Biological and Chemical Processes that Lead to Textile Malodour Development
Abstract
:1. Introduction
2. The Human Skin
2.1. Skin Microbiome
2.2. Body Odour
3. Clothing
3.1. Textile Volatilome
3.2. Textile Microbiome
4. Washing Machine
4.1. Impact of Washing Process on Volatiles and Microorganisms
4.2. The Washing Machine Volatilome and Microbiome
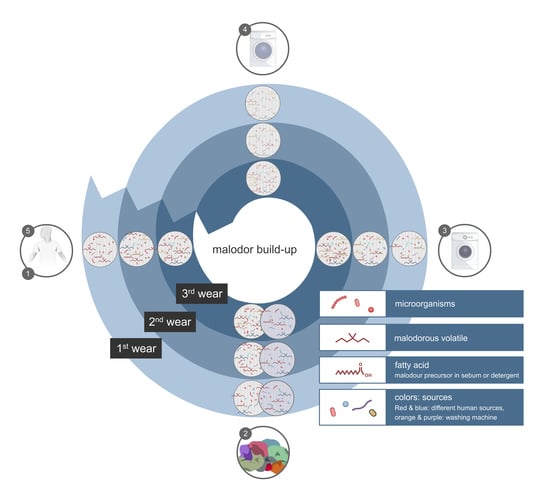
5. Factors that Determine Malodour Formation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Klepp, I.G.; Buck, M.; Laitala, K.; Kjeldsberg, M. What’s the problem? Odor-control and the smell of sweat in sportswear. Fash. Pract. 2016, 8, 296–317. [Google Scholar] [CrossRef]
- Kubota, H.; Mitani, A.; Niwano, Y.; Takeuchi, K.; Tanaka, A.; Yamaguchi, N.; Kawamura, Y.; Hitomi, J. Moraxella species are primarily responsible for generating malodor in laundry. Appl. Environ. Microbiol. 2012, 78, 3317–3324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honisch, M.; Stamminger, R.; Bockmuhl, D.P. Impact of wash cycle time, temperature and detergent formulation on the hygiene effectiveness of domestic laundering. J. Appl. Microbiol. 2014, 117, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Bockmuhl, D.P.; Schages, J.; Rehberg, L. Laundry and textile hygiene in healthcare and beyond. Microb. Cell 2019, 6, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Bockmuhl, D.P. Laundry hygiene-how to get more than clean. J. Appl. Microbiol. 2017, 122, 1124–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munk, S.; Johansen, C.; Stahnke, L.H.; Adler-Nissen, J. Microbial survival and odor in laundry. J. Surfactants Deterg. 2001, 4, 385–394. [Google Scholar] [CrossRef]
- Callewaert, C.; Van Nevel, S.; Kerckhof, F.M.; Granitsiotis, M.S.; Boon, N. Bacterial exchange in household washing machines. Front. Microbiol. 2015, 6, 1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denawaka, C.J.; Fowlis, I.A.; Dean, J.R. Source, impact and removal of malodour from soiled clothing. J. Chromatogr. A 2016, 1438, 216–225. [Google Scholar] [CrossRef] [Green Version]
- McQueen, R.H.; Keelan, M.; Xu, Y.; Mah, T. In vivo assessment of odour retention in an antimicrobial silver chloride-treated polyester textile. J. Text. Instit. 2013, 104, 108–117. [Google Scholar] [CrossRef]
- Nagoh, Y.; Tobe, S.; Watanabe, T.; Mukaiyama, T. Analysis of odorants produced from indoor drying laundries and effects of enzyme for preventing malodor generation. Tenside Surf. Det. 2005, 42, 7–12. [Google Scholar] [CrossRef]
- Gallo, R.L. Human skin is the largest epithelial surface for interaction with microbes. J. Investig. Dermatol. 2017, 137, 1213–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Program, N.C.S.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and temporal diversity of the human skin microbiome. Science (NY) 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial community variation in human body habitats across space and time. Science (NY) 2009, 326, 1694–1697. [Google Scholar] [CrossRef] [Green Version]
- Cundell, A.M. Microbial ecology of the human skin. Microb. Ecol. 2018, 76, 113–120. [Google Scholar] [CrossRef]
