UV-A Irradiation Increases Scytonemin Biosynthesis in Cyanobacteria Inhabiting Halites at Salar Grande, Atacama Desert
Abstract
:1. Introduction
2. Material and Methods
2.1. Sampling and Handling of Halites
2.2. Experimental Setup
2.3. Scytonemin and Chlorophyll Content
2.4. RNA Extraction and cDNA Synthesis
2.5. Genes and Primers for PCR Amplifications
2.6. Cloning and Sequencing of 16S rRNA and scyB Genes
2.7. Similarity Searches for Nucleotide Sequences of scyB and 16S rRNA Genes
2.8. Quantitative Real Time PCR
2.9. Validation of Results by the ∆∆CT Method
2.10. Statistical Treatment
3. Results and Discussion
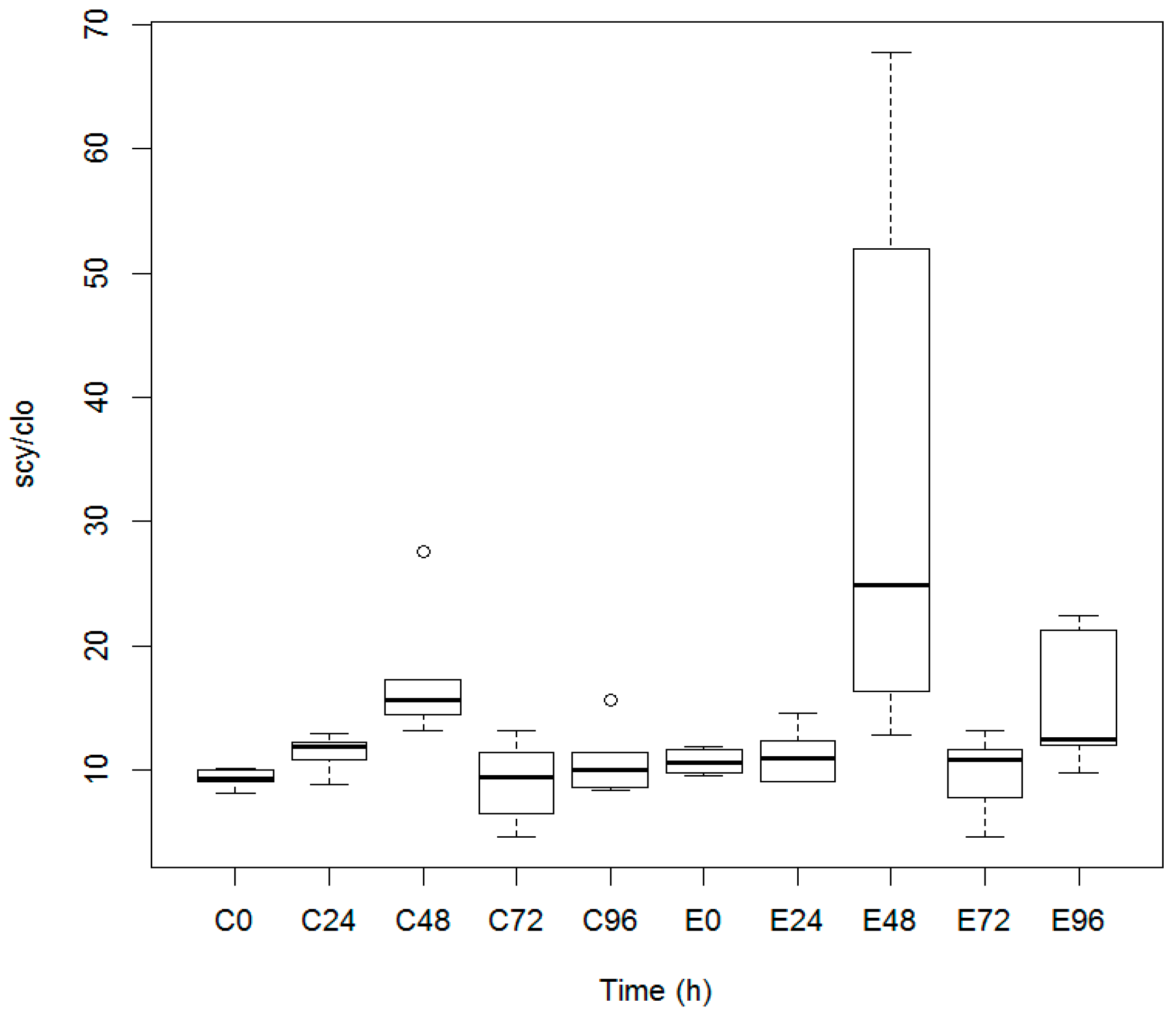
3.1. Quantification of Scytonemin
3.2. Amplification of scyB Gene
3.3. Relative Expression of the scyB Gene in Cyanobacterial Inhabiting Halites by qRT-PCR
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Houston, J.; Hartley, J. The central Andean West-slope rain shadow and its potential contribution to the origin hyperaridity in the Atacama Desert. Int. J. Climatol. 2003, 23, 1453–1464. [Google Scholar] [CrossRef]
- McKay, C.P.; Friedmann, E.I.; Gómez-Silva, B.; Cáceres-Villanueva, L.; Andersen, D.T.; Landheim, R. Temperature and moisture conditions for life in the extreme arid region of the Atacama desert: Four years of observations including the El Niño of 1997–1998. Astrobiology 2003, 3, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Silva, B.; Rainey, F.A.; Warren-Rhodes, K.A.; McKay, C.P.; Navarro-González, R. Atacama Desert Soil Microbiology. In Microbiology of Extreme Soils; Dion, P., Nautiyal, C.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 13, pp. 117–132. [Google Scholar] [CrossRef]
- Robinson, C.; Wierzchos, J.; Black, C.; Crits-Christoph, A.; Bing, M.; Ravel, J.; Ascaso, C.; Artieda, O.; Valea, S.; Roldán, M.; et al. Microbial diversity and the presence of algae in halite endolithic communities are correlated to atmospheric moisture in the hyper-arid zone of the Atacama Desert. Environ. Microbiol. 2015, 17, 299–315. [Google Scholar] [CrossRef]
- Bull, A.; Asenjo, J.; Goodfellow, M.; Gomez-Silva, B. Resources and the growing importance of novel microbial diversity. Annu. Rev. Microbiol. 2016, 8, 215–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uritskiy, G.; Getsein, S.; Munn, A.; Gómez-Silva, B.; Dávila, A.; Glass, B.; Taylor, J.; Di Ruggiero, J. Halophilic Microbial Community compositional shift after rare rainfall in the Atacama Desert. ISME J. 2019, 13, 2737–2749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores, N.; Hoyos, S.; Venegas, M.; Galetovic, A.; Zúñiga, L.; Fábrega, F.; Paredes, B.; Salazar-Ardiles, C.; Vilo, C.; Ascaso, C.; et al. Haloterrigena sp. Strain SGH1, a Bacterioruberin-Rich, Perchlorate-Tolerant Halophilic Archaeon Isolated from Halite Microbial Communities, Atacama Desert, Chile. