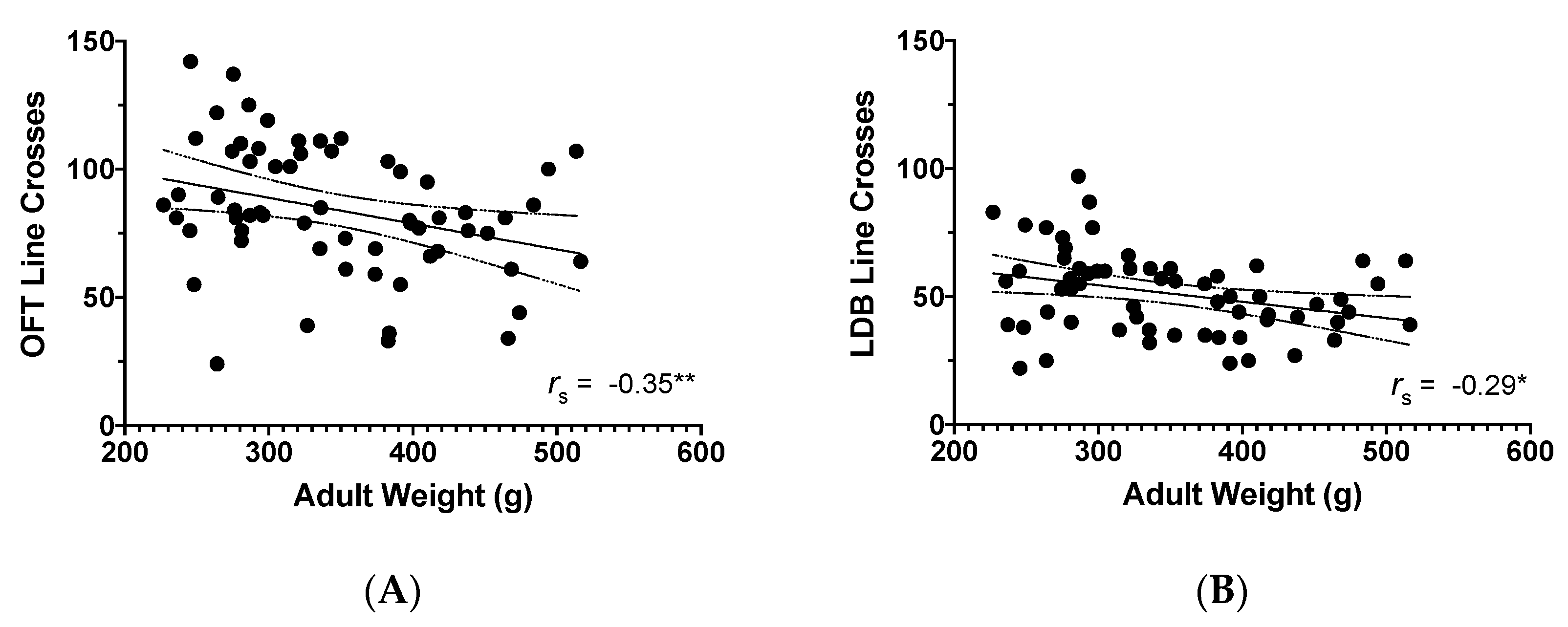
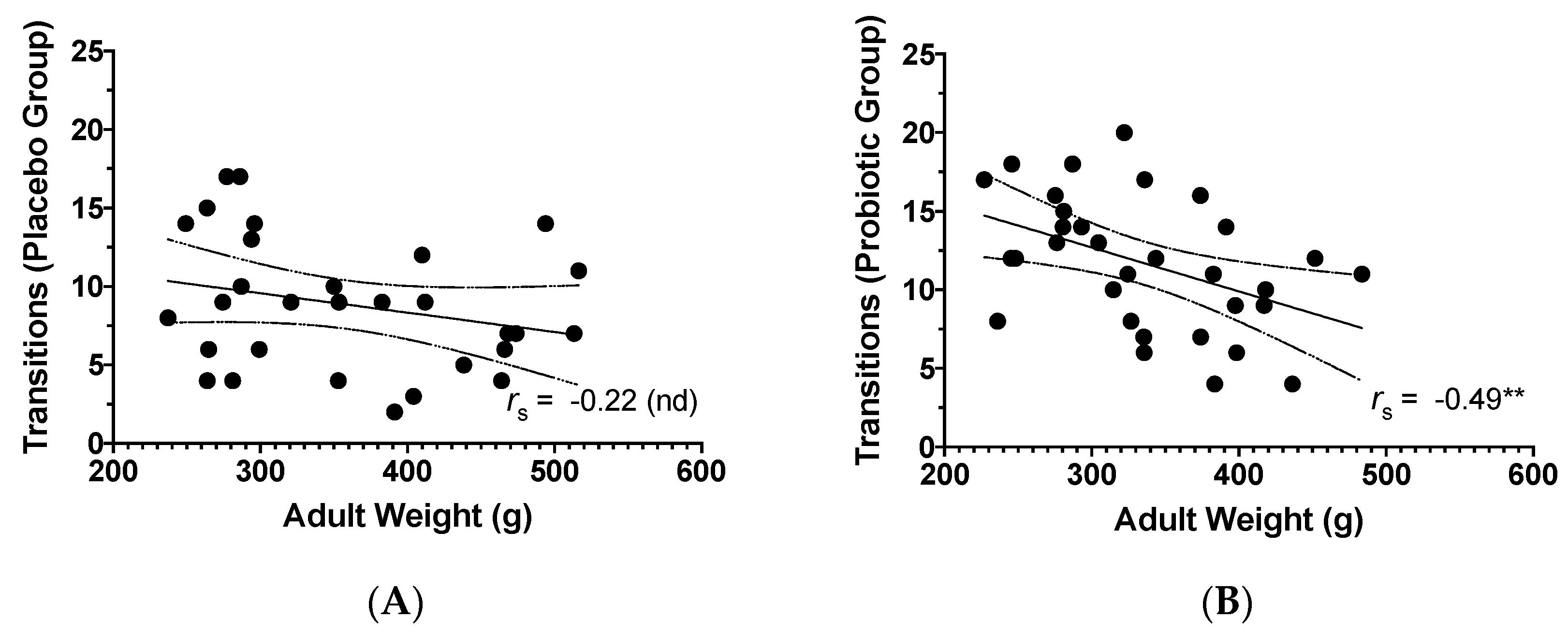
3.3. Adult Weight and Behavioural Findings
3.4. Plasma Inflammatory Analytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain. Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Moloney, R.D.; Desbonnet, L.; Clarke, G.; Dinan, T.G.; Cryan, J.F. The microbiome: Stress, health and disease. Mamm. Genome 2014, 25, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Fraser, C.M.; Ringel, Y.; Sanders, M.E.; Sartor, R.B.; Sherman, P.M.; Versalovic, J.; Young, V.; Finlay, B.B. Defining a healthy human gut microbiome: Current concepts, future directions, and clinical applications. Cell Host Microbe 2012, 12, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stecher, B.; Maier, L.; Hardt, W.D. “Blooming” in the gut: How dysbiosis might contribute to pathogen evolution. Nat. Rev. Microbiol. 2013, 11, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Vega Mejía, N.; Ponce Reyes, R.; Martinez, Y.; Carrasco, O.; Cerritos, R. Implications of the Western diet for agricultural production, health and climate change. Front. Sustain. Food Syst. 2018, 2, 88. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Li, H.; Zhao, L.; Nicholson, J.K. Gut microbiota: A potential new territory for drug targeting. Nat. Rev. Drug Discov. 2008, 7, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Traina, G.; Menchetti, L.; Rappa, F.; Casagrande-Proietti, P.; Barbato, O.; Leonardi, L.; Carini, F.; Piro, F.; Brecchia, G. Probiotic mixture supplementation in the preventive management of trinitrobenzenesulfonic acid-induced inflammation in a murine model. J. Biol. Regul. Homeost. Agents 2016, 30, 895–901. [Google Scholar] [PubMed]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruce-Keller, A.J.; Salbaum, J.M.; Berthoud, H.R. Harnessing gut microbes for mental health: Getting from here to there. Biol. Psychiatry 2018, 83, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Mahony, S.M. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol. Motil. 2011, 23, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.A.; Foster, J.A. Gut brain axis: Diet microbiota interactions and implications for modulation of anxiety and depression. Curr. Opin. Biotechnol. 2015, 32, 35–41. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Payard, I.; Rolland, C.; Harkat, C.; Braniste, V.; Théodorou, V.; Tompkins, T.A. Bifidobacterium longum and Lactobacillus helveticus synergistically suppress stress-related visceral hypersensitivity through hypothalamic-pituitary-adrenal axis modulation. J. Neurogastroenterol. Motil. 2018, 24, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Gareau, M.G.; Jury, J.; MacQueen, G.; Sherman, P.M.; Perdue, M.H. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 2007, 56, 1522–1528. [Google Scholar] [CrossRef] [Green Version]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, C.L.J.; Molin, G.; Fåk, F.; Johansson Hagslätt, M.L.; Jakesevic, M.; Håkansson, Å.; Jeppsson, B.; Weström, B.; Ahrné, S. Effects on weight gain and gut microbiota in rats given bacterial supplements and a high-energy-dense diet from fetal life through to 6 months of age. Br. J. Nutr. 2011, 106, 887–895. [Google Scholar] [CrossRef] [Green Version]
- An, H.M.; Park, S.Y.; Lee, D.K.; Kim, J.R.; Cha, M.K.; Lee, S.W.; Lim, H.T.; Kim, K.J.; Ha, N.J. Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids Health Dis. 2011, 10, 116. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.N.; Yu, Q.F.; Fu, N.; Liu, X.W.; Lu, F.G. Effects of four Bifidobacteria on obesity in high-fat diet induced rats. World J. Gastroenterol. 2010, 16, 3394–3401. [Google Scholar] [CrossRef] [PubMed]
- Ohland, C.L.; Kish, L.; Bell, H.; Thiesen, A.; Hotte, N.; Pankiv, E.; Madsen, K.L. Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology 2013, 38, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Avolio, E.; Fazzari, G.; Zizza, M.; de Lorenzo, A.; di Renzo, L.; Alò, R.; Facciolo, R.M.; Canonaco, M. Probiotics modify body weight together with anxiety states via pro-inflammatory factors in HFD-treated Syrian golden hamster. Behav. Brain Res. 2019, 356, 390–399. [Google Scholar] [CrossRef]
- Li, N.; Wang, Q.; Wang, Y.; Sun, A.; Lin, Y.; Jin, Y.; Li, X. Oral probiotics ameliorate the behavioral deficits induced by chronic mild stress in mice via the gut microbiota-inflammation axis. Front. Behav. Neurosci. 2018, 12, 266. [Google Scholar] [CrossRef] [Green Version]
- Dallman, M.F.; Pecoraro, N.C.; la Fleur, S.E. Chronic stress and comfort foods: Self-medication and abdominal obesity. Brain. Behav. Immun. 2005, 19, 275–280. [Google Scholar] [CrossRef]
- Pecoraro, N.; Reyes, F.; Gomez, F.; Bhargava, A.; Dallman, M.F. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology 2004, 145, 3754–3762. [Google Scholar] [CrossRef]
- Grissom, N.M.; George, R.; Reyes, T.M. The hypothalamic transcriptional response to stress is severely impaired in offspring exposed to adverse nutrition during gestation. Neuroscience 2017, 342, 200–211. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.M.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Qian, L.; Lu, L.; Huang, L.; Wen, Q.; Xie, J.; Jin, W.; Li, H.; Jiang, L. The effect of neonatal maternal separation on short-chain fatty acids and airway inflammation in adult asthma mice. Allergol. Immunopathol. 2019, 47, 2–11. [Google Scholar] [CrossRef]