- Oh, J.; Byrd, A.L.; Park, M.; Program, N.C.S.; Kong, H.H.; Segre, J.A. Temporal stability of the human skin microbiome. Cell 2016, 165, 854–866. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Byrd, A.L.; Deming, C.; Conlan, S.; Program, N.C.S.; Kong, H.H.; Segre, J.A. Biogeography and individuality shape function in the human skin metagenome. Nature 2014, 514, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Ying, S.; Zeng, D.N.; Chi, L.; Tan, Y.; Galzote, C.; Cardona, C.; Lax, S.; Gilbert, J.; Quan, Z.X. The influence of age and gender on skin-associated microbial communities in urban and rural human populations. PLoS ONE 2015, 10, e0141842. [Google Scholar] [CrossRef]
- Zeeuwen, P.L.; Boekhorst, J.; van den Bogaard, E.H.; de Koning, H.D.; van de Kerkhof, P.M.; Saulnier, D.M.; van Swam, I.I.; van Hijum, S.A.; Kleerebezem, M.; Schalkwijk, J.; et al. Microbiome dynamics of human epidermis following skin barrier disruption. Genome Biol. 2012, 13, R101. [Google Scholar] [CrossRef] [Green Version]
- McCall, L.I.; Callewaert, C.; Zhu, Q.; Song, S.J.; Bouslimani, A.; Minich, J.J.; Ernst, M.; Ruiz-Calderon, J.F.; Cavallin, H.; Pereira, H.S.; et al. Home chemical and microbial transitions across urbanization. Nat. Microbiol. 2020, 5, 108–115. [Google Scholar] [CrossRef]
- Harker, M.; Carvell, A.M.; Marti, V.P.; Riazanskaia, S.; Kelso, H.; Taylor, D.; Grimshaw, S.; Arnold, D.S.; Zillmer, R.; Shaw, J.; et al. Functional characterisation of a snp in the abcc11 allele—Effects on axillary skin metabolism, odour generation and associated behaviours. J. Dermatol. Sci. 2014, 73, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Claesen, J.S.J.; Ramos, S.F.; Kurita, K.L.; Byrd, A.L.; Aksenov, A.A.; Melnik, A.V.; Wong, W.R.; Wang, S.; Hernandez, R.D.; Donia, M.S.; et al. Cutibacterium acnesantibiotic production shapes niche competition in the human skin microbiome. BioRxiv Prepr. 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Callewaert, C.; De Maeseneire, E.; Kerckhof, F.M.; Verliefde, A.; Van de Wiele, T.; Boon, N. Microbial odor profile of polyester and cotton clothes after a fitness session. Appl. Environ. Microbiol. 2014, 80, 6611–6619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouslimani, A.; da Silva, R.; Kosciolek, T.; Janssen, S.; Callewaert, C.; Amir, A.; Dorrestein, K.; Melnik, A.V.; Zaramela, L.S.; Kim, J.N.; et al. The impact of skin care products on skin chemistry and microbiome dynamics. BMC Biol. 2019, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Budding, A.E.; van der Lugt-Degen, M.; Du-Thumm, L.; Vandeven, M.; Fan, A. The influence of age, gender and race/ethnicity on the composition of the human axillary microbiome. Int. J. Cosmet. Sci. 2019, 41, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Saathoff, M.; Kuhn, F.; Max, H.; Terstegen, L.; Natsch, A. A functional abcc11 allele is essential in the biochemical formation of human axillary odor. J. Investig. Dermatol. 2010, 130, 529–540. [Google Scholar] [CrossRef] [Green Version]
- Ellis, A.; Billings, S.D.; Khanna, U.; Warren, C.B.; Piliang, M.; Vij, A.; Ko, J.S.; Bergfeld, W.F.; Fernandez, A.P. Diagnoses of hospitalized patients with skin abnormalities prompting biopsy by consulting dermatologists: A 3-year review from a tertiary care center. J. Cutan. Pathol. 2020, 47, 346–356. [Google Scholar] [CrossRef]
- Troccaz, M.; Gaia, N.; Beccucci, S.; Schrenzel, J.; Cayeux, I.; Starkenmann, C.; Lazarevic, V. Mapping axillary microbiota responsible for body odours using a culture-independent approach. Microbiome 2015, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- James, A.G.; Austin, C.J.; Cox, D.S.; Taylor, D.; Calvert, R. Microbiological and biochemical origins of human axillary odour. FEMS Microbiol. Ecol. 2013, 83, 527–540. [Google Scholar] [CrossRef]
