Abstract
Malaria remains a significant disease, causing epic health problems and challenges all over the world, especially in sub-Saharan Africa. CD209 and CD28 genes act as co-stimulators and regulators of the immune system, while the STAT6 gene has been reported to mediate cytokine-induced responses. Single nucleotide polymorphisms of these genes might lead to differential disease susceptibility among populations at risk for malaria, due to alterations in the immune response. We aim to identify key drivers of the immune response to malaria infection among the three SNPs: CD209 (rs4804803), CD28 (rs35593994) and STAT6 (rs3024974). After approval and informed consent, we genotyped blood samples from a total of 531 children recruited from Nigeria using the Taqman SNP genotyping assay and performed comparative analysis of clinical covariates among malaria-infected children. Our results reveal the CD209 (rs4804803) polymorphism as a susceptibility factor for malaria infection, significantly increasing the risk of disease among children, but not CD28 (rs35593994) or STAT6 (rs3024974) polymorphisms. Specifically, individuals with the homozygous mutant allele (rs4804803G/G) for the CD209 gene have a significantly greater susceptibility to malaria, and presented with higher mean parasitemia. This observation may be due to a defective antigen presentation and priming, leading to an ineffective downstream adaptive immune response needed to combat infection, as well as the resultant higher parasitemia and disease manifestation. We conclude that the CD209 gene is a critical driver of the immune response during malaria infection, and can serve as a predictor of disease susceptibility or a biomarker for disease diagnosis.
1. Introduction
Malaria remains a parasitic disease with about 3.2 billion people at risk of infection [1,2], with an estimated annual death toll of ~400,000 people out of 219 million clinical cases of malaria [3]. Many of these deaths occur among young children and pregnant women from sub-Saharan Africa [4,5]. Such mortality rates among these vulnerable groups necessitates timely intervention and improved control initiatives [6]. Several factors have been adduced for the significant morbidity and mortality rates [7], including ethnic delineation [8,9], parasite species/genotype [10,11,12,13,14], pathogen load [15], but most importantly, host genetic factors, which have been shown to mediate disease susceptibility, severity, and clinical outcome [16,17,18]. More than 90% of malaria infection cases in Africa are caused by Plasmodium falciparum, with multiple studies implicating the asexual blood stages of the parasite for its infection pathology [19,20]. The immune response against P. falciparum, in context of the host’s genetic contribution to disease outcome, is a complex process, and requires stepwise dissection.
To establish a protective immune mechanism against infection, there is the need for an intricately organized, innate and adaptive response, involving dendritic cells, macrophages, and B and T lymphocytes [4,21,22]. The CD209 gene, also known as the dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) gene, encodes a transmembrane receptor on dendritic cells, and is a significant player in recognizing and presenting pathogens with a diverse evolutionary origin [23,24]. The CD209 protein has been shown to recognize pathogens through its N-terminal domain, binding to ligands on microbes in the process [25], as well as activating the signal transduction pathway in the process. Since DC’s are an essential component for antigen presentation, and for initiation of the process mediating adaptive immune responses, its polymorphisms, especially the rs4804803 (snp -336G/A) gene promoter polymorphism, have been shown to modulate disease susceptibility or severity [26,27,28,29,30,31,32]. We have shown previously that this polymorphism tracks ethnicity, with significant genotypic and allelic diversity between African and American sickle-cell disease patients [27]. Importantly, this study provides one explanatory mechanism for the epidemiologic evidence of sickle-cell disease as a protective factor against malarial infection. We demonstrated that the preponderance of the homozygous recessive variant among Africans reveal a possible impairment of the immune response to infectious diseases (due to poor antigen presentation), and that this diversity is a critical factor determining susceptibility to disease or severity within and between groups.
CD28, on the other hand, is an important costimulatory molecule, promoting transcription signaling, activation and differentiation of naive CD4 and CD8 T cells, as well as activation of cytokines or cytokine receptors [33,34]. Studies have shown the significant co-stimulation of CD28 in protective immune responses against various diseases [35,36,37,38], including malaria, with its polymorphisms also showing interethnic delineation mediating disease outcome. Though many of the single nucleotide polymorphisms have been identified and studied, only a few have been studied in the context of malaria infection [34,39]. Therefore, CD28 genetic polymorphisms may also serve an important role in the varying susceptibility to malaria.
Furthermore, published reports have shown that IL-10 production in response to a pathogenic stimulus is modulated by signal transducer and activator of transcription 6 (STAT6), enhancing IL-12 production in dendritic cells and driving a Th1 immune response in STAT6-/- mice [40,41]. The role of STAT6 in the immune response to malaria infection is currently limited to a handful of studies, with no definitive conclusion [41,42]. A study examining regulatory gene variants in Congo showed a disparate response with STAT6 and FOXP3 among malaria-infected children [41], with the STAT6 promoter variant rs3024944 being associated with uncomplicated malaria, and the FOXP3 SNP rs11091253 being associated with parasitemia. The role of these regulatory variants in association with disease or covariates of infection has been very challenging to elucidate.
