Optimized Production of a Redox Metabolite (pyocyanin) by Pseudomonas aeruginosa NEJ01R Using a Maize By-Product
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Statistical Design and Optimization by Response Surface
2.3. Culture Conditions
2.4. Biomass Determination
2.5. PYO Determination
2.6. Microbial Growth Kinetics and PYO Production
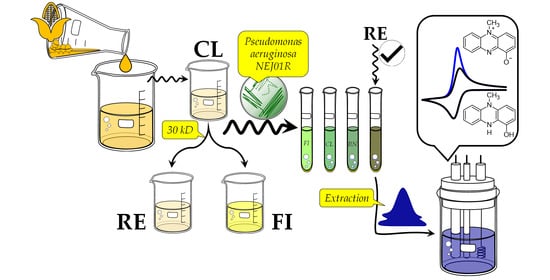
2.7. Valorization of Maize Wastewater
2.8. Characterization of Maize Wastewater and Its By-Products
2.9. Ferulic Acid Determination
2.10. PYO Identification
2.11. Electrochemical Study
3. Results and Discussion
3.1. Culture Condition Optimization in Defined Medium
3.2. Microbial Growth Kinetics and PYO Production
3.3. Maize Wastewater (Nejayote) as a Substrate to PYO Production
3.4. Characterization of Maize Wastewater Fractions
3.5. PYO Identification
3.6. Electrochemical Analysis of PYO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Samrot, A.V.; Rio, J.A.; Kumar, S.S.; Samanvitha, S.K. Bioprospecting studies of pigmenting Pseudomonas aeruginosa SU-1, Microvirga aerilata SU14 and Bacillus megaterium SU15 isolated from garden soil. Biocatal. Agric. Biotechnol. 2017, 11, 330–337. [Google Scholar] [CrossRef]
- Yong, X.Y.; Yan, Z.Y.; Shen, H.B.; Zhou, J.; Wu, X.Y.; Zhang, L.J.; Zheng, T.; Jiang, M.; Wei, P.; Jia, H.H.; et al. An integrated aerobic-anaerobic strategy for performance enhancement of Pseudomonas aeruginosa-inoculated microbial fuel cell. Bioresour. Technol. 2017, 241, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Narenkumar, J.; Sathishkumar, K.; Sarankumar, R.K.; Murugan, K.; Rajasekar, A. An anticorrosive study on potential bioactive compound produced by Pseudomonas aeruginosa TBH2 against the biocorrosive bacterial biofilm on copper metal. J. Mol. Liq. 2017, 243, 706–713. [Google Scholar] [CrossRef]
- Stancu, M.M. Production of some extracellular metabolites by a solvent-tolerant Pseudomonas aeruginosa strain. Waste Biomass Valor. 2018, 9, 1747–1755. [Google Scholar] [CrossRef]
- El-Fouly, M.Z.; Sharaf, A.M.; Shahin, A.A.M.; El-Bialy, H.A.; Omara, A.M.A. Biosynthesis of pyocyanin pigment by Pseudomonas aeruginosa. J. Radiat. Res. Appl. Sci. 2015, 8, 36–48. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, M.; D’Morris, S.; Vinod, P.; Warrier, S.; Vasudevan, A.K.; Vanuopadath, M.; Nair, S.S.; Paul-Prasanth, B.; Mohan, C.G.; Biswas, R. Mechanistic understanding of Phenyllactic acid mediated inhibition of quorum sensing and biofilm development in Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2017, 101, 8223–8236. [Google Scholar] [CrossRef]
- Kang, H.; Gan, J.; Zhao, J.; Kong, W.; Zhang, J.; Zhu, M.; Li, F.; Song, Y.; Qin, J.; Liang, H. Crystal structure of Pseudomonas aeruginosa RsaL bound to promoter DNA reaffirms its role as a global regulator involved in quorum-sensing. Nucleic Acids Res. 2017, 45, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.; Nikam, M.; Patil, H.; Anokhina, T.; Kochetkov, V.; Chaudhari, A. Bioactive pigment production by Pseudomonas spp. MCC 3145: Statistical media optimization, biochemical characterization, fungicidal and DNA intercalation-based cytostatic activity. Process. Biochem. 2017, 58, 298–305. [Google Scholar] [CrossRef]
- Price-Whelan, A.; Dietrich, L.; Newman, D. Rethinking ‘secondary’ metabolism: Physiological roles for phenazine antibiotics. Nat. Chem. Biol. 2006, 2, 71–78. [Google Scholar] [CrossRef]
- Oziat, J.; Gougis, M.; Malliaras, G.G.; Mailley, P. Electrochemical characterizations of four main redox–metabolites of Pseudomonas aeruginosa. Electroanalysis 2017, 29, 1332–1340. [Google Scholar] [CrossRef]
- Qiao, Y.; Qiao, Y.J.; Zou, L.; Ma, C.X.; Liu, J.H. Real-time monitoring of phenazines excretion in Pseudomonas aeruginosa microbial fuel cell anode using cavity microelectrodes. Bioresour. Technol. 2015, 198, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cayman Chemical Co. Home Page. Available online: https://www.caymanchem.com/product/10009594/pyocyanin (accessed on 4 September 2020).