- Carpenter, L.L.; Gawuga, C.E.; Tyrka, A.R.; Lee, J.K.; Anderson, G.M.; Price, L.H. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology 2010, 35, 2617–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in fear-and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 2017, 42, 254–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, C.T.; Araujo, E.P.; Bordin, S.; Ashimine, R.; Zollner, R.L.; Boschero, A.C.; Saad, M.J.A.; Velloso, L.A. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 2005, 146, 4192–4199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004, 25, 4–7. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory effects of probiotics on cytokine profiles. BioMed Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef] [Green Version]
- Korgan, A.C.; O’Leary, E.; King, J.L.; Weaver, I.C.G.; Perrot, T.S. Effects of paternal high-fat diet and rearing environment on maternal investment and development of defensive responses in the offspring. Psychoneuroendocrinology 2018, 91, 20–30. [Google Scholar] [CrossRef]
- Myles, E.M.; O’Leary, M.E.; Romkey, I.D.; Piano, A.; de Carvalho, V.; Tompkins, T.A.; Perrot, T.S. Guidelines for best practice in placebo-controlled experimental studies on probiotics in rodent animal models. Benef. Microbes 2020, 11, 245–254. [Google Scholar] [CrossRef]
- Tillmann, S.; Wegener, G. Syringe-feeding as a novel delivery method for accurate individual dosing of probiotics in rats. Benef. Microbes 2018, 9, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Mueller, N.T.; Bakacs, E.; Combellick, J.; Grigoryan, Z.; Dominguez-Bello, M.G. The infant microbiome development: Mom matters. Trends Mol. Med. 2015, 21, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Rautava, S. Probiotic intervention through the pregnant and breastfeeding mother to reduce disease risk in the child. Breastfeed. Med. 2018, 13, S14–S15. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalueff, A.V.; Tuohimaa, P. Experimental modeling of anxiety and depression. Acta Neurobiol. Exp. 2004, 64, 439–448. [Google Scholar] [PubMed]
- Barrow, P.; Leconte, I. The influence of body weight on open field and swimming maze performance during the post-weaning period in the rat. Lab. Anim. 1996, 30, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Young, J.W.; Halberstadt, A.L.; Masten, V.L.; Geyer, M.A. Four factors underlying mouse behavior in an open field. Behav. Brain Res. 2012, 233, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Sturman, O.; Germain, P.L.; Bohacek, J. Exploratory rearing: A context- and stress-sensitive behavior recorded in the open-field test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef]