We have previously demonstrated the importance of the CD14 promoter gene polymorphism (rs2569190C/T) [17] in malaria infection, showing that clinical malaria among Nigerian children is significantly regulated by this gene, in addition to its correlation with parasitemia, and thus a marker of disease severity. However, CD14 gene polymorphisms are only one component of the complex host–parasite interaction, and thus constitute only a piece of the genetic puzzle required for a complete picture of the immune response to malaria. Considering the paucity of available data, what are the roles of CD209, CD28 and STAT6 gene polymorphisms, either individually or in combination, with malaria infection among children, and how are they associated with markers of disease severity (age, anemia and parasitemia)? Do these polymorphisms serve as susceptibility factors for malaria infection, or do they play any critical role in regulating disease covariates such as anemia and parasitemia in West Africa? To answer these questions, we evaluated the genetic variability of CD209 (rs4804803), CD28 (rs35593994) and STAT6 (rs3024974) single nucleotide polymorphisms among malaria-infected children in Nigeria and extrapolated any association with markers of infection. Our data suggest that CD209 (rs4804803) is a susceptibility factor for clinical malaria that also influences disease pathogenesis, but not CD28 (rs35593994) or STAT6 (rs3024974). We also show that despite the preponderance of CD28 (rs35593994) wild type variants that would assist in T cell differentiation and parasite clearance, this was not the case. We postulate that a defective antigen presentation, depicted by the higher frequency of CD209 mutant alleles, renders downstream immune response and parasite clearance moot. It also appears that any role for STAT6 (rs3024974) variants to influence malaria pathogenesis occurs via a completely different mechanism, and this polymorphism is potentially still in flux.
2. Materials and Methods
2.1. Study Population and Genomic DNA Isolation
This study population consisted of febrile children recruited at St Mary Catholic Hospital, Ibadan, and in a rural primary health care center (Idi-Ayunre, Oluyole Local Government Area, Oyo State), southwest Nigeria, from November 2013 to November 2014, and in accordance with the 1975 Helsinki declaration. Detailed demographic, geographic and clinical parameters, as well as the genomic DNA extraction process, including modifications, are as described in (Ojurongbe et al., 2017). Approval was obtained from the University of Ibadan/University College Hospital Institutional Review Committee (approval number UI/EC/12/0279). Two hundred and thirty-one children presenting with malaria infection, for whom informed consent was obtained, were included into the study. Additionally, 330 matched uninfected children were recruited as controls, as part of the original study. Genomic DNA quality was accessed with a Nanodrop 2000 (ThermoFisher Scientific, Waltham, MA, USA). Axillary body temperature (in Celsius) and packed cell volume (expressed as a percentage), indicative of fever and anemia, respectively, were determined. Parasite count was evaluated per international standards [43].
2.2. TaqMan SNP Genotyping Assay
To perform the SNP genotyping analysis, our designed custom assay for STAT6 (rs3024974; -G > A), alongside pre-made assays for CD209 (rs4804803; -383A > G) and CD28 (rs35593994; -372G > A) SNPs, were utilized. Briefly, a 10 µL reaction mixture was prepared for each assay containing 1 µL of the 20X Taqman SNP genotyping assay, 5 µL of 2× Taqman Mastermix (Thermo Fisher Scientific, Waltham, MA, USA), 1 µL of 20 ng genomic DNA and 3 µL of nuclease-free water. The reactions were set up for SNP genotyping using the CFXconnect (Bio-Rad, Hercules, CA, USA) real-time PCR machine with the following reaction conditions: 90 °C for 10 min, followed by 90 °C for 30 s, 56 °C for 30 s and 72 °C for 50 s, with a final extension of 72 °C for 5 min. Melt analysis was performed to confirm assay specificity; temperatures varied from 65 °C to 95 °C with increments of 0.5 °C for 5 s. We generated allele calls with the CFX Manager software.
2.3. Statistical Analysis
The program SNPstats [44] was used to estimate allelic and genotypic frequencies, testing the Hardy–Weinberg equilibrium for the three loci under study: CD28 (rs3559399), CD209 (rs4804803) and STAT6 (rs3024974), with SNP’s rejected on the threshold of p < 0.05, as described by [45]; association analysis was performed for each SNP separately. Allelic and genotypic frequencies between controls and malaria-infected individuals were as previously described. To examine the association between malaria and the genetic variants of the loci under study with malaria, we utilized a binary logistic regression, to evaluate the association between gene variants and age, fever, PCV and parasitemia. Likewise, haplotype analysis was performed for the three SNPs; individuals who were heterozygous at more than one locus were excluded from the analysis.
