Dysregulated Immune Responses by ASK1 Deficiency Alter Epithelial Progenitor Cell Fate and Accelerate Metaplasia Development during H. pylori Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Helicobacter Infection Model
2.2. Reagents
2.3. Bone Marrow Chimeric Mice Generation
2.4. Cell Lines
2.5. Immunostaining
2.6. Western Blotting, Immunoprecipitation, and ELISA
2.7. RNA Analysis
2.8. Statistical Analyses
3. Results
3.1. Loss of ASK1 Exacerbated Atrophic and Metaplastic Changes in H. pylori-Infected Stomach
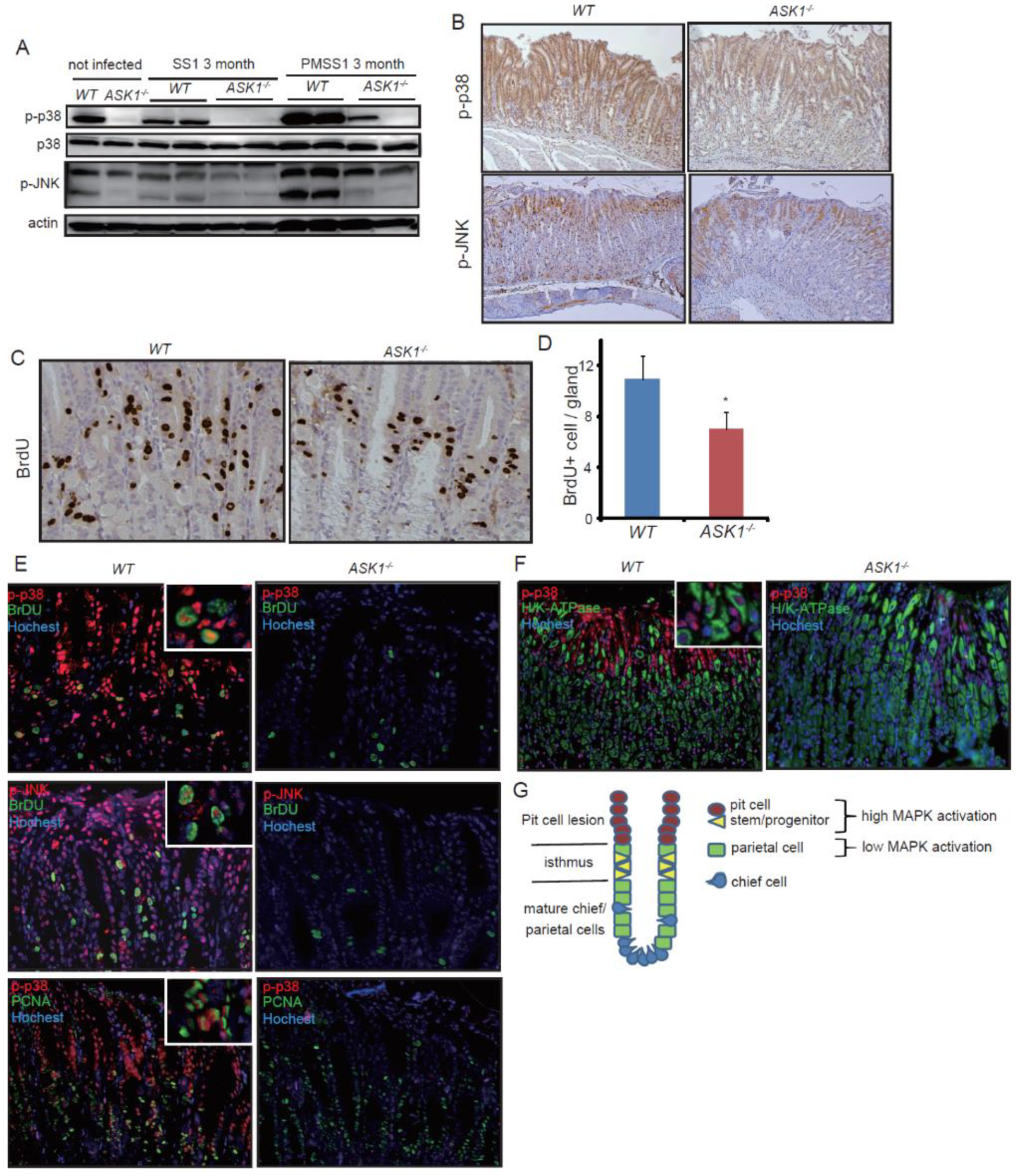
3.2. ASK1 Regulates Stem/Progenitor Cell Proliferation Through Downstream MAPK Activation
3.3. ASK1 Deficiency Enhanced NF-κB and STAT1 Activation in the Hp Infected Stomach
3.4. Epithelial ASK1 Did Not Affect Inflammatory and Metaplastic Changes After H. pylori Infection
3.5. ASK1 Deficiency Promotes TH1-Dependent Immune Response and Recruits Immature Gr-1+CD11b+ Cells
3.6. Lack of ASK1 and p38 Activation in Myeloid Lineage Induced Atrophic and Metaplastic Changes through NF-κB and STAT1 Activation
3.7. ASK1 and p38 Suppressed Macrophage Cell Death and IL-1β Secretion
3.8. ASK1 Deficiency Induced the Expansion of Facultative Gastric Progenitor Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parsonnet, J.; Friedman, G.D.; Vandersteen, D.P.; Chang, Y.; Vogelman, J.H.; Orentreich, N.; Sibley, R.K. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991, 325, 1127–1131. [Google Scholar] [CrossRef]
- Marshall, B.J.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 1, 1311–1315. [Google Scholar] [CrossRef]
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process--first american cancer society award lecture on cancer epidemiology and prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar]
- Canfield, V.; West, A.B.; Goldenring, J.R.; Levenson, R. Genetic ablation of parietal cells in transgenic mice: A new model for analyzing cell lineage relationships in the gastric mucosa. Proc. Natl. Acad. Sci. USA 1996, 93, 2431–2435. [Google Scholar] [CrossRef]
- Nam, K.T.; Lee, H.J.; Sousa, J.F.; Weis, V.G.; O’Neal, R.L.; Finke, P.E.; Romero-Gallo, J.; Shi, G.; Mills, J.C.; Peek, R.M.; et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 2010, 139, 2028–2037.e2029. [Google Scholar] [CrossRef]
- Han, S.; Fink, J.; Jorg, D.J.; Lee, E.; Yum, M.K.; Chatzeli, L.; Merker, S.R.; Josserand, M.; Trendafilova, T.; Andersson-Rolf, A.; et al. Defining the identity and dynamics of adult gastric isthmus stem cells. Cell Stem Cell 2019, 25, 342–356. [Google Scholar] [CrossRef]
- Hata, M.; Kinoshita, H.; Hayakawa, Y.; Konishi, M.; Tsuboi, M.; Oya, Y.; Kurokawa, K.; Hayata, Y.; Nakagawa, H.; Tateishi, K.; et al. Gpr30-expressing gastric chief cells do not dedifferentiate but are eliminated via pdk-dependent cell competition during development of metaplasia. Gastroenterology 2020, 158, 1650–1666.e1615. [Google Scholar] [CrossRef]
- Barker, N.; Huch, M.; Kujala, P.; van de Wetering, M.; Snippert, H.J.; van Es, J.H.; Sato, T.; Stange, D.E.; Begthel, H.; van den Born, M.; et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010, 6, 25–36. [Google Scholar] [CrossRef]