- Bridgewater, L.C.; Zhang, C.; Wu, Y.; Hu, W.; Zhang, Q.; Wang, J.; Li, S.; Zhao, L. Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Sci. Rep. 2017, 7, 10776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soulis, G.; Papalexi, E.; Kittas, C.; Kitraki, E. Early impact of a fat-enriched diet on behavioral responses of male and female rats. Behav. Neurosci. 2007, 121, 483–490. [Google Scholar] [CrossRef]
- Arrant, A.E.; Schramm-Sapyta, N.L.; Kuhn, C.M. Use of the light/dark test for anxiety in adult and adolescent male rats. Behav. Brain Res. 2013, 256, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.M.; Piasecki, C.C.; Lonstein, J.S. Use of the light-dark box to compare the anxiety-related behavior of virgin and postpartum female rats. Pharmacol. Biochem. Behav. 2011, 100, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [Green Version]
- Olejnik, S.; Algina, J. Generalized eta and omega squared statistics: Measures of effect size for some common research designs. Psychol. Methods 2003, 8, 434–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.R.; Hem, V.; Katz, K.S.; Ovetsky, M.; Wallin, C.; Ermolaeva, O.; Tolstoy, I.; Tatusova, T.; Pruitt, K.D.; Maglott, D.R.; et al. Gene: A gene-centered information resource at NCBI. Nucleic Acids Res. 2015, 43, D36–D42. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Chang, A.; Zavvarian, M.M.; Wang, J.; Liu, Y.; Fehlings, M.G. Level-specific differences in systemic expression of pro-and anti-inflammatory cytokines and chemokines after spinal cord injury. Int. J. Mol. Sci. 2018, 19, 2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Fragala, M.S.; McElhaney, J.E.; Kuchel, G.A. Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 541–547. [Google Scholar] [CrossRef] [Green Version]
- Bode, H.; Schmitz, H.; Fromm, M.; Scholz, P.; Riecken, E.O.; Schulzke, J.D. IL-1β and TNF-α, but not IFN-α, IFN-γ, IL-6 or IL-8, are secretory mediators in human distal colon. Cytokine 1998, 10, 457–465. [Google Scholar] [CrossRef]
- Chio, C.C.; Lin, H.J.; Tian, Y.F.; Chen, Y.C.; Lin, M.T.; Lin, C.H.; Chang, C.P.; Hsu, C.C. Exercise attenuates neurological deficits by stimulating a critical HSP70/NF-ΚB/IL-6/synapsin I axis in traumatic brain injury rats. J. Neuroinflamm. 2017, 14, 90. [Google Scholar] [CrossRef] [Green Version]
- Cuneo, A.; Autieri, M. Expression and function of anti-inflammatory interleukins: The other side of the vascular response to injury. Curr. Vasc. Pharmacol. 2009, 7, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.K.; Wood, C.S.; Lombard, C.M.; Lee, C.S.; Zhang, X.Y.; Finnell, J.E.; Valentino, R.J. Inflammatory factors mediate vulnerability to a social stress-induced depressive-like phenotype in passive coping rats. Biol. Psychiatry 2015, 78, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Ushach, I.; Zlotnik, A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J. Leukoc. Biol. 2016, 100, 481–489. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Adams, D.H. Chemokines and chemokine receptors as therapeutic targets in inflammatory bowel disease; Pitfalls and promise. J. Crohn’s Colitis 2018, 12, S641–S652. [Google Scholar] [CrossRef] [Green Version]