- Focus Biomolecules Home Page. Available online: https://focusbiomolecules.com/pyocyanin-ros-generator/ (accessed on 4 September 2020).
- Santa Cruz Biotechnology Home Page. Available online: https://www.scbt.com/p/pyocyanin-85-66-5 (accessed on 4 September 2020).
- Sigma-Aldrich Home Page. Available online: https://www.sigmaaldrich.com/catalog/product/sigma/p0046?lang=es®ion=MX (accessed on 4 September 2020).
- Bacame-Valenzuela, F.J.; Pérez-Garcia, J.A.; Castañeda-Zaldívar, F.; Reyes-Vidal, Y. Pyocyanin biosynthesis by Pseudomonas aeruginosa using a biodiesel byproduct. Mex. J. Biotechnol. 2020, 5, 1–16. [Google Scholar] [CrossRef]
- DeBritto, S.; Gajbar, T.D.; Satapute, P.; Sundaram, L.; Lakshmikantha, R.Y.; Jogaiah, S.; Ito, S. Isolation and characterization of nutrient dependent pyocyanin from Pseudomonas aeruginosa and its dye and agrochemical properties. Sci. Rep. 2020, 10, 1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valderrama-Bravo, C.; Domínguez-Pacheco, F.; Hernández-Aguilar, C.; Flores-Saldaña, N.; Villagran-Ortiz, P.; Pérez-Reyes, C.; Sánchez-Hernández, G.; Oaxaca-Luna, A. Effect of nixtamalized maize with lime water (nejayote) on rheological and microbiological properties of masa. J. Food Process. Preserv. 2017, 41, e12748. [Google Scholar] [CrossRef] [Green Version]
- Villada, J.A.; Sánchez-Sinencio, F.; Zelaya-Ángel, O.; Gutiérrez-Cortes, E.; Rodríguez-García, M.E. Study of the morphological, structural, thermal, and pasting corn transformation during the traditional nixtamalization process: From corn to tortilla. J. Food Eng. 2017, 212, 242–251. [Google Scholar] [CrossRef]
- Rojas-García, C.; García-Lara, S.; Serna-Saldivar, S.O.; Gutiérrez-Uribe, J.A. Chemopreventive effects of free and bound phenolics associated to steep waters (Nejayote) obtained after nixtamalization of different maize types. Plant. Foods Hum. Nutr. 2012, 67, 94–99. [Google Scholar] [CrossRef]
- Argun, M.S.; Argun, M.E. Treatment and alternative usage possibilities of a special wastewater: Nejayote. J. Food Process. Eng. 2017, 48, e12609. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fila, V.; Durán-Páramo, E. A review of the primary by-product (Nejayote) of the nixtamalization during maize processing: Potential reuses. Waste Biomass Valor. 2019, 10, 13–22. [Google Scholar] [CrossRef]
- Gutiérrez-Uribe, J.A.; Rojas-García, C.; García-Lara, S.; Serna-Saldivar, S.O. Phytochemical analysis of wastewater (nejayote) obtained after lime-cooking of different types of maize kernels processed into masa for tortillas. J. Cereal Sci. 2010, 52, 410–416. [Google Scholar] [CrossRef]
- López-Pacheco, I.Y.; Carrillo-Nieves, D.; Salinas-Salazar, C.; Silva-Núñez, A.; Arévalo-Gallegos, A.; Barceló, D.; Afewerki, S.; Izbal, H.M.N.; Parra-Saldívar, R. Combination of nejayote and swine wastewater as a medium for Arthrospira maxima and Chlorella vulgaris production and wastewater treatment. Sci. Total Environ. 2019, 676, 356–367. [Google Scholar] [CrossRef]
- Asaff-Torres, A.J.; Reyes-Vidal, M.Y. Un Método y un Sistema para el Tratamiento Integral de Aguas Residuales de una Industria del Maíz. WO/2014/119990. 2014. Available online: https://patentscope.wipo.int/search/es/detail.jsf?docId=WO2014119990 (accessed on 28 September 2020).