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Galetović, A.; Seura, F.; Gallardo, V.; Graves, R.; Cortés, J.; Valdivia, C.; Núñez, J.; Tapia, C.; Neira, I.; Sanzana, S.; et al. Use of Phycobiliproteins from Atacama Cyanobacteria as Food Colorants in a Dairy Beverage Prototype. Foods 2020, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Chong Diaz, G.; Mendoza, M.; García-Veigas, J.; Pueyo, J.; Turnerd, P. Evolution and geochemical signatures in a Neogene fore-arc evaporitic basin: The Salar Grande (Central Andes of Chile). Palaeogeo. Palaeoclim. Palaeocol. 1999, 151, 39–54. [Google Scholar] [CrossRef]
- Roldán, M.; Ascaso, C.; Wierzchos, J. Fluorescent Fingerprints of Endolithic Phototrophic Cyanobacteria Living within Halite Rocks in the Atacama Desert. Appl. Environ. Microbiol. 2014, 80, 2998–3006. [Google Scholar] [CrossRef] [Green Version]
- De los Ríos, A.; Valea, S.; Ascaso, C.; Davila, A.; Kastovsky, J.; McKay, C.; Gomez-Silva, B.; Wierzchos, J. Comparative analysis of the microbial communities inhabiting halite evaporites of the Atacama Desert. Int. Microbiol. 2010, 13, 79–89. [Google Scholar] [CrossRef]
- Gómez-Silva, B.; Vilo-Muñoz, C.; Galetović, A.; Dong, Q.; Castelán-Sánchez, H.G.; Pérez-Llano, Y.; Sánchez-Carbente, M.D.R.; Dávila-Ramos, S.; Cortes-López, N.G.; Martínez-Ávila, L.; et al. Metagenomics of Atacama lithobiontic extremophile life unveils highlights on fungal communities, biogeochemical cycles and carbohydrate-active enzymes. Microorganisms 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Silva, B.J. Lithobiontic life: “Atacama rocks are well and alive”. Anton. Leeuw, J. Microbiol 1333. [Google Scholar] [CrossRef]
- Wierzchos, J.; Cámara, A.; De Los Ríos, A.; Dávila, A.F.; Sánchez Almazo, I.M.; Artieda, O.; Wierzchos, K.; Gomez-Silva, B.; Mckay, C.; Ascaso, C. Microbial Colonization of Ca-sulfates crusts in Hyperarid core of Atacama Desert. Geobiology 2011, 9, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Davila, A.; Gomez-Silva, B.; De Los Ríos, A.; Ascaso, C.; Olivares, H.; McKay, C.; Wierzchos, J. Facilitation of endolithic microbial survival in hyperarid core of Atacama Desert by mineral deliquescence. J. Geophys. Res. 2008, 113, 1–9. [Google Scholar] [CrossRef] [Green Version]
- García-Pichel, F.; Nubel, U.; Muyzer, G. The phylogeny of unicellular, extremely halotolerant cyanobacteria. Arch. Microbiol. 1998, 169, 469–482. [Google Scholar] [CrossRef]
- Dress, K.; Neilson, J.; Betancourt, J.; Henderson, D.; Pryor, B.; Maier, R. Bacterial community structure in the hyper arid core of the Atacama Desert, Chile. Appl. Environ. Microbiol. 2006, 72, 7902–7908. [Google Scholar] [CrossRef] [Green Version]