3. Results
We genotyped blood samples collected from a total of 561 individuals (231 malaria-infected patients and 330 uninfected, control individuals) for STAT6 (rs3024974), CD28 (rs35593994) and CD209 (rs4804803) gene polymorphisms using Taqman SNP genotyping assays. We also performed association analyses of genetic variants with clinical variables among the malaria-infected group. The genotypic frequencies of STAT6, CD28 and CD209 gene promoter polymorphisms in malaria patients were compared to the control group with logistic regression analysis (Table 1, Table 2 and Table 3). Our results show significant differences in genotypic frequencies between malaria and control groups for CD28 (rs35593994; p = 0.0001) and CD209 (rs4804803; p = 0.0001) but not STAT6 (rs3024974; p = 0.30) gene polymorphisms. Although there was no significant difference in genotypic frequency between groups for the STAT6 (rs3024974) gene, we did however observe a higher frequency of wild type G/G (74.4%) and heterozygote G/A (20.1%) variants in the control group, while the opposite (higher frequency of homozygous recessive variant A/A) was seen in the malaria group (8.7% versus 5.6% for the malaria and control groups, respectively (Table 1).

Table 1.
Genotypic frequency of the STAT6 gene promoter polymorphism between malaria-infected and control groups.

Table 2.
Genotypic frequency of the CD28 gene promoter polymorphism between malaria-infected and control groups.

Table 3.
Genotypic frequency of the CD209 gene promoter polymorphism between malaria-infected and control groups.
Examining genotypic frequency of the CD28 (snp: -372G/A; rs35593994) gene polymorphism, we observed a higher frequency of homozygous mutant variants (-372A/A), though statistically insignificant in the control group compared to the malaria-infected group (16.8% versus 12.4% for the control and malaria groups, respectively) (Table 2); however, this difference was statistically insignificant. On the other hand, significantly more heterozygotes (-372G/A) were present in the malaria group (65.5%) compared to the control group (28.2%). Similarly, significantly more mutant variants (-383G/G) were observed in the malaria group (46.0%) compared to the control group (22.7%) for the CD209 (snp: -383A/G; rs4804803) gene, depicting the homozygous mutant variant as a susceptibility factor for clinical malaria among children in southwestern Nigeria. This observation was not seen for other genes, except for CD28 gene heterozygous variants (372G/A). The opposite was the case among those with the CD209 gene homozygous dominant variant (-383A/A) (20.7% versus 52.1% for the malaria and control groups, respectively) (Table 3).
Our inheritance test model revealed that the STAT6 (rs3024974) gene polymorphism did not increase the risk of malaria with the dominant (G/A-A/A versus G/G; OR = 0.98; 95% CI = 0.67–1.44) or recessive allele (G/G-G/A versus A/A; OR = 0.62; 95% CI = 0.32–1.20). However, in the case of the CD28 (rs35593994) gene polymorphism, the dominant allele (G/A-A/A versus G/G; OR = 0.23; 95% CI = 0.16–0.34) demonstrated a significant increase in risk of malaria infection (p = 0.0001) but not the recessive allele (G/G-G/A versus A/A; OR = 1.43; 95% CI = 0.87–2.34) (Table 2). In the case of the CD209 (rs4804803) gene polymorphism, a significantly increased risk of malaria infection was observed with both dominant and recessive alleles (p = 0.0001; Table 3).
To delineate the haplotype combination of the loci under study with the highest risk for malaria, we constructed a haplotype table, producing a total of eight haplotype combinations common to both the disease and control groups (Table 4). From our analysis, four out of the eight haplotype groups (haplotypes H2: GGA; H3: GAG; H4: GGG; and H8: AGG) showed a significant difference in haplotype frequencies between groups; others were insignificant. Based on the odds ratio, the same haplotypes (H2, H3, H4 and H8) in addition to haplotype H5 (AAA; p = 0.038) showed the highest/increased risk of malaria compared to others (Table 4), though the risk of malaria infection is significantly less with haplotype H8 (OR 0.12; 95% CI: 0.04–0.35). The linkage disequilibrium (LD) analysis showed the strongest association between CD28 (rs35593994) and CD209 (rs4804803) gene polymorphisms in the malaria group (0.77; p = 0.0022; Table 5).

Table 4.
Estimated haplotype frequencies of the selected loci between the malaria-infected and control groups.

Table 5.
Linkage disequilibrium analysis of STAT6, CD209 and CD28 genetic polymorphisms.