- Ayala-Soto, F.; Serna-Saldívar, S.O.; García-Lara, S.; Pérez-Carrillo, E. Hydroxycinnamic acids, sugar composition and antioxidant capacity of arabinoxylans extracted from different maize fiber sources. Food Hydrocoll. 2014, 35, 471–475. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Cerón-Montes, G.I.; Barragán-Huerta, B.E.; Yáñez-Fernández, J. Recovery of carbohydrates from nixtamalization wastewaters (Nejayote) by ultrafiltration. Rev. Mex. Ing. Quim. 2015, 14, 735–744. [Google Scholar]
- Castro-Muñoz, R.; Yáñez-Fernández, J. Valorization of nixtamalization wastewaters by integrated membrane process. Food Bioprod. Process. 2015, 95, 7–18. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Carvajal-Millán, E.; Lizardi, J.; Rascon-Chu, A.; Marquez-Escalante, J.A.; Gardea, A.; Martinez-López, A.L.; Guerrero, V. Maize processing waste water arabinoxylans: Gelling capability and cross-linking content. Food Chem. 2009, 115, 1286–1290. [Google Scholar] [CrossRef]
- Rocha-Pizaña, M.R.; Chen, W.N.; Lee, J.J.L.; Bultimea-Cantúa, N.E.; González-Nimi, E.; Gutierrez-Uribe, J.A. Production of a potential collagenolytic protease by nejayote fermentation with Aspergillus oryzae. Int. J. Food Sci. Technol. 2020, 55, 3289–3296. [Google Scholar] [CrossRef]
- Ramírez-Romero, G.; Reyes-Velazquez, M.; Cruz-Guerrero, A. Study of nejayote as culture medium for probiotics and production of bacteriocins. Rev. Mex. Ing. Quim. 2013, 12, 463–471. [Google Scholar]
- Sanchez-Gonzalez, M.; Blanco-Gamez, A.; Escalante, A.; Valladares, A.G.; Olvera, C.; Parra, R. Isolation and characterization of new facultative alkaliphilic Bacillus flexus strains from maize processing waste water (nejayote). Lett. Appl. Microbiol. 2011, 52, 413–419. [Google Scholar] [CrossRef]
- Baqueiro-Peña, I.; Contreras-Jácquez, V.; Kirchmayr, M.R.; Mateos-Díaz, J.C.; Valenzuela-Soto, E.M.; Asaff-Torres, A. Isolation and characterization of a new ferulic-acid-biotransforming Bacillus megaterium from maize alkaline wastewater (nejayote). Curr. Microbiol. 2019, 76, 1215–1224. [Google Scholar] [CrossRef]
- García-Depraect, O.; Gómez-Romero, J.; León-Becerril, E.; López-López, A. A novel biohydrogen production process: Co-digestion of vinasse and nejayote as complex raw substrates using a robust inoculum. Int. J. Hydrog. Energy. 2017, 42, 5820–5831. [Google Scholar] [CrossRef]
- Wilson, K. Preparation of genomic DNA from bacteria. Curr. Protoc. Mol. Biol. 2001, 56, 2–4. [Google Scholar] [CrossRef]
- El-Shouny, W.A.; Al-Baidani, A.R.H.; Hamza, W.T. Antimicrobial activity of pyocyanin produced by Pseudomonas aeruginosa isolated from surgical wound-infections. Intl. J. Pharm. Med. Sci. 2011, 1, 1–7. [Google Scholar]
- Castro-Muñoz, R.; Barragán-Huerta, B.E.; Fila, V.; Denis, P.C.; Ruby-Figueroa, R. Current role of membrane technology: From the treatment of agro-industrial by-products up to the valorization of valuable compounds. Waste Biomass Valor. 2018, 9, 513–529. [Google Scholar] [CrossRef]