- Lever, C.; Burton, S.; O’Keefe, J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev. Neurosci. 2006, 17, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Wegener, G.; Lund, S. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology 2017, 79, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Ait-Belgnaoui, A.; Colom, A.; Braniste, V.; Ramalho, L.; Marrot, A.; Cartier, C.; Houdeau, E.; Theodorou, V.; Tompkins, T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 2014, 26, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Genaro, G.; Schmidek, W.R. Exploratory activity of rats in three different environments. Ethology 2000, 106, 849–859. [Google Scholar] [CrossRef]
- Cagampang, F.R.; Poore, K.R.; Hanson, M.A. Developmental origins of the metabolic syndrome: Body clocks and stress responses. Brain. Behav. Immun. 2011, 25, 214–220. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Ebbeling, C.B. The carbohydrate-insulin model of obesity: Beyond “calories in, calories out”. JAMA Intern. Med. 2018, 178, 1098–1103. [Google Scholar] [CrossRef]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.; Prasad, C. Short-term consumption of a diet rich in fat decreases anxiety response in adult male rats. Physiol. Behav. 1996, 60, 1039–1042. [Google Scholar] [CrossRef]
- Kobyliak, N.; Conte, C.; Cammarota, G.; Haley, A.P.; Styriak, I.; Gaspar, L.; Fusek, J.; Rodrigo, L.; Kruzliak, P. Probiotics in prevention and treatment of obesity: A critical view. Nutr. Metab. 2016, 13, 14. [Google Scholar] [CrossRef] [Green Version]
- Falcinelli, S.; Rodiles, A.; Hatef, A.; Picchietti, S.; Cossignani, L.; Merrifield, D.L.; Unniappan, S.; Carnevali, O. Dietary lipid content reorganizes gut microbiota and probiotic L. rhamnosus attenuates obesity and enhances catabolic hormonal milieu in zebrafish. Sci. Rep. 2017, 7, 5512. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs. placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Watkins, S.M.; Lorenzo, C.; Wagenknecht, L.E.; Il’Yasova, D.; Chen, Y.D.I.; Haffner, S.M.; Hanley, A.J. Branched-chain amino acids and insulin metabolism: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2016, 39, 582–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Clegg, D.J. Sex differences in the regulation of body weight. Physiol. Behav. 