- Fredrich, E.; Barzantny, H.; Brune, I.; Tauch, A. Daily battle against body odor: Towards the activity of the axillary microbiota. Trends Microbiol. 2013, 21, 305–312. [Google Scholar] [CrossRef]
- Shelley, W.B.; Hurley, H.J., Jr. The physiology of the human axillary apocrine sweat gland. J. Investig. Dermatol. 1953, 20, 285–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, K.T.; Bojar, R.A. Cosmetics: What is their influence on the skin microflora? Am. J. Clin. Dermatol. 2002, 3, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, R.; Hodgins, M.B.; Van der Kwast, T.H.; Brinkmann, A.O.; Boersma, W.J. Localization of androgen receptors in human skin by immunohistochemistry: Implications for the hormonal regulation of hair growth, sebaceous glands and sweat glands. J. Endocrinol. 1992, 133, 467–475. [Google Scholar] [CrossRef]
- Toyoda, Y.; Sakurai, A.; Mitani, Y.; Nakashima, M.; Yoshiura, K.; Nakagawa, H.; Sakai, Y.; Ota, I.; Lezhava, A.; Hayashizaki, Y.; et al. Earwax, osmidrosis, and breast cancer: Why does one snp (538g>a) in the human abc transporter abcc11 gene determine earwax type? FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 2001–2013. [Google Scholar] [CrossRef]
- Noel, F.; Pierard-Franchimont, C.; Pierard, G.E.; Quatresooz, P. Sweaty skin, background and assessments. Int. J. Dermatol. 2012, 51, 647–655. [Google Scholar] [CrossRef]
- Wilke, K.; Martin, A.; Terstegen, L.; Biel, S.S. A short history of sweat gland biology. Int. J. Cosmet. Sci. 2007, 29, 169–179. [Google Scholar] [CrossRef]
- Emter, R.; Natsch, A. The sequential action of a dipeptidase and a beta-lyase is required for the release of the human body odorant 3-methyl-3-sulfanylhexan-1-ol from a secreted cys-gly-(s) conjugate by corynebacteria. J. Biol. Chem. 2008, 283, 20645–20652. [Google Scholar] [CrossRef] [Green Version]
- Troccaz, M.; Starkenmann, C.; Niclass, Y.; van de Waal, M.; Clark, A.J. 3-methyl-3-sulfanylhexan-1-ol as a major descriptor for the human axilla-sweat odour profile. Chem. Biodivers. 2004, 1, 1022–1035. [Google Scholar] [CrossRef]
- Bawdon, D.; Cox, D.S.; Ashford, D.; James, A.G.; Thomas, G.H. Identification of axillary staphylococcus sp. Involved in the production of the malodorous thioalcohol 3-methyl-3-sufanylhexan-1-ol. FEMS Microbiol. Lett. 2015, 362, fnv111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minhas, G.S.; Bawdon, D.; Herman, R.; Rudden, M.; Stone, A.P.; James, A.G.; Thomas, G.H.; Newstead, S. Structural basis of malodour precursor transport in the human axilla. eLife 2018, 7, e34995. [Google Scholar] [CrossRef] [PubMed]
- James, A.G.; Hyliands, D.; Johnston, H. Generation of volatile fatty acids by axillary bacteria. Int. J. Cosmet. Sci. 2004, 26, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Natsch, A.; Gfeller, H.; Gygax, P.; Schmid, J.; Acuna, G. A specific bacterial aminoacylase cleaves odorant precursors secreted in the human axilla. J. Biol. Chem. 2003, 278, 5718–5727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, K.; Yabuki, M.; Hasegawa, Y. Review of odorants in human axillary odour and laundry malodour: The importance of branched c7 chain analogues in malodours perceived by humans. Flavour Fragr. J. 2013, 28, 223–230. [Google Scholar] [CrossRef]