Since the CD209 (rs4804803) gene polymorphisms presented the strongest association with malaria, we set out to examine the possibility that its genetic variants may be modulating the clinical covariates associated with disease (age, temperature (indicative of fever), packed cell volume, PCV (indicative of anemia) and parasitemia). Comparing wild type versus homozygous mutant variants, we report significant differences (rs4804803A/A < rs4804803GG) with age (p = 0.002; 37 versus 58 months; Figure 1) and parasitemia (p = 0.04; 12,139 versus 26,028 parasites per μL of blood; Figure 2). Malaria patients with the CD209 gene wild type variants were much younger and had a lower mean parasitemia compared to patients with the mutant variant (older in age and a higher mean parasitemia). A similar observation with age and parasitemia, in addition to PCV (p = 0.005) was made when we compared patients with wild type variants (rs4804803A/A) to those with heterozygous (rs4804803A/G) variants (Table 6). To our surprise, the STAT6 (rs3024974) gene showed a significant correlation between age and all its variants, and to some degree, with temperature, PCV and parasitemia (Table 6). The heterozygous variant of the STAT6 gene promoter (rs3024974G/A) presented with the most protection against malaria infection (lowest mean age, PCV and parasitemia), possibly implying that the evolution of this gene in the context of malaria infection is still ongoing.
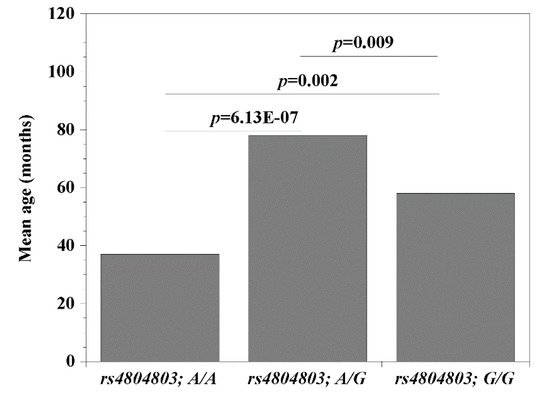
Figure 1.
Mean age among malaria patients with CD209 (snp: -383A > G; rs4804803) wild type, heterozygous and mutant variants. Odds ratios were calculated by Fisher’s two-tailed exact tests. A p-value < 0.05 was considered significant.
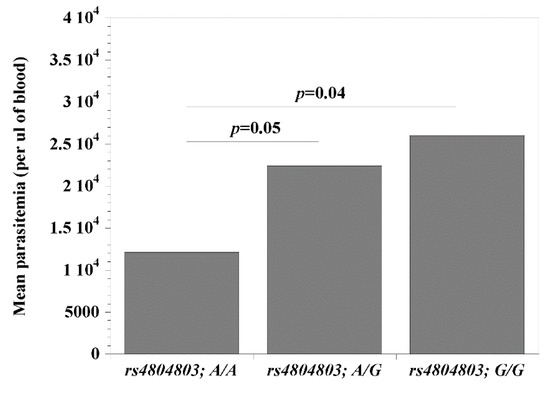
Figure 2.
Mean parasitemia among malaria patients with CD209 (snp: -383A > G; rs4804803) wild type, heterozygous and mutant variants. Odds ratios were calculated by Fisher’s two-tailed exact tests. A p-value < 0.05 was considered significant.

Table 6.
Comparative analysis of disease covariates with genetic variants of selected loci among malaria-infected patients.
4. Discussion
Malaria is a life-threatening disease, causing global morbidity and mortality among endemic populations and non-immune individuals travelling to such locations, with the most deaths among children under five years of age [3,4,46]. Reports have shown that infection among children could lead to severe clinical episodes, therefore a better understanding of immunoregulation and/or the development of immunity post-infection is imperative. This is especially imperative in sub-Saharan Africa, where Plasmodium falciparum, the most virulent of the species, is endemic. Protective immunity can be acquired naturally after consecutive malaria infections when dendritic cells, serving as antigen-presenting cells, prime and activate the T cells necessary for downstream adaptive response, for which CD209 and CD28 molecules are significant participants [47,48,49]. Likewise, the STAT6 gene is known to regulate the expression of regulatory T cells [50], while potentially modulating the immune response to malaria [41,42]. Therefore, elucidating the mechanism mediating susceptibility to or resistance against malaria would be significant in combatting disease pathogenesis. Studies on gene polymorphisms and genetic linkage analysis have shown differing relationships between genes or gene variants and association with disease susceptibility [41,51]. To this end, we investigated the role of STAT6 (rs3024974), CD28 (rs35593994) and CD209 (rs4804803) gene polymorphisms in modulating disease susceptibility and infection outcome, as well as their relationship to clinical covariates of disease among children diagnosed with malaria in southwestern Nigeria. Remarkably, we show that the CD209 (rs4804803) gene polymorphism is a susceptibility factor for malaria among the three genes under study, showing a significant disease-related association. The majority of malaria-infected children had the homozygous mutant variant (rs4804803G/G), while the control (uninfected) individuals predominantly had the wild type phenotype (rs4804803A/A), confirming our hypothesis that the CD209 gene is a susceptibility factor for clinical malaria.