- Herald, P.J.; Davidson, P.M. Antibacterial activity of selected hydroxycinnamic acids. J. Food Sci. 1983, 48, 1378–1379. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Ugurlu, A.; Yagci, A.K.; Ulusoy, S.; Aksu, B.; Bosgelmez-Tinaz, G. Phenolic compounds affect production of pyocyanin, swarming motility and biofilm formation of Pseudomonas aeruginosa. Asian Pac. J. Trop. Biomed. 2016, 6, 698–701. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.A.; Lee, S.J.; Park, N.H.; Mechesso, A.F.; Birhanu, B.T.; Kang, J.; Reza, A.; Suh, J.W.; Park, S.C. Impact of phenolic compounds in the acyl homoserine lactone-mediate quorum sensing regulatory pathways. Sci. Rep. 2017, 7, 10618. [Google Scholar] [CrossRef] [Green Version]
- Castro-Muñoz, R.; Barragán-Huerta, B.E.; Yáñez-Fernández, J. The use of nixtamalization waste waters clarified by ultrafiltration for production of a fraction rich in phenolic compounds. Waste Biomass Valor. 2016, 7, 1167–1176. [Google Scholar] [CrossRef]
- Özcan, D.; Kahraman, H. Pyocyanin production in the presence of calcium ion in Pseudomonas aeruginosa and recombinant bacteria. Turkish J. Sci. Technol. 2015, 10, 13–19. [Google Scholar]
- Kerr, J.R.; Taylor, G.W.; Rutman, A.; HØiby, N.; Cole, P.J.; Wilson, R. Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J. Clin. Pathol. 1999, 52, 385–387. [Google Scholar] [CrossRef] [Green Version]
- Yasmin, S.; Hafeez, F.Y.; Mirza, M.S.; Rasul, M.; Arshad, H.M.I.; Zubair, M.; Iqbal, M. Biocontrol of bacterial leaf blight of rice and profiling of secondary metabolites produced by rhizospheric Pseudomonas aeruginosa BRp3. Front. Microbiol. 2017, 8, 1895. [Google Scholar] [CrossRef] [Green Version]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Harris, D., Swain, E., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Wopschall, R.H.; Shain, I. Effects of adsorption of electroactive species in stationary electrode polarography. Anal. Chem. 1967, 39, 1514–1527. [Google Scholar] [CrossRef]
- Friedheim, E.; Michaelis, L. Potentiometric study of piocyanine. J. Biol. Chem. 1931, 91, 355–368. [Google Scholar]
- Wang, Y.; Newman, D.K. Redox reactions of phenazine antibiotics with ferric (hydr)oxides and molecular oxygen. Environ. Sci. Technol. 2008, 42, 2380–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Unit | Experimental Code Factors | Results | |||||
|---|---|---|---|---|---|---|---|
| Temperature (X1) | pH (X2) | Agitation (X3) | Biomass g L−1 | PYO µg mL−1 | |||
| 1 | 0 | 0 | 0 | 0.368 | 0.380 | 1.5903 | 1.4260 |
| 2 | −1 | −1 | 1 | 0.512 | 0.484 | 0.0001 | 0.0001 |
| 3 | 1 | −1 | −1 | 0.480 | 0.330 | 0.6600 | 0.5800 |
| 4 | 1 | 1 | −1 | 0.318 | 0.278 | 0.4788 | 0.3420 |
| 5 | 0 | 0 | −1.47119 | 0.600 | 0.710 | 2.8200 | 2.7800 |
| 6 | 0 | 0 | 0 | 0.388 | 0.380 | 1.5732 | 1.4312 |
| 7 | 0 | 0 | 0 | 0.368 | 0.366 | 1.6416 | 1.6758 |
| 8 | −1.47119 | 0 | 0 | 0.440 | 0.470 | 0.5814 | 0.4275 |