- Vítek, P.; Jehlička, J.; Ascaso, C.; Vlastimil, M.; Gómez-Silva, B.; Olivares, H.; Wierzchos, J. Distribution of scytonemin in endolithic microbial communities from halite crusts in the hyperarid zone of the Atacama Desert, Chile. FEMS Microbiol. Ecol. 2014, 90, 351–366. [Google Scholar] [CrossRef]
- Nägeli, C. Gattungen einzelliger Algen, physiologisch und systematisch bearbeitet Neue Denkschrift. Allg. Schweiz. Nat. Ges. 1849, 10, 1–138. [Google Scholar] [CrossRef]
- Karsten, U.; Sawall, T.; Hanelt, D.; Bischof, K.; Figueroa, L. An Inventory of UV-Absorbing like mycosporine aminoacids in microalgae from polar to warm- temperate regions. Bot. Mar. 1998, 41, 443–453. [Google Scholar] [CrossRef]
- Castenholz, R.W.; Garcia-Pichel, F. Cyanobacterial Responses to UV Radiation. In Ecology of Cyanobacteria II; Whitton, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 481–499. [Google Scholar] [CrossRef]
- Bultel-Ponce, V.; Felix-Theodose, F.; Sarthou, C.; Ponge, G.-F.; Bodo, B. New pigments from terrestrial cyanobacterium Scytonema sp. Collected on Mitaraka Inselberg, French Gutyana. J. Nat. Prod. 2004, 67, 678–681. [Google Scholar] [CrossRef] [Green Version]
- Proteau, P.; Sorrels, C.; Gerwick, W. Organization, Evolution, and Expression Analysis of the Biosynthetic Gene Cluster for Scytonemin a Cyanobacterial UV-Absorbing Pigment. Appl. Environ. Microbiol. 2009, 75, 4861–4869. [Google Scholar] [CrossRef] [Green Version]
- Balskus, E.; Walsh, C. Investigating the initial steps in the biosynthesis of cyanobacterial sunscreen scytonemin. NIH 2008, 130, 15260. [Google Scholar] [CrossRef] [Green Version]
- Soule, T.; Stout, V.; Swingley, D.; Meeks, J.; Garcia-Pichel, F. Molecular genetics and genomic analysis of scytonemin biosynthesis in Nostoc punctiforme ATCC 29133. J. Bacteriol. 2007, 12, 4465–4472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soule, T.; Palmer, K.; Gao, Q.; Potrafka, M.; Stout, V.; Garcia-Pichel, F. A comparative genomics approach to understanding the biosynthesis of the sunscreen scytonemin in cyanobacteria. BMC Genom. 2009, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshy, D.; Mohandass, C.; Dhale, M. Effect of UV-B Radiation and Desiccation Stress on Photoprotective Compounds Accumulation in Marine Leptolyngbya sp. Appl. Biochem. Biotech. 2015, 184, 35–47. [Google Scholar] [CrossRef]
- Gao, Q.; García-Pichel, F. Microbial ultraviolet sunscreens. Nat. Microbiol. 2011, 9, 791–802. [Google Scholar] [CrossRef]
- Warren-Rhodes, K.; Pointing, S.; Ewing, S.; Lacap, D.; Gomez-Silva, B.; Amundson, R.; Friedmann, E.; McKay, C. Hypo lithic cyanobacteria, dry limit of photosynthesis, and microbial ecology in the hyper arid Atacama Desert. Microb. Ecol. 2006, 52, 389–398. [Google Scholar] [CrossRef]