- Arnold, K.; Sarkar, A.; Yram, M.A.; Polo, J.M.; Bronson, R.; Sengupta, S.; Seandel, M.; Geijsen, N.; Hochedlinger, K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 2011, 9, 317–329. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Ariyama, H.; Stancikova, J.; Sakitani, K.; Asfaha, S.; Renz, B.W.; Dubeykovskaya, Z.A.; Shibata, W.; Wang, H.; Westphalen, C.B.; et al. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell 2015, 28, 800–814. [Google Scholar] [CrossRef]
- Sheng, W.; Malagola, E.; Nienhuser, H.; Zhang, Z.; Kim, W.; Zamechek, L.; Sepulveda, A.; Hata, M.; Hayakawa, Y.; Zhao, C.M.; et al. Hypergastrinemia expands gastric ecl cells through cck2r+ progenitor cells via erk activation. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 434–449.e1. [Google Scholar] [CrossRef]
- Lee, Y.; Urbanska, A.M.; Hayakawa, Y.; Wang, H.; Au, A.S.; Luna, A.M.; Chang, W.; Jin, G.; Bhagat, G.; Abrams, J.A.; et al. Gastrin stimulates a cholecystokinin-2-receptor-expressing cardia progenitor cell and promotes progression of barrett’s-like esophagus. Oncotarget 2016, 8, 203. [Google Scholar] [CrossRef]
- Chang, W.; Wang, H.; Kim, W.; Liu, Y.; Deng, H.; Liu, H.; Jiang, Z.; Niu, Z.; Sheng, W.; Napoles, O.C.; et al. Hormonal suppression of stem cells inhibits symmetric cell division and gastric tumorigenesis. Cell Stem Cell 2020, 26, 739–754.e738. [Google Scholar] [CrossRef]
- Nienhuser, H.; Kim, W.; Malagola, E.; Ruan, T.; Valenti, G.; Middelhoff, M.; Bass, A.; Der, C.J.; Hayakawa, Y.; Wang, T.C. Mist1+ gastric isthmus stem cells are regulated by wnt5a and expand in response to injury and inflammation in mice. Gut 2020, in press. [Google Scholar] [CrossRef]
- Crabtree, J.E.; Shallcross, T.M.; Heatley, R.V.; Wyatt, J.I. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with helicobacter pylori associated gastritis. Gut 1991, 32, 1473–1477. [Google Scholar] [CrossRef]
- Peek, R.M., Jr.; Miller, G.G.; Tham, K.T.; Perez-Perez, G.I.; Zhao, X.; Atherton, J.C.; Blaser, M.J. Heightened inflammatory response and cytokine expression in vivo to caga+ helicobacter pylori strains. Lab. Invest. 1995, 73, 760–770. [Google Scholar]
- Fox, J.G.; Sheppard, B.J.; Dangler, C.A.; Whary, M.T.; Ihrig, M.; Wang, T.C. Germ-line p53-targeted disruption inhibits helicobacter-induced premalignant lesions and invasive gastric carcinoma through down-regulation of th1 proinflammatory responses. Cancer Res. 2002, 62, 696–702. [Google Scholar] [PubMed]
- Tu, S.; Bhagat, G.; Cui, G.; Takaishi, S.; Kurt-Jones, E.A.; Rickman, B.; Betz, K.S.; Penz-Oesterreicher, M.; Bjorkdahl, O.; Fox, J.G.; et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 2008, 14, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, T.; Hirata, Y.; Hayakawa, Y.; Suzuki, N.; Sakitani, K.; Hikiba, Y.; Ihara, S.; Kinoshita, H.; Nakagawa, H.; Tateishi, K.; et al. Gastric metaplasia induced by helicobacter pylori is associated with enhanced sox9 expression via interleukin-1 signaling. Infect. Immun. 2015, 84, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Hirata, Y.; Nakagawa, H.; Sakamoto, K.; Hayakawa, Y.; Takahashi, R.; Nakata, W.; Sakitani, K.; Serizawa, T.; Hikiba, Y.; et al. Interleukin-6 mediates epithelial-stromal interactions and promotes gastric tumorigenesis. PLoS ONE 2013, 8, e60914. [Google Scholar] [CrossRef] [PubMed]
- Eaton, K.A.; Mefford, M.; Thevenot, T. The role of t cell subsets and cytokines in the pathogenesis of helicobacter pylori gastritis in mice. J. Immunol. 2001, 166, 7456–7461. [Google Scholar] [CrossRef] [PubMed]