- Dravnieks, A.; Krotoszynski, B.K.; Lieb, W.E.; Jungermann, E. Influence of an antibacterial soap on various effluents from axillae. J. Soc. Cosmet. Chem. 1968, 19, 611–626. [Google Scholar]
- McQueen, H.R.; Harynuk, J.J.; Wismer, V.W.; Keelan, M.; Xu, Y.; Paulina, A. Axillary odour build-up in knit fabrics following multiple use cycles. Int. J. Cloth. Sci. Technol. 2014, 26, 274–290. [Google Scholar] [CrossRef]
- Chung, H.; Seok, H.J. Populations of malodor-forming bacteria and identification of volatile components in triolein-soiled cotton fabric. Fibers Polym. 2012, 13, 740–747. [Google Scholar] [CrossRef]
- Chi, Y.S.; Obendorf, S.K. Aging of oily soils on textile materials: A literature review. J. Surf. Deterg. 1998, 1, 407–418. [Google Scholar] [CrossRef]
- Bowers, C.A.; Chantrey, G. Factors controlling soiling of white polyestercotton fabrics: Part I: Laboratory studies. Text. Res. J. 1969, 39, 1–11. [Google Scholar] [CrossRef]
- Yao, L.; Laing, R.M.; Bremer, P.J.; Silcock, P.J.; Leus, M.J. Measuring textile adsorption of body odor compounds using proton-transfer-reaction mass spectrometry. Text. Res. J. 2015, 85, 1817–1826. [Google Scholar] [CrossRef]
- Prada, P.A.; Curran, A.M.; Furton, K.G. The evaluation of human hand odor volatiles on various textiles: A comparison between contact and noncontact sampling methods. J. Forensic Sci. 2011, 56, 866–881. [Google Scholar] [CrossRef]
- Obendorf, S.K.; Webb, J.J. Detergency study—Distribution of natural soils on shirt collars. Text. Res. J. 1987, 57, 557–563. [Google Scholar] [CrossRef]
- Obendorf, S.K.; Namaste, Y.M.N.; Durnam, D.J. A microscopical study of residual oily soil distribution on fabrics of varying fiber content. Text. Res. J. 1983, 53, 375–383. [Google Scholar] [CrossRef]
- Hammer, T.R.; Berner-Dannenmann, N.; Hoefer, D. Quantitative and sensory evaluation of malodour retention of fibre types by use of artificial skin, sweat and radiolabelled isovaleric acid. Flavour Fragr. J. 2013, 28, 238–244. [Google Scholar] [CrossRef]
- Abdul-Bari, M.M.; McQueen, R.H.; Nguyen, H.; Wismer, W.V.; de la Mata, A.P.; Harynuk, J.J. Synthetic clothing and the problem with odor: Comparison of nylon and polyester fabrics. Cloth. Text. Res. J. 2018, 36, 251–266. [Google Scholar] [CrossRef]
- McQueen, R.H.; Laing, R.M.; Brooks, H.J.L.; Niven, B.E. Odor intensity in apparel fabrics and the link with bacterial populations. Text. Res. J. 2007, 77, 449–456. [Google Scholar] [CrossRef]
- Wang, J.; Lu, X.; Wang, J.; Wang, X. Quantitative and sensory evaluation of odor retention on polyester wool blends. Text. Res. J. 2018, 89, 2729–2738. [Google Scholar] [CrossRef]
- Rathinamoorthy, R.; Thilagavathi, G. Optimisation of process conditions of cotton fabric treatment with terminalia chebula extract for antibacterial application. Indian J. Fibre Text. 2013, 38, 293–303. [Google Scholar]
- Rathinamoorthy, R.; Thilagavathi, G. Gc-ms analysis of worn textile for odour formation. Fibers Polym. 2016, 17, 917–924. [Google Scholar] [CrossRef]
- Ara, K.; Hama, M.; Akiba, S.; Koike, K.; Okisaka, K.; Hagura, T.; Kamiya, T.; Tomita, F. Foot odor due to microbial metabolism and its control. Can. J. Microbiol. 2006, 52, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Obendorf, S.K.; Kim, J.; Koniz, R.F. Measurement of odor development due to bacterial action on antimicrobial polyester fabrics. Aatcc Rev. 2007, 7, 35–40. [Google Scholar]