CD209 encodes dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN), and has been shown to bind various ligands including T-lymphocytes, thus contributing to activation of signal transduction pathways and recognition of infectious agents. Several studies have shown its polymorphic variants to be associated with tolerance or susceptibility to diseases, such as tuberculosis, schistosomiasis, leishmaniasis, and sickle-cell disease [23,27,30,51,52,53], asserting that the G allele significantly increased the risk of disease. Our results confirm this observation, indicating that mutation of the CD209 gene has a considerable impact on malaria pathogenesis. Under normal circumstances, this gene protects against infection, but among the significant number of individuals with the mutant variant, this protection is lost, rendering these individuals more susceptible to disease. We show that a defective CD209 response (mediated by G mutant allele) presented with significantly higher parasitemia and anemia (indicated by a low PCV) compared to the wild type variant, demonstrating its role in driving immune responses to malaria, more so than STAT6 (rs3024974) or CD28 (rs35593994) polymorphisms. This observation complements our previous report showing that the preponderance of the G mutant allele leads to a defective antigen presentation, weakening protective immune responses [27] and, as such, an unhindered parasitemia increase. On the other hand, the preponderance of the wild type A allele strengthens antigen presentation, T cell priming and downstream T cell differentiation, leading to an adaptive immune response that modulates disease (i.e., properly differentiated T cells drive a Th1 response that controls disease and parasitemia).
Though we found a notable diversity in CD28 gene polymorphism, whereby the heterozygous variant was most common among malaria patients, and the homozygous dominant was most common among controls, this does not appear to be a factor in malaria pathogenesis. This observation implies a balanced immune response, expected to elicit a proper T cell response and control of parasitemia. The fact that this is not the case confirms our suspicion that higher parasitemia among malaria patients is hinged on the preponderance of the CD209 mutant G allele, mediating a poor antigen presentation and an unmitigated disaster in the immune response. For the STAT6 (rs3024974) gene, our analysis did not show any significant diversity in the genotypic frequency of the homozygous dominant (G/G), heterozygote (G/A) and the homozygous recessive (A/A) variants between the malaria and control groups (rs3024974G/G > rs3024974G/A > rs3024974A/A), except when analyzed in the context of disease covariates. The odds ratio showed no indication of an increased risk of malaria infection with the STAT6 (rs3024974) gene polymorphism. This observation, however, contradicts previous studies associating this polymorphism with malaria pathogenesis among Congolese children [41], as well as cerebral malaria among children from Ghana [42]. As shown from our findings, the STAT6 (rs3024974) polymorphism did not have any effect on disease susceptibility. Rather than driving a severe disease outcome as reported from Ghana, we propose a heterozygous advantage among malaria patients when analyzed with disease covariates. Patients with this variant, despite being the youngest (mean age: 46 months) and therefore expected to show the most severe disease outcome, had the lowest mean parasite count (8891 parasites per μL of blood compared to 24,952 and 22,523 parasites per μL of blood for homozygous dominant and homozygous recessive, respectively). This observation demonstrates the significantly higher relative fitness in endemic communities, endowed by the heterozygote genotype, but not seen in dominant or recessive groups, similar to that conferred by the sickle cell trait [27,54].
On the other hand, we report a low genotypic frequency of the CD28 (rs35593994) homozygote recessive (A/A) variant in the malaria group, though statistically insignificant, implying that this variant possibly has a protective effect against infection. A published report has shown that low level parasitemia is retained in CD28 knockout mice infected with malaria [55], suggesting that parasite control occurs via additional immune processes. It was reported that T cell memory is independent of CD28, and that CD28-deficient mice showed efficient type 1 and type 2 responses during infection with Leishmania major and Heligmosoides polygyrus, respectively [56,57]. The higher frequency of the heterozygote G/A variant in the malaria group might imply a potential additive effect producing a negative interaction of allele G and A, leading to a higher risk of disease in infected but not control groups. Therefore, the heterozygote variant in this case may have a negative effect on the immune response to disease and contribute to susceptibility, while the recessive variant (A/A) seems protective. Teutsch et al. (2004) described the CD28 (rs35593994) SNP through identification of transcription factor binding sites, showing that the A allele possesses a CCAAT enhancer-binding protein site, which is lacking in allele G. Additional reports showed that this variant influences gene transcription and immune alteration in breast cancer [58,59]. We recommend that this polymorphism be further elucidated in the context of malaria and associated covariates of disease.