2009, 97, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tennoune, N.; Legrand, R.; Ouelaa, W.; Breton, J.; Lucas, N.; Bole-Feysot, C.; do Rego, J.C.; Déchelotte, P.; Fetissov, S.O. Sex-related effects of nutritional supplementation of Escherichia coli: Relevance to eating disorders. Nutrition 2015, 31, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Taraschenko, O.D.; Maisonneuve, I.M.; Glick, S.D. Sex differences in high fat-induced obesity in rats: Effects of 18-methoxycoronaridine. Physiol. Behav. 2011, 103, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Caraballo, Y.; Hodgson, K.J.; Morgan, S.A.; Ferrario, C.R.; Vollbrecht, P.J. Enhanced anxiety-like behavior emerges with weight gain in male and female obesity-susceptible rats. Behav. Brain Res. 2019, 360, 81–93. [Google Scholar] [CrossRef]
- Harris, R.B.S.; Bowen, H.M.; Mitchell, T.D. Leptin resistance in mice is determined by gender and duration of exposure to high-fat diet. Physiol. Behav. 2003, 78, 543–555. [Google Scholar] [CrossRef]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Leptin resistance: Underlying mechanisms and diagnosis. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.; Zheng, C.Q.; Jiang, M.; Ma, X.Y.; Jiang, L.J. Probiotics and irritable bowel syndrome. World J. Gastroenterol. 2013, 19, 5973–5980. [Google Scholar] [CrossRef]
- Bisson, J.F.; Hidalgo, S.; Rozan, P.; Messaoudi, M. Preventive effects of different probiotic formulations on travelers’ diarrhea model in wistar rats: Preventive effects of probiotics on TD. Dig. Dis. Sci. 2010, 55, 911–919. [Google Scholar] [CrossRef]
- Goujon, E.; Parnet, P.; Laye, S.; Combe, C.; Kelley, K.W.; Dantzer, R. Stress downregulates lipopolysaccharide-induced expression of proinflammatory cytokines in the spleen, pituitary, and brain of mice. Brain Behav. Immun. 1995, 9, 292–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goshen, I.; Yirmiya, R. Interleukin-1 (IL-1): A central regulator of stress responses. Front. Neuroendocrinol. 2009, 30, 30–45. [Google Scholar] [CrossRef]
- Wagner, R.D.; Johnson, S.J. Probiotic lactobacillus and estrogen effects on vaginal epithelial gene expression responses to Candida albicans. J. Biomed. Sci. 2012, 19, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferris, H.A.; Kahn, C.R. New mechanisms of glucocorticoid-induced insulin resistance: Make no bones about it. J. Clin. Invest. 2012, 122, 3854–3857. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Akabayashi, A.; Dourmashkin, J.; Yu, H.J.; Alexander, J.T.; Chae, H.J.; Leibowitz, S.F. Neuropeptide Y in relation to carbohydrate intake, corticosterone and dietary obesity. Brain Res. 1998, 802, 75–88. [Google Scholar] [CrossRef]