- Akhiani, A.A.; Pappo, J.; Kabok, Z.; Schon, K.; Gao, W.; Franzen, L.E.; Lycke, N. Protection against helicobacter pylori infection following immunization is il-12-dependent and mediated by th1 cells. J. Immunol. 2002, 169, 6977–6984. [Google Scholar] [CrossRef] [PubMed]
- Sayi, A.; Kohler, E.; Hitzler, I.; Arnold, I.; Schwendener, R.; Rehrauer, H.; Müller, A. The cd4+ t cell-mediated ifn-gamma response to helicobacter infection is essential for clearance and determines gastric cancer risk. J. Immunol. 2009, 182, 7085–7101. [Google Scholar] [CrossRef] [PubMed]
- Kurt-Jones, E.A.; Cao, L.; Sandor, F.; Rogers, A.B.; Whary, M.T.; Nambiar, P.R.; Cerny, A.; Bowen, G.; Yan, J.; Takaishi, S.; et al. Trefoil family factor 2 is expressed in murine gastric and immune cells and controls both gastrointestinal inflammation and systemic immune responses. Infect. Immun. 2007, 75, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Quante, M.; Tu, S.P.; Tomita, H.; Gonda, T.; Wang, S.S.; Takashi, S.; Baik, G.H.; Shibata, W.; Diprete, B.; Betz, K.S.; et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 2011, 19, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Hitzler, I.; Sayi, A.; Kohler, E.; Engler, D.B.; Koch, K.N.; Hardt, W.D.; Muller, A. Caspase-1 has both proinflammatory and regulatory properties in helicobacter infections, which are differentially mediated by its substrates il-1beta and il-18. J. Immunol. 2012, 188, 3594–3602. [Google Scholar] [CrossRef]
- El-Omar, E.M.; Carrington, M.; Chow, W.H.; McColl, K.E.; Bream, J.H.; Young, H.A.; Herrera, J.; Lissowska, J.; Yuan, C.C.; Rothman, N.; et al. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature 2001, 412, 99. [Google Scholar] [CrossRef]
- El-Omar, E.M.; Rabkin, C.S.; Gammon, M.D.; Vaughan, T.L.; Risch, H.A.; Schoenberg, J.B.; Stanford, J.L.; Mayne, S.T.; Goedert, J.; Blot, W.J.; et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology 2003, 124, 1193–1201. [Google Scholar] [CrossRef]
- Maeda, S.; Yoshida, H.; Ogura, K.; Mitsuno, Y.; Hirata, Y.; Yamaji, Y.; Akanuma, M.; Shiratori, Y.; Omata, M.H. Pylori activates nf-kappab through a signaling pathway involving ikappab kinases, nf-kappab-inducing kinase, traf2, and traf6 in gastric cancer cells. Gastroenterology 2000, 119, 97–108. [Google Scholar] [CrossRef]
- Mitsuno, Y.; Yoshida, H.; Maeda, S.; Ogura, K.; Hirata, Y.; Kawabe, T.; Shiratori, Y.; Omata, M. Helicobacter pylori induced transactivation of sre and ap-1 through the erk signalling pathway in gastric cancer cells. Gut 2001, 49, 18–22. [Google Scholar] [CrossRef]
- Higashi, H.; Tsutsumi, R.; Muto, S.; Sugiyama, T.; Azuma, T.; Asaka, M.; Hatakeyama, M. Shp-2 tyrosine phosphatase as an intracellular target of helicobacter pylori caga protein. Science 2002, 295, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Ohmae, T.; Shibata, W.; Maeda, S.; Ogura, K.; Yoshida, H.; Kawabe, T.; Omata, M. Myd88 and tnf receptor-associated factor 6 are critical signal transducers in helicobacter pylori-infected human epithelial cells. J. Immunol. 2006, 176, 3796–3803. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Hayakawa, Y.; Koike, K. Metaplasia in the stomach-precursor of gastric cancer? Int. J. Mol. Sci. 2017, 18, 2063. [Google Scholar] [CrossRef] [PubMed]