Interestingly, our linkage disequilibrium analysis revealed that CD209 (rs4804803) and CD28 (rs35593994) polymorphisms are significantly linked, justifying their co-stimulatory activity with pro-inflammatory cytokines such as IL-4, IL-10, IL17F and TNF-α. In the same vein, the haplotype combinations that identified haplotypes H2 (GGA), H3 (GAG) and H5 (AAA) as important for malaria might be as a result of the additive effect of the three loci under study favoring disease susceptibility. Importantly, the mutant allele G is significantly implicated among the haplotype definitions, implying that a change from an A to G as in the CD209 (rs4804803) gene polymorphism will hamper downstream expression of the gene, thereby affecting the immune response and contributing to malaria pathogenesis, which was not seen in the STAT6 (rs3024974) polymorphism. However, it is not impossible that STAT6 (rs3024974) and CD28 (rs35593994) polymorphisms may mediate malaria pathogenesis through a different mechanism not examined in this study. Overall, the CD209 (rs4804803) gene polymorphism presenting the mutant allele G is critical in determining disease susceptibility, thereby identifying this gene and its polymorphism (A > G; rs4804803) as an important driver of immune response during infection.
Author Contributions
B.N.T. conceived and designed experiments; C.O.F. and O.O. recruited patients, collected samples and confirmed microscopy; J.L.M., M.E.H., A.L.C., B.N.S. and O.B.M. carried out Taqman molecular genotyping and PCR-RFLP confirmatory studies, allele calling and statistical analysis; O.B.M. and B.N.T. wrote the initial draft of the manuscript; C.O.F., O.O. and B.N.T. contributed to the discussion and scientific content. All authors read and approved the final version of the manuscript.
Funding
This research was funded through Faculty Development Award, College of Health Sciences and Technology, Rochester Institute of Technology” and “The APC was discounted by MDPI”
Acknowledgments
We are grateful to the children/their parents who participated in this study, as well as Katlyn Delaney for technical assistance. Funding from the Miller Chair in International Education, Rochester Institute of Technology (BNT) facilitated the collaboration with Ladoke Akintola University of Technology. OBM was supported through the American Association of Immunologists Careers in Immunology Fellowship Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare we have no conflicts of interest.
References
- Snow, R.W.; Guerra, C.A.; Noor, A.M.; Myint, H.Y.; Hay, S.I. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 2005, 434, 214–217. [Google Scholar] [CrossRef]
- Langhorne, J.; Ndungu, F.; Sponaas, A.; Marsh, K. Immunity to malaria: More questions than answers. Nat. Immunol. 2008, 9, 725–732. [Google Scholar] [CrossRef] [PubMed]
- WHO. Malaria Fact Sheet 2016. Available online: http://www.who.int/mediacentre/factsheets/fs094/en/ (accessed on 1 December 2019).
- Noone, C.; Parkinson, M.; Dowling, D.J.; Aldridge, A.; Kirwan, P.; Molloy, S.F.; Asaolu, S.O.; Holland, C.; O’Neill, S.M. Plasma cytokines, chemokines and cellular immune responses in pre-school Nigerian children infected with Plasmodium falciparum. Malar. J. 2013, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Lawal, O.A.; Adegoke, S.A.; Oseni, S.B.; Oyelami, O.A. Low serum vitamin A is prevalent in under five children with severe malaria and is associated with increased risk of death. J. Infect. Dev. Ctries. 2018, 12, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Arama, C.; Maiga, B.; Dolo, A.; Kouriba, B.; Traoré, B.; Crompton, P.D.; Pierce, S.K.; Troye-Blomberg, M.; Miller, L.H.; Doumbo, O.K. Ethnic differences in susceptibility to malaria: What have we learned from immuno-epidemiological studies in West Africa? Acta Trop. 2015, 146, 152–156. [Google Scholar] [CrossRef]
- Fortin, A.; Stevenson, M.M.; Gros, P. Susceptibility to malaria as a complex trait: Big pressure from a tiny creature. Hum. Mol. Genet. 2002, 11, 2469–2478. [Google Scholar] [CrossRef][Green Version]