- Pyter, L.M.; Kelly, S.D.; Harrell, C.S.; Neigh, G.N. Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain. Behav. Immun. 2013, 30, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Heck, A.L.; Handa, R.J. Sex differences in the hypothalamic–pituitary–adrenal axis’ response to stress: An important role for gonadal hormones. Neuropsychopharmacology 2019, 44, 45–58. [Google Scholar] [CrossRef] [Green Version]
- Schulster, M.; Bernie, A.M.; Ramasamy, R. The role of estradiol in male reproductive function. Asian J. Androl. 2016, 18, 435–440. [Google Scholar] [CrossRef]


| Experimental Group | ||||||||
|---|---|---|---|---|---|---|---|---|
| PR-WD-M | PR-WD-F | PR-CD-M | PR-CD-F | PL-WD-M | PL-WD-F | PL-CD-M | PL-CD-F | |
| Sample size | 8 | 8 | 8 | 8 | 7 | 8 | 7 | 8 |
| Max. n per litter | 3 | 3 | 2 | 3 | 5 | 4 | 3 | 3 |
| Represented litters | 4 of 4 | 3 of 4 | 4 of 4 | 3 of 4 | 2 of 3 | 2 of 3 | 3 of 3 | 3 of 3 |
| Behaviour, df | Probiotic M(SD) | Placebo M (SD) | F-Value | p-Value | Effect Size (ηp2) |
| Total Rearing, F1, 54 | 20.22 (8.69) | 29.03 (11.85) | 11.19 | p = 0.002 | ηp2 = 0.172 |
| Supported Rearing, F1, 53 | 15.81 (6.11) | 24.07 (9.59) | 17.21 | p < 0.001 | ηp2 = 0.245 |
| Behaviour, df | Male M (SD) | Female M(SD) | F-Value | p-Value | Effect Size (ηp2) |
| Line Crosses, F1, 54 | 72.73 (21.39) | 94.69 (24.25) | 14.06 | p < 0.001 | ηp2 = 0.207 |
| Total Rearing, F1, 54 | 21.73 (10.21) | 27.31 (11.62) | 4.17 | p = 0.046 | ηp2 = 0.072 |
| Supported Rearing, F1, 53 | 17.63 (8.19) | 21.77 (9.24) | 4.39 | p = 0.041 | ηp2 = 0.076 |
| Behaviour, df | Probiotic M(SD) | Placebo M (SD) | F-Value | p-Value | Effect Size (ηp2) |
| Supported Rearing, F1, 54 | 22.97 (8.22) | 27.73 (8.16) | 6.61 | p = 0.013 | ηp2 = 0.109 |
| Transitions, F1, 54 | 11.69 (4.21) | 8.83 (4.19) | 10.21 | p = 0.002 | ηp2 = 0.159 |
| Behaviour, df | Male M (SD) | Female M (SD) | F-Value | p-Value | Effect Size (ηp2) |
| Line Crosses, F1, 54 | 43.30 (10.92) | 58.97 (16.81) | 18.63 | p < 0.001 | ηp2 = 0.256 |
| Supported Rearing, F1, 54 | 22.23 (7.84) | 28.13 (8.15) | 9.41 | p = 0.003 | ηp2 = 0.148 |
| Transitions, F1, 54 | 8.17 (3.53) | 12.31 (4.24) | 20.66 | p < 0.001 | ηp2 = 0.277 |
| Latency to Light, F1, 53 | 50.39 (44.06) | 17.90 (26.37) | 13.55 | p < 0.001 | ηp2 = 0.204 |
| Analyte, df | Probiotic M (SD) | Placebo M (SD) | F-Value | p-Value | Effect Size (ηp2) |
|---|---|---|---|---|---|
| IL-1α, F1, 53 | 163.79 (126.86) | 94.81 (95.64) | 5.54 | p = 0.022 | ηp2 = 0.095 |
| IL-10, F1, 53 | 100.15 (94.01) | 37.04 (63.77) | 8.95 | p = 0.004 | ηp2 = 0.145 |
| M-CSF, F1, 53 | 24.97 (11.51) | 13.54 (11.22) | 14.40 | p < 0.001 | ηp2 = 0.214 |
| MIP-3α, F1, 51 | 31.40 (11.49) | 19.93 (6.53) | 20.85 | p < 0.001 | ηp2 = 0.290 |
| Analyte, df | Western M (SD) | Control M (SD) | F-Value | p-Value | Effect Size (ηp2) |
|---|---|---|---|---|---|
| IL-1β, F1, 53 | 416.93 (441.58) | 175.24 (166.16) | 12.00 | p = 0.001 | ηp2 = 0.185 |