| 9 | 1 | −1 | 1 | 0.440 | 0.370 | 1.1900 | 1.2800 |
| 10 | 0 | 1.47119 | 0 | 0.036 | 0.020 | 0.0500 | 0.0400 |
| 11 | 1.47119 | 0 | 0 | 0.252 | 0.188 | 0.0342 | 0.0223 |
| 12 | 0 | 0 | 0 | 0.340 | 0.380 | 1.5390 | 1.4483 |
| 13 | 0 | 0 | 0 | 0.358 | 0.346 | 1.5323 | 1.4141 |
| 14 | −1 | 1 | 1 | 0.320 | 0.398 | 1.4706 | 1.5561 |
| 15 | −1 | −1 | −1 | 0.705 | 0.386 | 0.9498 | 1.0602 |
| 16 | 0 | 0 | 1.47119 | 0.974 | 0.484 | 0.6669 | 0.7011 |
| 17 | 1 | 1 | 1 | 0.370 | 0.240 | 0.1300 | 0.2200 |
| 18 | −1 | 1 | −1 | 0.364 | 0.356 | 1.4193 | 1.2996 |
| 19 | 0 | −1.47119 | 0 | 0.380 | 0.342 | 2.3127 | 2.3598 |
| Source | Biomass | PYO | ||
|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | |
| Constant (X0) | 0.3674 | 1.5832 | ||
| Temperature (X1) | −0.0390 | 0.0732 | −0.1539 | 0.01757 |
| pH (X2) | −0.0655 | 0.0042 | −0.2053 | 0.0744 |
| Agitation (X3) | 0.0227 | 0.2869 | −0.2712 | 0.0210 |
| Temperature:temperature (X12) | −0.0138 | 0.5700 | −0.6030 | 0.0001 |
| Temperature:pH (X1X2) | 0.0413 | 0.1236 | −0.3620 | 0.0138 |
| Temperature:agitation (X1X3) | 0.0336 | 0.2074 | 0.1838 | 0.1919 |
| pH:pH (X22) | −0.0799 | 0.0026 | −0.1760 | 0.1769 |
| pH:agitation (X2X3) | 0.0333 | 0.2107 | 0.0686 | 0.6215 |
| Agitation:agitation (X32) | 0.1499 | 0.000 | 0.0787 | 0.5405 |
| Response | Biomass Calculated, 0.71 g L−1 PYO Calculated, 2.21 µg mL−1 (Desirability = 0.9084) | |
|---|---|---|
| Factor | Code | Real Value |
| Temperature (°C) | −0.3220 | 29.6 |
| pH | −0.5007 | 6.92 |
| Agitation (rpm) | 1.4711 | 223.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacame-Valenzuela, F.J.; Pérez-Garcia, J.A.; Figueroa-Magallón, M.L.; Espejel-Ayala, F.; Ortiz-Frade, L.A.; Reyes-Vidal, Y. Optimized Production of a Redox Metabolite (pyocyanin) by Pseudomonas aeruginosa NEJ01R Using a Maize By-Product. Microorganisms 2020, 8, 1559. https://doi.org/10.3390/microorganisms8101559
Bacame-Valenzuela FJ, Pérez-Garcia JA, Figueroa-Magallón ML, Espejel-Ayala F, Ortiz-Frade LA, Reyes-Vidal Y. Optimized Production of a Redox Metabolite (pyocyanin) by Pseudomonas aeruginosa NEJ01R Using a Maize By-Product. Microorganisms. 2020; 8(10):1559. https://doi.org/10.3390/microorganisms8101559
Chicago/Turabian StyleBacame-Valenzuela, Francisco Javier, Jesús Alberto Pérez-Garcia, Mayra Leticia Figueroa-Magallón, Fabricio Espejel-Ayala, Luis Antonio Ortiz-Frade, and Yolanda Reyes-Vidal. 2020. "Optimized Production of a Redox Metabolite (pyocyanin) by Pseudomonas aeruginosa NEJ01R Using a Maize By-Product" Microorganisms 8, no. 10: 1559. https://doi.org/10.3390/microorganisms8101559
APA StyleBacame-Valenzuela, F. J., Pérez-Garcia, J. A., Figueroa-Magallón, M. L., Espejel-Ayala, F., Ortiz-Frade, L. A., & Reyes-Vidal, Y. (2020). Optimized Production of a Redox Metabolite (pyocyanin) by Pseudomonas aeruginosa NEJ01R Using a Maize By-Product. Microorganisms, 8(10), 1559. https://doi.org/10.3390/microorganisms8101559