- Modiano, D.; Luoni, G.; Sirima, B.S.; Lanfrancotti, A.; Petrarca, V.; Cruciani, F.; Simpore, J.; Siminelli, B.M.; Foglietta, E.; Gristanti, P.; et al. The lower susceptibility to Plasmodium falciparum malaria of Fulani of Burkina Faso (west Africa) is associated with low frequencies of classic malaria-resistance genes. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 149–152. [Google Scholar] [CrossRef]
- Arama, C.; Quin, J.E.; Kouriba, B.; Östlund Farrants, A.K.; Troye-Blomberg, M.; Doumbo, O.K. Epigenetics and malaria susceptibility/protection: A missing piece of the puzzle. Front. Immunol. 2018, 9, 1733. [Google Scholar] [CrossRef]
- Funwei, R.I.; Thomas, B.N.; Falade, C.O.; Ojurongbe, O. Extensive diversity in the allelic frequency of Plasmodium falciparum merozoite surface proteins and glutamate-rich protein between rural and urban settings in south-western Nigeria. Malar. J. 2018, 17, 1. [Google Scholar] [CrossRef]
- Funwei, R.; Nderu, D.; Nguetse, C.N.; Thomas, B.N.; Falade, C.; Velavan, T.P.; Ojurongbe, O. Deletion of Plasmodium falciparum histidine rich proteins 2 (Pfhrp2) and 3 (Pfhrp3) gene in Nigerian isolates. Acta Trop. 2019, 196, 121–125. [Google Scholar] [CrossRef]
- Mace, K.E.; Arguin, P.M.; Tan, K.R. Malaria surveillance—United States, 2015. MMWR Surveill. Summ. 2018, 67, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Nguetse, C.N.; Ojo, J.A.; Nchotebah, C.; Ikegbunam, M.N.; Thomas, B.N.; Velavan, T.P.; Ojurongbe, O. Genetic diversity of the Plasmodium falciparum glutamate rich protein R2 before and twelve years after introduction of artemisinin combination therapies among febrile children in Nigeria. Am. J. Trop. Med. Hyg. 2018, 98, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Prior, K.F.; O’Donnell, A.J.; Rund, S.S.C.; Savill, N.J.; van der Veen, D.R.; Reece, S.E. Host circadian rhythms are disrupted during malaria infection in parasite genotype-secific manners. Sci. Rep. 2019, 9, 10905. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Georgiadou, A.; Walther, M.; Nwakanma, D.; Stewart, L.B.; Levin, M.; Otto, T.D.; Conway, D.J.; Coin, L.J.; Cunnington, A.J. Integrated pathogen load and dual transcriptome analysis of systemic host-pathogen interactions in severe malaria. Sci. Transl. Med. 2018, 10, eaar3619. [Google Scholar] [CrossRef]
- Boutlis, C.S.; Yeo, T.W.; Anstey, N.M. Malaria tolerance—For whom the cell tolls? Trends Parasitol. 2006, 22, 371–377. [Google Scholar] [CrossRef]
- Ojurongbe, O.; Funwei, R.I.; Snyder, T.; Aziz, N.; Li, Y.; Falade, C.; Thomas, B.N. Genetic diversity of CD14 promoter gene polymorphism (rs2569190) is associated with regulation of parasitemia but not susceptibility to Plasmodium falciparum infection. Infect. Dis. Res. Treat. 2017, 10, 1178633617726781. [Google Scholar] [CrossRef]
- Ojurongbe, O.; Funwei, R.I.; Snyder, T.; Farid, I.; Aziz, N.; Li, Y.; Falade, C.; Thomas, B.N. Genetic variants of tumor necrosis factor alpha -308G/A (rs1800629) but not Toll-interacting proteins or vitamin D receptor genes enhance susceptibility and severity of malaria infection. Immunogenetics 2018, 70, 135–140. [Google Scholar] [CrossRef]
- Bijker, E.M.; Bastiaens, G.J.; Teirlinck, A.C.; Van Gemert, G.J.; Graumans, W.; van de Vegte-Bolmer, M.; Siebelink-Stoter, R.; Arens, T.; Teelen, K.; Nahrendorf, W.; et al. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 7862–7867. [Google Scholar] [CrossRef]
- Bijker, E.M.; Schats, R.; Obiero, J.M.; Behet, M.C.; van Gemert, G.-J.; van de Vegte-Bolmer, M.; Graumans, W.; van Lieshout, L.; Bastiaens, G.J.; Teelen, K.; et al. Sporozoite immunization of human volunteers under mefloquine prophylaxis is safe, immunogenic and protective: A double-blind randomized controlled clinical trial. PLoS ONE 2014, 9, e112910. [Google Scholar] [CrossRef]