- Wierzchos, J.; Ascaso, C.; Mckay, C. Endolithic Cyanobacteria in Halite Rocks from the Hyperarid Core of the Atacama Desert. Astrobiology 2006, 4, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Dávila, A.F.; Hawes, I.; Araya, J.G.; Gelsinger, D.R.; DiRuggiero, J.; Ascaso, C.; Osano, A.; Wierzchos, J. In situ metabolism in halite endolithic microbial communities of the hyperarid Atacama Desert. Front. Microbiol. 2015, 6, 1035. [Google Scholar] [CrossRef] [Green Version]
- Soule, T.; García-Pichel, F.; Stout, V. Gene expression patterns associated with the biosynthesis of the sunscreen scytonemin in Nostoc punctiforme ATCC 29133 in response to UV-A radiation. J. Bacteriol. 2009, 191, 4639–4646. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Pathak, J.; Singh, D.K.; Ahmed, H.; Singh, V.; Kumar, D.; Sinha, R.P. Photoprotective role of UV-screening pigment scytonemin against UV-B-induced damages in the heterocyst-forming cyanobacterium Nostoc sp. strain HKAR-2. Braz. J. Bot. 2020, 43. [Google Scholar] [CrossRef]
- Wollenhaupt, N.C.; Wolkowski, R.P. Grid Soil Sampling. Better Crops Fall 1994, 78, 6–9. [Google Scholar]
- Brenowitz, S.; Castenholz, R. Long-terms UV-effect and visible irradiance on natural populations of scytonemin-containing cyanobacterium (Calotrix sp.). FEMS 1997, 4, 343–352. [Google Scholar] [CrossRef]
- The R Development Core Team. R: A Language and Environment for Statistical Computing, Vienna, Austria. 2017 Version 2.6.2.. Available online: http://softlibre.unizar.es/manuales/aplicaciones/r/fullrefman.pdf (accessed on 24 March 2019).
- Nubel, U.; Garcia-Pichel, F.; Muyzer, G. PCR Primers to amplify 16S rRNA Genes from cyanobacteria. Appl. Environ. Microbiol. 1997, 8, 3327–3332. [Google Scholar] [CrossRef] [Green Version]
- Pinto, F.; Pacheco, C.; Ferreira, D.; Moradas-Ferreira, P.; Tamagnini, P. Selection of suitable reference genes for RT-qPCR analyses in cyanobacteria. PLoS ONE 2012, 4, e34983. [Google Scholar] [CrossRef]
- Atschul, S.; Gish, W.; Miller, W.; Myers, E.; Lipman, D. Basic Local Alignment Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Cheregi, O.; Sicora, C.; Kós, P.; Barker, M.; Nixon, P. The role of the FtsH and Deg proteases in the repair of UV-B radiation-damaged Photosystem II in the cyanobacterium Synechocystis PCC 6803. Biochem. Biophys. Acta 2007, 1767, 820–828. [Google Scholar] [CrossRef] [Green Version]
- Applied Biosystems. Guide to Performing Relative Quantification of Gene Expression Using Real-Time Quantitative PCR. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/cms_042380.pdf (accessed on 23 May 2018).