- Ichijo, H.; Nishida, E.; Irie, K.; ten Dijke, P.; Saitoh, M.; Moriguchi, T.; Takagi, M.; Matsumoto, K.; Miyazono, K.; Gotoh, Y. Induction of apoptosis by ask1, a mammalian mapkkk that activates sapk/jnk and p38 signaling pathways. Science 1997, 275, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Hirata, Y.; Nakagawa, H.; Sakamoto, K.; Hikiba, Y.; Kinoshita, H.; Nakata, W.; Takahashi, R.; Tateishi, K.; Tada, M.; et al. Apoptosis signal-regulating kinase 1 and cyclin d1 compose a positive feedback loop contributing to tumor growth in gastric cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Hirata, Y.; Sakitani, K.; Nakagawa, H.; Nakata, W.; Kinoshita, H.; Takahashi, R.; Takeda, K.; Ichijo, H.; Maeda, S.; et al. Apoptosis signal-regulating kinase-1 inhibitor as a potent therapeutic drug for the treatment of gastric cancer. Cancer Sci. 2012, 103, 2181–2185. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Hirata, Y.; Nakagawa, H.; Sakamoto, K.; Hikiba, Y.; Otsuka, M.; Ijichi, H.; Ikenoue, T.; Tateishi, K.; Akanuma, M.; et al. Apoptosis signal-regulating kinase 1 regulates colitis and colitis-associated tumorigenesis by the innate immune responses. Gastroenterology 2010, 138, 1055–1067. [Google Scholar] [CrossRef]
- Takahashi, R.; Hirata, Y.; Sakitani, K.; Nakata, W.; Kinoshita, H.; Hayakawa, Y.; Nakagawa, H.; Sakamoto, K.; Hikiba, Y.; Ijichi, H.; et al. Therapeutic effect of c-jun n-terminal kinase inhibition on pancreatic cancer. Cancer Sci. 2013, 104, 337–344. [Google Scholar] [CrossRef]
- Nakagawa, H.; Hirata, Y.; Takeda, K.; Hayakawa, Y.; Sato, T.; Kinoshita, H.; Sakamoto, K.; Nakata, W.; Hikiba, Y.; Omata, M.; et al. Apoptosis signal-regulating kinase 1 inhibits hepatocarcinogenesis by controlling the tumor-suppressing function of stress-activated mitogen-activated protein kinase. Hepatology 2011, 54, 185–195. [Google Scholar] [CrossRef]
- Matsuzawa, A.; Saegusa, K.; Noguchi, T.; Sadamitsu, C.; Nishitoh, H.; Nagai, S.; Koyasu, S.; Matsumoto, K.; Takeda, K.; Ichijo, H. Ros-dependent activation of the traf6-ask1-p38 pathway is selectively required for tlr4-mediated innate immunity. Nat. Immunol. 2005, 6, 587–592. [Google Scholar] [CrossRef]
- Kundu, M.; Pathak, S.K.; Kumawat, K.; Basu, S.; Chatterjee, G.; Pathak, S.; Noguchi, T.; Takeda, K.; Ichijo, H.; Thien, C.B.; et al. A tnf- and c-cbl-dependent flip(s)-degradation pathway and its function in mycobacterium tuberculosis-induced macrophage apoptosis. Nat. Immunol. 2009, 10, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Tobiume, K.; Matsuzawa, A.; Takahashi, T.; Nishitoh, H.; Morita, K.; Takeda, K.; Minowa, O.; Miyazono, K.; Noda, T.; Ichijo, H. Ask1 is required for sustained activations of jnk/p38 map kinases and apoptosis. EMBO Rep. 2001, 2, 222–228. [Google Scholar] [CrossRef]
- Takeda, K.; Shimozono, R.; Noguchi, T.; Umeda, T.; Morimoto, Y.; Naguro, I.; Tobiume, K.; Saitoh, M.; Matsuzawa, A.; Ichijo, H. Apoptosis signal-regulating kinase (ask) 2 functions as a mitogen-activated protein kinase kinase kinase in a heteromeric complex with ask1. J. Biol. Chem. 2007, 282, 7522–7531. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; O’Rourke, J.; De Ungria, M.C.; Robertson, B.; Daskalopoulos, G.; Dixon, M.F. A standardized mouse model of helicobacter pylori infection: Introducing the sydney strain. Gastroenterology 1997, 112, 1386–1397. [Google Scholar] [CrossRef]