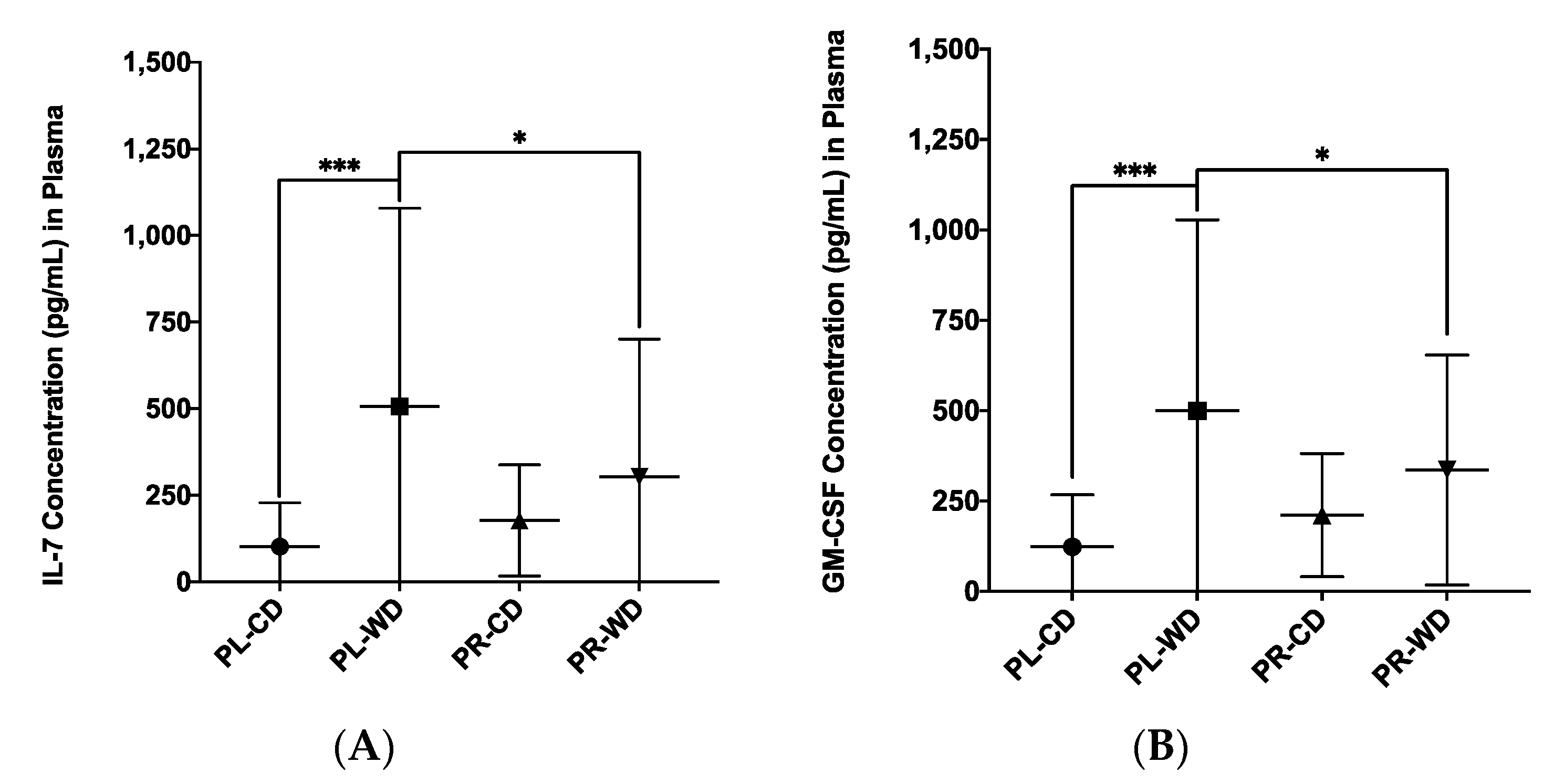
| IL-7, F1, 53 | 398.52 (488.85) | 141.26 (147.92) | 12.91 | p < 0.001 | ηp2 = 0.196 |
| GM-CSF, F1, 53 | 412.78 (429.38) | 168.43 (161.61) | 14.99 | p < 0.001 | ηp2 = 0.220 |
| GRO/KC, F1, 53 | 291.18 (200.57) | 193.62 (142.54) | 6.00 | p = 0.018 | ηp2 = 0.102 |
| MIP-1α, F1, 53 | 115.89 (121.43) | 57.20 (50.43) | 8.03 | p = 0.006 | ηp2 = 0.132 |
| VEGF, F1, 52 | 456.43 (383.96) | 250.07 (265.76) | 5.83 | p = 0.019 | ηp2 = 0.101 |
| MCP-1, F1, 53 | 2014.98 (1476.12) | 1321.93 (689.83) | 8.10 | p = 0.006 | ηp2 = 0.133 |
| Analyte, df | Male M (SD) | Female M (SD) | F-Value | p-Value | Effect Size (ηp2) |
|---|---|---|---|---|---|
| IL-1β, F1, 53 | 372.48 (461.38) | 223.07 (186.10) | 4.60 | p = 0.037 | ηp2 = 0.080 |
| IL-7, F1, 53 | 351.46 (511.02) | 191.94 (170.06) | 5.16 | p = 0.027 | ηp2 = 0.089 |
| GM-CSF, F1, 53 | 365.50 (450.16) | 218.91 (183.12) | 5.59 | p = 0.022 | ηp2 = 0.095 |
| MCP-1, F1, 53 | 2091.35 (1451.34) | 1274.37 (710.64) | 9.83 | p = 0.003 | ηp2 = 0.157 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myles, E.M.; O’Leary, M.E.; Smith, R.; MacPherson, C.W.; Oprea, A.; Melanson, E.H.; Tompkins, T.A.; Perrot, T.S. Supplementation with Combined Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 Across Development Reveals Sex Differences in Physiological and Behavioural Effects of Western Diet in Long–Evans Rats. Microorganisms 2020, 8, 1527. https://doi.org/10.3390/microorganisms8101527
Myles EM, O’Leary ME, Smith R, MacPherson CW, Oprea A, Melanson EH, Tompkins TA, Perrot TS. Supplementation with Combined Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 Across Development Reveals Sex Differences in Physiological and Behavioural Effects of Western Diet in Long–Evans Rats. Microorganisms. 2020; 8(10):1527. https://doi.org/10.3390/microorganisms8101527
Chicago/Turabian StyleMyles, Elizabeth M., M. Elizabeth O’Leary, Rylan Smith, Chad W. MacPherson, Alexandra Oprea, Emma H. Melanson, Thomas A. Tompkins, and Tara S. Perrot. 2020. "Supplementation with Combined Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 Across Development Reveals Sex Differences in Physiological and Behavioural Effects of Western Diet in Long–Evans Rats" Microorganisms 8, no. 10: 1527. https://doi.org/10.3390/microorganisms8101527
APA StyleMyles, E. M., O’Leary, M. E., Smith, R., MacPherson, C. W., Oprea, A., Melanson, E. H., Tompkins, T. A., & Perrot, T. S. (2020). Supplementation with Combined Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 Across Development Reveals Sex Differences in Physiological and Behavioural Effects of Western Diet in Long–Evans Rats. Microorganisms, 8(10), 1527. https://doi.org/10.3390/microorganisms8101527