- Dumas, E.R.; Michaud, A.E.; Bergeron, C.; Lafrance, J.L.; Mortillo, S.; Gafner, S. Deodorant effects of a supercritical hops extract: Antibacterial activity against corynebacterium xerosis and staphylococcus epidermidis and efficacy testing of a hops/zinc ricinoleate stick in humans through the sensory evaluation of axillary deodorancy. J. Cosmet. Dermatol. 2009, 8, 197–204. [Google Scholar] [CrossRef]
- Gower, D.B.; Mallet, A.I.; Watkins, W.J.; Wallace, L.M.; Calame, J.P. Capillary gas chromatography with chemical ionization negative ion mass spectrometry in the identification of odorous steroids formed in metabolic studies of the sulphates of androsterone, dha and 5 alpha-androst-16-en-3 beta-ol with human axillary bacterial isolates. J. Steroid Biochem. 1997, 63, 81–89. [Google Scholar]
- Natsch, A.; Gfeller, H.; Gygax, P.; Schmid, J. Isolation of a bacterial enzyme releasing axillary malodor and its use as a screening target for novel deodorant formulations. Int. J. Cosmet. Sci. 2005, 27, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Dayan, C.L.B.N. Corynebacterium species and their role in the generation of human malodor. In Innate Immune System of Skin and Oral Mucosa: Properties and Impact in Pharmaceutics, Cosmetics, and Personal Care Products; Wertz, N.D.P.W., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Brooksbank, B.W.; Brown, R.; Gustafsson, J.A. The detection of 5alpha-androst-16-en-3alpha-ol in human male axillary sweat. Experientia 1974, 30, 864–865. [Google Scholar] [CrossRef] [PubMed]
- Claus, R.; Alsing, W. Occurrence of 5alpha-androst-16en-3-one, a boar pheromone, in man and its relationship to testosterone. J. Endocrinol. 1976, 68, 483–484. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.J. Chemical composition of household malodours—An overview. Flavour Fragr. J. 2013, 28, 251–261. [Google Scholar] [CrossRef]
- Stapleton, K.; Dean, J.R. A preliminary identification and determination of characteristic volatile organic compounds from cotton, polyester and terry-towel by headspace solid phase microextraction gas chromatography-mass spectrometry. J. Chromatogr. A 2013, 1295, 147–151. [Google Scholar] [CrossRef]
- Teufel, L.; Redl, B. Improved methods for the investigation of the interaction between textiles and microorganisms. Lenzing. Ber. 2006, 85, 54–60. [Google Scholar]
- Teufel, L.; Pipal, A.; Schuster, K.C.; Staudinger, T.; Redl, B. Material-dependent growth of human skin bacteria on textiles investigated using challenge tests and DNA genotyping. J. Appl. Microbiol. 2010, 108, 450–461. [Google Scholar] [CrossRef]
- Takashima, M.; Shirai, F.; Sageshima, M.; Ikeda, N.; Okamoto, Y.; Dohi, Y. Distinctive bacteria-binding property of cloth materials. Am. J. Infect. Control 2004, 32, 27–30. [Google Scholar] [CrossRef]
- Lucassen, R.; Merettig, N.; Bockmühl, D.P. Antimicrobial efficacy of hygiene rinsers under consumer-related conditions. Tenside Surfactant Deterg. 2013, 50, 259–262. [Google Scholar] [CrossRef]
- Bellante, S.; Engel, A.; Hatice, T.; Neumann, A.; Okyay, G.; Vossebein, L. Hygienische aufbereitung von textilien in privathaushalten-eine studie aus der praxis. Hyg. Med. 2011, 36, 300–305. [Google Scholar]
- Bloomfield, S.F.; Exner, M.; Signorelli, C.; Scott, E.A. Effectiveness of Laundering Processes Used in Domestic (Home) Settings October 2013; International Science Forum on Home Hygien: Somerset, UK, 2013; pp. 1–62. [Google Scholar]