- Riedy, M.; Timm, E.; Stewart, C. Quantitative Measure qRT-PCR gene expression. Biotechniques 1995, 18, 70–76. [Google Scholar]
- Hoaglin, D.C.; John, W. Tukey and Data Analysis. Stat. Sci. 2003, 18, 311–318. [Google Scholar] [CrossRef]
- Cordero, R.; Damiani, A.; Seckmeyer, G.; Jorquera, J.; Caballero, M.; Rowe, P.; Ferrer, J.; Mubarak, R.; Carrasco, J.; Rondanelli, R.; et al. The solar spectrum in the Atacama Desert. Nat. Rev. 2018. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Bilger, W.; Scherer, S. UV-B-Induced Synthesis of Photoprotective Pigments and Extracellular Polysaccharides in the Terrestrial Cyanobacterium Nostoc Commune. J. Bacteriol. 1997, 179, 1940–1945. [Google Scholar] [CrossRef] [Green Version]
- Moon, Y.; Kim, S.; Chung, Y. Sensing and responding to UV-A in cyanobacteria. Int. J. Mol. Sci. 2012, 13, 16303–16332. [Google Scholar] [CrossRef]
- Rastogi, R.; Marward, D.; Incharoensakdi, A. Bloom Dynamics of Cyanobacteria and Their Toxins: Environmental Health Impacts and Mitigation Strategies. Front. Microbiol. 2015, 6, 1551–1563. [Google Scholar] [CrossRef] [Green Version]
- Fleming, E.D.; Castenholz, R.W. Effects of periodic desiccation on the synthesis of the UV--screening compound, scytonemin, in cyanobacteria. Environ. Microbiol. 2007, 6, 1448–1455. [Google Scholar] [CrossRef]
- Varnali, T.; Edwards, H. Reduced and oxidized scytonemin: Theoretical protocol for Raman spectroscopic identification of potential key biomolecules for astrobiology. Spectrochem. Acta Part A Mol. Biol. Spect. 2014, 117, 72–77. [Google Scholar] [CrossRef]
- Mishra, A.; Tandon, R.; Kesarwani, S.; Singh, R.; Tiwari, G.L. Emerging applications of cyanobacterial ultraviolet protecting compound scytonemin. J. Appl. Phycol. 2015, 27, 1045–1051. [Google Scholar] [CrossRef]