- Arnold, I.C.; Lee, J.Y.; Amieva, M.R.; Roers, A.; Flavell, R.A.; Sparwasser, T.; Muller, A. Tolerance rather than immunity protects from helicobacter pylori-induced gastric preneoplasia. Gastroenterology 2011, 140, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Ogura, K.; Maeda, S.; Nakao, M.; Watanabe, T.; Tada, M.; Kyutoku, T.; Yoshida, H.; Shiratori, Y.; Omata, M. Virulence factors of helicobacter pylori responsible for gastric diseases in mongolian gerbil. J. Exp. Med. 2000, 192, 1601–1610. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Maeda, S.; Nakagawa, H.; Hikiba, Y.; Shibata, W.; Sakamoto, K.; Yanai, A.; Hirata, Y.; Ogura, K.; Muto, S.; et al. Effectiveness of ikappab kinase inhibitors in murine colitis-associated tumorigenesis. J. Gastroenterol. 2009, 44, 935–943. [Google Scholar] [CrossRef]
- Crabtree, J.E.; Ferrero, R.L.; Kusters, J.G. The mouse colonizing helicobacter pylori strain ss1 may lack a functional cag pathogenicity island. Helicobacter 2002, 7, 139–140. [Google Scholar] [CrossRef]
- Goldenring, J.R.; Nomura, S. Differentiation of the gastric mucosa iii. Animal models of oxyntic atrophy and metaplasia. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G999–G1004. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Fox, J.; Gonda, T.; Worthley, D.; Muthupalani, S.; Wang, T. Mouse models of gastric cancer. Cancers 2013, 5, 92–130. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Fox, J.G.; Wang, T.C. The origins of gastric cancer from gastric stem cells: Lessons from mouse models. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Fox, J.G.; Wang, T.C. Isthmus stem cells are the origins of metaplasia in the gastric corpus. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Derijard, B.; Hibi, M.; Wu, I.H.; Barrett, T.; Su, B.; Deng, T.; Karin, M.; Davis, R.J. Jnk1: A protein kinase stimulated by uv light and ha-ras that binds and phosphorylates the c-jun activation domain. Cell 1994, 76, 1025–1037. [Google Scholar] [CrossRef]
- Park, J.M.; Greten, F.R.; Wong, A.; Westrick, R.J.; Arthur, J.S.; Otsu, K.; Hoffmann, A.; Montminy, M.; Karin, M. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis--creb and nf-kappab as key regulators. Immunity 2005, 23, 319–329. [Google Scholar] [CrossRef]
- Ashwell, J.D. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat. Rev. Immunol. 2006, 6, 532–540. [Google Scholar] [CrossRef]
- Maeda, S.; Kamata, H.; Luo, J.L.; Leffert, H.; Karin, M. Ikkbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 2005, 121, 977–990. [Google Scholar] [CrossRef]
- Sengupta, T.K.; Talbot, E.S.; Scherle, P.A.; Ivashkiv, L.B. Rapid inhibition of interleukin-6 signaling and stat3 activation mediated by mitogen-activated protein kinases. Proc. Natl. Acad. Sci. USA 1998, 95, 11107–11112. [Google Scholar] [CrossRef]
- Sakamoto, K.; Hikiba, Y.; Nakagawa, H.; Hayakawa, Y.; Yanai, A.; Akanuma, M.; Ogura, K.; Hirata, Y.; Kaestner, K.H.; Omata, M.; et al. Inhibitor of kappab kinase beta regulates gastric carcinogenesis via interleukin-1alpha expression. Gastroenterology 2010, 139, 226–238.e226. [Google Scholar] [CrossRef]
- Nakata, W.; Hayakawa, Y.; Nakagawa, H.; Sakamoto, K.; Kinoshita, H.; Takahashi, R.; Hirata, Y.; Maeda, S.; Koike, K. Anti-tumor activity of the proteasome inhibitor bortezomib in gastric cancer. Int. J. Oncol. 2011, 39, 1529–1536. [Google Scholar] [CrossRef]