- Hammer, T.R.; Mucha, H.; Hoefer, D. Infection risk by dermatophytes during storage and after domestic laundry and their temperature-dependent inactivation. Mycopathologia 2011, 171, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Hirakawa, H.; Morita, Y.; Tomida, J.; Sato, J.; Matsumura, Y.; Mitani, A.; Niwano, Y.; Takeuchi, K.; Kubota, H.; et al. Complete genome sequence of moraxella osloensis strain kmc41, a producer of 4-methyl-3-hexenoic acid, a major malodor compound in laundry. Genome Announc. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Jacksch, S.; Thota, J.; Shetty, S.; Smidt, H.; Schnell, S.; Egert, M. Metagenomic analysis of regularly microwave-treated and untreated domestic kitchen sponges. Microorganisms 2020, 8, 736. [Google Scholar] [CrossRef]
- International Association for Soaps, Detergents and Maintenance Products. Pan-European Consumer Habits Survey 2017: Perceptions of Cleanliness and Hygiene—Cleaning Habits, Sustainability and Safety; International Association for Soaps, Detergents and Maintenance Products: Brussels, Belgium, 2017. [Google Scholar]
- Meredith, C. How Long Does a Washing Machine Take?—Cycle Times & More. Available online: https://blog.bellinghamelectric.com/ (accessed on 5 June 2020).
- Kimberly, J. Laundry Tips That Will Save You Time. Available online: https://www.consumerreports.org/ (accessed on 5 June 2020).
- Nix, I.D.; Frontzek, A.; Bockmühl, D.P. Characterization of microbial communitiesin household washing machines. Tenside Surfactant Deterg. 2015, 52, 432–440. [Google Scholar] [CrossRef]
- Gattlen, J.; Amberg, C.; Zinn, M.; Mauclaire, L. Biofilms isolated from washing machines from three continents and their tolerance to a standard detergent. Biofouling 2010, 26, 873–882. [Google Scholar] [CrossRef]
- Babic, M.N.; Zalar, P.; Zenko, B.; Schroers, H.J.; Dzeroski, S.; Gunde-Cimerman, N. Candida and fusarium species known as opportunistic human pathogens from customer-accessible parts of residential washing machines. Fungal Biol. 2015, 119, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Terpstra, M.J. The correlation between sustainable development and home hygiene. Am. J. Infect. Control 2001, 29, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmithausen, R.M.; Sib, E.; Exner, M.; Hack, S.; Rosing, C.; Ciorba, P.; Bierbaum, G.; Savin, M.; Bloomfield, S.F.; Kaase, M.; et al. The washing machine as a reservoir for transmission of extended-spectrum-beta-lactamase (ctx-m-15)-producing klebsiella oxytoca st201 to newborns. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [Green Version]
- Boonstra, M.B.; Spijkerman, D.C.M.; Voor, A.F.; van der Laan, R.J.; Bode, L.G.M.; van Vianen, W.; Klaassen, C.H.W.; Vos, M.C.; Severin, J.A. An outbreak of st307 extended-spectrum beta-lactamase (esbl)-producing klebsiella pneumoniae in a rehabilitation center: An unusual source and route of transmission. Inf. Control Hosp. Epidemiol. 2020, 41, 31–36. [Google Scholar] [CrossRef] [PubMed]
- McQueen, R.H.; Laing, R.M.; Wilson, C.A.; Niven, B.E.; Nelahunty, C.M. Odor retention on apparel fabrics: Development of test methods for sensory detection. Text. Res. J. 2007, 77, 642–652. [Google Scholar] [CrossRef]
- Xu, Y.; McQueen, R.; Wismer, W. A preliminary study on the collection and detection of axillary odour within textiles. J. Text. Appar. Technol. Manag. 2013, 8, 1–13. [Google Scholar]