- Ferreira, D.; García Pichel, F. Mutational Studies of Putative Biosynthetic Genes for the Cyanobacterial Sunscreen Scytonemin in Nostoc punctiforme ATCC 29133. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Williamson, L.; Saponaro, M.; Boeing, S.; East, P.; Mitter, R.; Kantidakis, T.; Kelly, G.; Lobley, A.; Walker, J.; Spencer-Dene, B.; et al. UV Irradiation induces a Non-coding RNA that functionally opposes the protein encoded by the same gene. Cell 2017, 168, 843–855. [Google Scholar] [CrossRef] [Green Version]


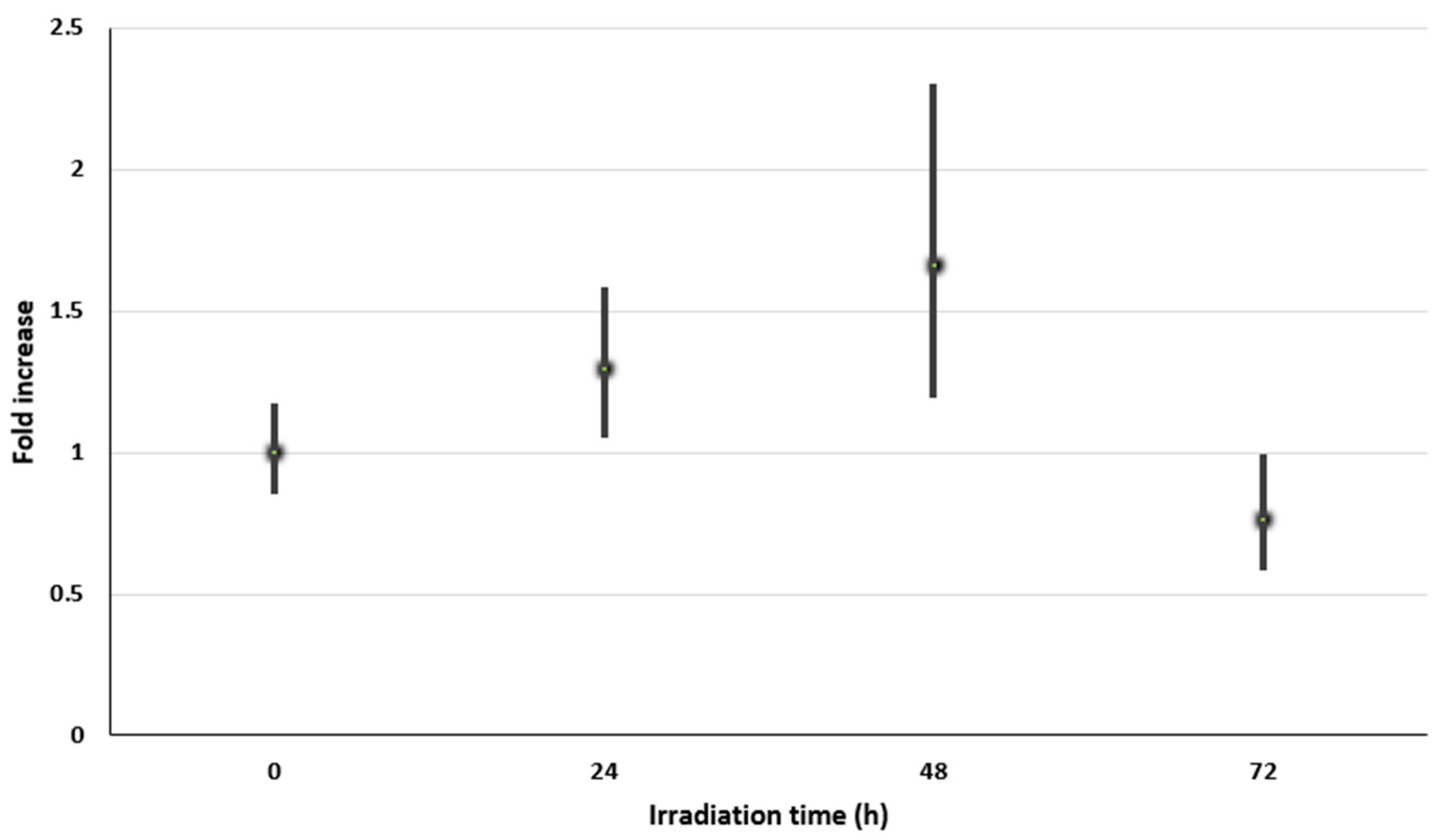
| UV-A Irradiation Time (h) | scyB (Mean CT) | rnpB (Mean CT) | ∆CT (scyB-rnpB) | ∆∆ CT (*) | Fold Increase (**) |
|---|---|---|---|---|---|
| 0 | 29.67 | 32.68 | −2.51 | 0 | 1 (0.85–1.17) |
| 24 | 29.24 | 32.12 | −2.88 | −0.37 | 1.29 (1.05–1.58) |
| 48 | 29.49 | 32.73 | −3.24 | −0.73 | 1.66 (1.19–2.31) |
| 72 | 29.84 | 31.96 | −2.12 | 0.39 | 0.76 (0.58–0.99) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orellana, G.; Gómez-Silva, B.; Urrutia, M.; Galetović, A. UV-A Irradiation Increases Scytonemin Biosynthesis in Cyanobacteria Inhabiting Halites at Salar Grande, Atacama Desert. Microorganisms 2020, 8, 1690. https://doi.org/10.3390/microorganisms8111690
Orellana G, Gómez-Silva B, Urrutia M, Galetović A. UV-A Irradiation Increases Scytonemin Biosynthesis in Cyanobacteria Inhabiting Halites at Salar Grande, Atacama Desert. Microorganisms. 2020; 8(11):1690. https://doi.org/10.3390/microorganisms8111690
Chicago/Turabian StyleOrellana, Gabriela, Benito Gómez-Silva, Milton Urrutia, and Alexandra Galetović. 2020. "UV-A Irradiation Increases Scytonemin Biosynthesis in Cyanobacteria Inhabiting Halites at Salar Grande, Atacama Desert" Microorganisms 8, no. 11: 1690. https://doi.org/10.3390/microorganisms8111690