- Berg, D.J.; Lynch, N.A.; Lynch, R.G.; Lauricella, D.M. Rapid development of severe hyperplastic gastritis with gastric epithelial dedifferentiation in helicobacter felis-infected il-10(-/-) mice. Am. J. Pathol. 1998, 152, 1377–1386. [Google Scholar]
- Boselli, D.; Losana, G.; Bernabei, P.; Bosisio, D.; Drysdale, P.; Kiessling, R.; Gaston, J.S.; Lammas, D.; Casanova, J.L.; Kumararatne, D.S.; et al. Ifn-gamma regulates fas ligand expression in human cd4+ t lymphocytes and controls their anti-mycobacterial cytotoxic functions. Eur. J. Immunol. 2007, 37, 2196–2204. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Stoicov, C.; Li, H.; Carlson, J.; Whary, M.; Fox, J.G.; Houghton, J. Overcoming fas-mediated apoptosis accelerates helicobacter-induced gastric cancer in mice. Cancer Res. 2005, 65, 10912–10920. [Google Scholar] [CrossRef] [PubMed]
- Rudi, J.; Kuck, D.; Strand, S.; von Herbay, A.; Mariani, S.M.; Krammer, P.H.; Galle, P.R.; Stremmel, W. Involvement of the cd95 (apo-1/fas) receptor and ligand system in helicobacter pylori-induced gastric epithelial apoptosis. J. Clin. Invest. 1998, 102, 1506–1514. [Google Scholar] [CrossRef]
- Yang, X.D.; Ai, W.; Asfaha, S.; Bhagat, G.; Friedman, R.A.; Jin, G.; Park, H.; Shykind, B.; Diacovo, T.G.; Falus, A.; et al. Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of cd11b+ly6g+ immature myeloid cells. Nat. Med. 2011, 17, 87–95. [Google Scholar] [CrossRef]
- Dubeykovskaya, Z.; Si, Y.; Chen, X.; Worthley, D.L.; Renz, B.W.; Urbanska, A.M.; Hayakawa, Y.; Xu, T.; Westphalen, C.B.; Dubeykovskiy, A.; et al. Neural innervation stimulates splenic tff2 to arrest myeloid cell expansion and cancer. Nat. Commun. 2016, 7, 10517. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Arkan, M.C.; Bollrath, J.; Hsu, L.C.; Goode, J.; Miething, C.; Goktuna, S.I.; Neuenhahn, M.; Fierer, J.; Paxian, S.; et al. Nf-kappab is a negative regulator of il-1beta secretion as revealed by genetic and pharmacological inhibition of ikkbeta. Cell 2007, 130, 918–931. [Google Scholar] [CrossRef]
- Okumura, T.; Ericksen, R.E.; Takaishi, S.; Wang, S.S.; Dubeykovskiy, Z.; Shibata, W.; Betz, K.S.; Muthupalani, S.; Rogers, A.B.; Fox, J.G.; et al. K-ras mutation targeted to gastric tissue progenitor cells results in chronic inflammation, an altered microenvironment, and progression to intraepithelial neoplasia. Cancer Res. 2010, 70, 8435–8445. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Seno, H.; Fukuoka, A.; Ueo, T.; Yamaga, Y.; Maruno, T.; Nakanishi, N.; Kanda, K.; Komekado, H.; Kawada, M.; et al. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat. Genet. 2013, 45, 98–103. [Google Scholar] [CrossRef]
- Westphalen, C.B.; Asfaha, S.; Hayakawa, Y.; Takemoto, Y.; Lukin, D.J.; Nuber, A.H.; Brandtner, A.; Setlik, W.; Remotti, H.; Muley, A.; et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J. Clin. Investig. 2014, 124, 1283–1295. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Hirata, Y.; Kinoshita, H.; Sakitani, K.; Nakagawa, H.; Nakata, W.; Takahashi, R.; Sakamoto, K.; Maeda, S.; Koike, K. Differential roles of ask1 and tak1 in helicobacter pylori-induced cellular responses. Infect. Immun. 2013, 81, 4551–4560. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Asim, M.; Romero-Gallo, J.; Barry, D.P.; Hoge, S.; de Sablet, T.; Delgado, A.G.; Wroblewski, L.E.; Piazuelo, M.B.; Yan, F.; et al. Spermine oxidase mediates the gastric cancer risk associated with helicobacter pylori caga. Gastroenterology 2011, 141, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Uchida, T.; Tsukamoto, Y.; Watada, M.; Yamaguchi, N.; Yamamoto, K.; Shiota, S.; Moriyama, M.; Graham, D.Y.; Yamaoka, Y. Helicobacter pylori infection introduces DNA double-strand breaks in host cells. Infect. Immun. 2014, 82, 4182–4189. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.T.; O’Neal, R.L.; Coffey, R.J.; Finke, P.E.; Barker, N.; Goldenring, J.R. Spasmolytic polypeptide-expressing metaplasia (spem) in the gastric oxyntic mucosa does not arise from lgr5-expressing cells. Gut 2012, 61, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Hayakawa, Y.; Koike, K. Gastric stem cell and cellular origin of cancer. Biomedicines 2018, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Hayakawa, Y.; Niu, Z.; Konishi, M.; Hata, M.; Tsuboi, M.; Hayata, Y.; Hikiba, Y.; Ihara, S.; Nakagawa, H.; et al. Mature gastric chief cells are not required for the development of metaplasia. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G583–G596. [Google Scholar] [CrossRef]
- Yoshioka, T.; Fukuda, A.; Araki, O.; Ogawa, S.; Hanyu, Y.; Matsumoto, Y.; Yamaga, Y.; Nakanishi, Y.; Kawada, K.; Sakai, Y.; et al. Bmi1 marks gastric stem cells located in the isthmus in mice. J. Pathol. 2019, 248, 179–190. [Google Scholar] [CrossRef]
- Worthley, D.L.; Churchill, M.; Compton, J.T.; Tailor, Y.; Rao, M.; Si, Y.; Levin, D.; Schwartz, M.G.; Uygur, A.; Hayakawa, Y.; et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 2015, 160, 269–284. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Chang, W.; Jin, G.; Wang, T.C. Gastrin and upper gi cancers. Curr. Opin. Pharmacol. 2016, 31, 31–37. [Google Scholar] [CrossRef]
- Sigal, M.; Logan, C.Y.; Kapalczynska, M.; Mollenkopf, H.J.; Berger, H.; Wiedenmann, B.; Nusse, R.; Amieva, M.R.; Meyer, T.F. Stromal r-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature 2017, 548, 451–455. [Google Scholar] [CrossRef]
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef]
- Rabben, H.L.; Zhao, C.M.; Hayakawa, Y.; Wang, T.C.; Chen, D. Vagotomy and gastric tumorigenesis. Curr. Neuropharmacol. 2016, 14, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Sakitani, K.; Konishi, M.; Asfaha, S.; Niikura, R.; Tomita, H.; Renz, B.W.; Tailor, Y.; Macchini, M.; Middelhoff, M.; et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 2017, 31, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Hayakawa, Y.; Koike, K. Role of muscarinic acetylcholine signaling in gastrointestinal cancers. Biomedicines 2019, 7, 58. [Google Scholar] [CrossRef]
- Sancho, R.; Nateri, A.S.; de Vinuesa, A.G.; Aguilera, C.; Nye, E.; Spencer-Dene, B.; Behrens, A. Jnk signalling modulates intestinal homeostasis and tumourigenesis in mice. EMBO J. 2009, 28, 1843–1854. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- He, D.; Wu, H.; Xiang, J.; Ruan, X.; Peng, P.; Ruan, Y.; Chen, Y.G.; Wang, Y.; Yu, Q.; Zhang, H.; et al. Gut stem cell aging is driven by mtorc1 via a p38 mapk-p53 pathway. Nat. Commun. 2020, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.S.; Yoon, J.H.; Ann, E.J.; Ahn, J.S.; Baek, H.J.; Lee, H.J.; Kim, S.H.; Kim, Y.D.; Kim, M.Y.; Park, H.S. Notch1 modulates oxidative stress induced cell death through suppression of apoptosis signal-regulating kinase 1. Proc. Natl. Acad. Sci. USA 2013, 110, 6865–6870. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Shivdasani, R.A. Notch signaling in stomach epithelial stem cell homeostasis. J. Exp. Med. 2011, 208, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Shibata, W.; Maeda, S.; Hikiba, Y.; Yanai, A.; Sakamoto, K.; Nakagawa, H.; Ogura, K.; Karin, M.; Omata, M. C-jun nh2-terminal kinase 1 is a critical regulator for the development of gastric cancer in mice. Cancer Res. 2008, 68, 5031–5039. [Google Scholar] [CrossRef] [PubMed]
- Sakitani, K.; Hayakawa, Y.; Deng, H.; Ariyama, H.; Kinoshita, H.; Konishi, M.; Ono, S.; Suzuki, N.; Ihara, S.; Niu, Z.; et al. Cxcr4-expressing mist1(+) progenitors in the gastric antrum contribute to gastric cancer development. Oncotarget 2017, 8, 111012–111025. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Jin, G.; Wang, H.; Chen, X.; Westphalen, C.B.; Asfaha, S.; Renz, B.W.; Ariyama, H.; Dubeykovskaya, Z.A.; Takemoto, Y.; et al. Cck2r identifies and regulates gastric antral stem cell states and carcinogenesis. Gut 2015, 64, 544–553. [Google Scholar] [CrossRef]
- Sade-Feldman, M.; Kanterman, J.; Ish-Shalom, E.; Elnekave, M.; Horwitz, E.; Baniyash, M. Tumor necrosis factor-alpha blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity 2013, 38, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Gallina, G.; Dolcetti, L.; Serafini, P.; De Santo, C.; Marigo, I.; Colombo, M.P.; Basso, G.; Brombacher, F.; Borrello, I.; Zanovello, P.; et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on cd8+ t cells. J. Clin. Investig. 2006, 116, 2777–2790. [Google Scholar] [CrossRef] [PubMed]
- Oya, Y.; Hayakawa, Y.; Koike, K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020, 111, 2696. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayakawa, Y.; Hirata, Y.; Hata, M.; Tsuboi, M.; Oya, Y.; Kurokawa, K.; Abe, S.; Arai, J.; Suzuki, N.; Nakagawa, H.; et al. Dysregulated Immune Responses by ASK1 Deficiency Alter Epithelial Progenitor Cell Fate and Accelerate Metaplasia Development during H. pylori Infection. Microorganisms 2020, 8, 1995. https://doi.org/10.3390/microorganisms8121995
Hayakawa Y, Hirata Y, Hata M, Tsuboi M, Oya Y, Kurokawa K, Abe S, Arai J, Suzuki N, Nakagawa H, et al. Dysregulated Immune Responses by ASK1 Deficiency Alter Epithelial Progenitor Cell Fate and Accelerate Metaplasia Development during H. pylori Infection. Microorganisms. 2020; 8(12):1995. https://doi.org/10.3390/microorganisms8121995
Chicago/Turabian StyleHayakawa, Yoku, Yoshihiro Hirata, Masahiro Hata, Mayo Tsuboi, Yukiko Oya, Ken Kurokawa, Sohei Abe, Junya Arai, Nobumi Suzuki, Hayato Nakagawa, and et al. 2020. "Dysregulated Immune Responses by ASK1 Deficiency Alter Epithelial Progenitor Cell Fate and Accelerate Metaplasia Development during H. pylori Infection" Microorganisms 8, no. 12: 1995. https://doi.org/10.3390/microorganisms8121995
APA StyleHayakawa, Y., Hirata, Y., Hata, M., Tsuboi, M., Oya, Y., Kurokawa, K., Abe, S., Arai, J., Suzuki, N., Nakagawa, H., Fujiwara, H., Tateishi, K., Maeda, S., & Koike, K. (2020). Dysregulated Immune Responses by ASK1 Deficiency Alter Epithelial Progenitor Cell Fate and Accelerate Metaplasia Development during H. pylori Infection. Microorganisms, 8(12), 1995. https://doi.org/10.3390/microorganisms8121995