- Krifa, M.; Rajaganesh, S.; Fahy, W. Perspectives on textile cleanliness—Detecting human sebum residues on worn clothing. Text. Res. J. 2019, 89, 5226–5237. [Google Scholar] [CrossRef]
- McQueen, R.H.; Vaezafshar, S. Odor in textiles: A review of evaluation methods, fabric characteristics, and odor control technologies. Text. Res. J. 2020, 90, 1157–1173. [Google Scholar] [CrossRef]
- Bloomfield, S.F. Preventing infectious disease in the domestic setting: A risk-based approach. Am. J. Infect. Control 2001, 29, 207–210. [Google Scholar] [CrossRef]
- Fijan, S.; Sostar-Turk, S.; Cencic, A. Implementing hygiene monitoring systems in hospital laundries in order to reduce microbial contamination of hospital textiles. J. Hosp. Inf. 2005, 61, 30–38. [Google Scholar] [CrossRef]
- Fijan, S.; Koren, S.; Cencic, A.; Sostar-Turk, S. Antimicrobial disinfection effect of a laundering procedure for hospital textiles against various indicator bacteria and fungi using different substrates for simulating human excrements. Diagn. Microbiol. Inf. Dis. 2007, 57, 251–257. [Google Scholar] [CrossRef]
- Nordstrom, J.M. Evaluation of the Occurrence and Risk of Microbes in Laundry and Laundry-Associated Surfaces; The University of Arizona: Tucson, Arizona, 2009. [Google Scholar]
- Patel, S.N.; Murray-Leonard, J.; Wilson, A.P. Laundering of hospital staff uniforms at home. J. Hosp. Inf. 2006, 62, 89–93. [Google Scholar] [CrossRef]
- Laitala, K.; Boks, C.; Klepp, I.G. Potential for environmental improvements in laundering. Int. J. Consum. Stud. 2011, 35, 254–264. [Google Scholar] [CrossRef]
- Tano, E.; Melhus, A. Level of decontamination after washing textiles at 60 degrees c or 70 degrees c followed by tumble drying. Inf. Ecol. Epidemio. 2014, 4, 24314. [Google Scholar]
- Brands, B.; Honisch, M.; Wegner, S.; Bockmühl, D.P. The effect of drying processes on the microbial load of laundry brands. Househ. Pers. Care Today 2016, 11, 24–27. [Google Scholar]
- Amichai, B.; Grunwald, M.H.; Davidovici, B.; Shemer, A. Sunlight is said to be the best of disinfectants: The efficacy of sun exposure for reducing fungal contamination in used clothes. Isr. Med. Assoc. J. IMAJ 2014, 16, 431–433. [Google Scholar] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. Uv radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [Green Version]

| Volatile Group | Odorous Volatiles | Involved Microorganisms | Analysis before and/or after Washing, FD-value | Literature |
|---|---|---|---|---|
| Fatty acids | Ethanoic acid (acetic acid) | Propionibacterium sp. Staphylococcus sp. | after wearing | [41,58] |
| Propanoic acid | Propionibacterium sp. Staphylococcus sp. | after wearing | [41,58] | |
| 2-methylpropanoic acid (isobutyric acid) | Bacillus subtilis | after wearing | [59] | |
| Butanoic acid | after wearing | [8] | ||
| 2-methylbutanoic acid | Bacillus subtilis | after wearing | [59] | |
| 3-methylbutanoic acid (isovaleric acid) | Bacillus subtilis Staphylococcus epidermidis | after wearing and after washing | [6,38,43,59,60,61,62] | |
| 3-methyl-2-hexenoic acid | Corynebacterium sp. Micrococcus sp. | after wearing | [42,58,60,63,64] | |
| 4-methyl-3-hexenoic acid (4M3H) | Moraxella osloensis | after washing | [2,43] | |
| 5-methyl-4-hexenoic acid | after washing | [43] | ||
| 3-methyl-3-hydroxy-hexanoic acid | Corynebacterium bovis, Corynebacterium jeijeikum, Corynebacterium striatum | after wearing | [42,60,63,64] | |
| 6-heptenoic acid | after washing | [43] | ||
| 4-methyloctanoic acid | after washing | [6] | ||
| 4-ethyloctanoic acid | after washing; FD > 256 | [6] | ||
| Steroid compounds | 5-α-androstenol | after wearing | [65] | |
| 5-α-androstenone | after wearing | [66] | ||
| 5-α-androst-2-en-17-one | Staphylococcus sp. Corynebacterium sp. | after wearing and after washing | [6,38,60,61,62,67] | |
| 5-α-androst-16-ene-3-one | after wearing | [58] | ||
| Sulfur compounds | 3-methyl-3-sulfanyl-hexan-1-ol (3M3SH) | Staphylococcus haemolyticus; Staphylococcus hominis | after wearing | [38,39,42,60,63,64] |
| Dimethyl disulphides | after washing | [8,68] | ||
| Dimethyl trisulphides | after washing | [8,68] | ||
| Benzyl mercaptan | Corynebacterium sp. | after wearing | [39] | |
| Ketones | 1-hexen-3-one | after washing | [6] | |
| 2-heptanone | after wearing | [8] | ||
| 2-octanone | after wearing | [8] | ||
| 1-octen-3-one | after wearing and after washing | [6,67] | ||
| 2-nonanone | after wearing | [8] | ||
| Medium-chain ketones (undetermined) | [10] | |||
| Esters | Ethyl-2-methylpropanoate | after washing; FD > 256 | [6] | |
| Ethyl butanoate | after wearing and after washing; FD > 256 | [6,67] | ||
| Methyl-3-methyl-hexanoate | after wearing | [58] | ||
| Methyl laurate | after wearing | [58] | ||
| Methyl myristate | after wearing | [58] | ||
| 2-Aminoacetophenone | after wearing; FD > 256 | [6] | ||
| Diethyl phthalate | after wearing | [58] | ||
| Aldehydes | Methional | after washing | [6] | |
| Hexanal | after washing | [46] | ||
| (Z)-4-heptenal | after washing; FD > 256 | [6] | ||
| Octanal | after washing; FD > 256 | [6] | ||
| after washing | [46] | |||
| (E)-2-octenal | after washing | [6] | ||
| Cis/trans-2-nonenal | after wearing and after washing; FD > 256 | [6,46] | ||
| (E,Z)-2,4-nonadienal | after washing; FD > 256 | [6] | ||
| (E,Z)-2,6-nonadienal | after washing; FD > 256 | [6] | ||
| Decanal | after washing | [43] | ||
| (E,E)-2,4-decadienal | after washing | [6] | ||
| (E)-4,5-epoxy-E-2-decenal | after wearing; FD > 256 | [6] | ||
| 4-methoxybenzaldehyde | after washing; FD > 256 | [6,68] | ||
| Medium-chain aldehydes (not determined) | [10] | |||
| Alcohols | Oct-1-en-3-ol | after washing | [67] | |
| 2-Nonanol | after wearing | [58] | ||
| 1-Decanol | after washing | [68] | ||
| 1-Dodecanol | after washing; FD > 256 | [68] | ||
| 2-Phenylethanol | after wearing and after washing; FD > 256 | [67,68] | ||
| 2-Methoxyphenol (guaiacol) | after wearing and after washing | [6,67] | ||
| Others | Naphthalene | after wearing | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Herreweghen, F.; Amberg, C.; Marques, R.; Callewaert, C. Biological and Chemical Processes that Lead to Textile Malodour Development. Microorganisms 2020, 8, 1709. https://doi.org/10.3390/microorganisms8111709
Van Herreweghen F, Amberg C, Marques R, Callewaert C. Biological and Chemical Processes that Lead to Textile Malodour Development. Microorganisms. 2020; 8(11):1709. https://doi.org/10.3390/microorganisms8111709
Chicago/Turabian StyleVan Herreweghen, Florence, Caroline Amberg, Rita Marques, and Chris Callewaert. 2020. "Biological and Chemical Processes that Lead to Textile Malodour Development" Microorganisms 8, no. 11: 1709. https://doi.org/10.3390/microorganisms8111709