- Roestenberg, M.; Teirlinck, A.C.; McCall, M.B.; Teelen, K.; Makamdop, K.N.; Wiersma, J.; Arens, T.; Beckers, P.; van Gemert, G.; van de Vegte-Bolmer, M.; et al. Long-term protection against malaria after experimental sporozoite inoculation: An open-label follow-up study. Lancet 2011, 377, 1770–1776. [Google Scholar] [CrossRef]
- Roestenberg, M.; de Vlas, S.J.; Nieman, A.E.; Sauerwein, R.W.; Hermsen, C.C. Efficacy of preerythrocytic and blood-stage malaria vaccines can be assessed in small sporozoite challenge trials in human volunteers. J. Infect. Dis. 2012, 206, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chen, Y.; Hao, L.; Zhang, Y. DC-SIGN and immunoregulation. Cell. Mol. Immunol. 2006, 3, 279–283. [Google Scholar] [PubMed]
- Yu, H.R.; Chang, W.P.; Wang, L.; Lin, Y.; Liang, C.; Yang, K.D.; Kuo, C.; Huang, Y.; Chang, W.; Kuo, H. DC-SIGN (CD209) promoter-336 A/G (rs4804803) polymorphism associated with susceptibility of Kawasaki disease. Sci. World J. 2012, 2012, 634835. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, D.; Ng, W.Y.; Holz, L.E.; Ma, J.; Zaid, A.; Wong, T.C.; Lau, L.S.; Mollard, V.; Cozinjsen, A.; Collins, N.; et al. Liver-resident memory CD8+ T cells form a front-line defense against malaria liver-stage infection. Immunity 2016, 45, 889–902. [Google Scholar] [CrossRef]
- Sakuntabhai, A.; Turbpaiboon, C.; Casadémont, I.; Chuansumrit, A.; Lowhnoo, T.; Kajaste-Rudnitski, A.; Kalayanarooj, S.M.; Tangnararatchakit, K.; Tangthawornchaikul, N.; Vasanawathana, S.; et al. A variant in the CD209 promoter is associated with severity of dengue disease. Nat. Genet. 2005, 37, 507–513. [Google Scholar] [CrossRef]
- Noble, J.A.; Duru, K.C.; Guindo, A.; Yi, L.; Imumorin, I.G.; Diallo, D.A.; Thomas, B.N. Interethnic diversity of the CD209 (rs4804803) gene promoter polymorphism in African but not American sickle cell disease. Peer J. 2015, 3, e799. [Google Scholar] [CrossRef][Green Version]
- Herrero, R.; Pineda, J.A.; Rivero-Juarez, A.; Echbarthi, M.; Real, L.M.; Camacho, A.; Macias, J.; Fibla, J.; Rivero, A.; Caruz, A. Common haplotypes in CD209 promoter and susceptibility to HIV-1 infection in intravenous drug users. Infect. Genet. Evol. 2016, 45, 20–25. [Google Scholar] [CrossRef]
- Zupin, L.; Polesello, V.; Alberi, G.; Moratelli, G.; Crocè, S.L.; Masutti, F.; Pozzato, G.; Crovella, S.; Segat, L. CD209 promoter polymorphisms associate with HCV infection and pegylated-interferon plus ribavirin treatment response. Mol. Immunol. 2016, 76, 49–54. [Google Scholar] [CrossRef]
- Afifi, R.A.; Kamal, D.; Sayed, R.E.; Ekladious, S.M.M.; Shaheen, G.H.; Yousry, S.M.; Hussein, R.E. CD209-336A/G promotor polymorphism and its clinical associations in sickle cell disease Egyptian Pediatric patients. Hematol. Oncol. Stem Cell Ther. 2018, 11, 75–81. [Google Scholar] [CrossRef]
- He, Y.X.; Ye, C.L.; Zhang, P.; Li, Q.; Park, C.G.; Yang, K.; Jiang, L.Y.; Lv, Y.; Ying, X.L.; Ding, H.H.; et al. Yersinia pseudotuberculosis exploits CD209 receptors for promoting host dissemination and infection. Infect. Immun. 2018, 87, e00654. [Google Scholar] [CrossRef]
- Pabalan, N.; Chaisri, S.; Tabunhan, S.; Phumyen, A.; Jarjanazi, H.; Steiner, T.S. Associations of DC-SIGN (CD209) promoter -336G/A polymorphism (rs4804803) with dengue infection: A systematic review and meta-analyses. Acta Trop. 2018, 177, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H.; Freeman, G.J. The B7-CD28 superfamily. Nat. Rev. Immunol. 2002, 2, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.; Sardinha, L.R.; Bastos, K.R.B.; Zago, C.A.; da Silva, A.P.F.; Alvarez, J.M.; Lima, M.R.D. Role of CD28 in polyclonal and specific T and B cell responses required for protection against blood stage malaria. J. Immunol. 2005, 174, 790–799. [Google Scholar] [CrossRef]
- Butty, V.; Roy, M.; Sabeti, P.; Besse, W.; Benoist, C.; Mathis, D. Signatures of strong population differentiation shape extended haplotypes across the human CD28, CTLA4, and ICOS costimulatory genes. Proc. Natl. Acad. Sci. USA 2007, 104, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Zhang, S.; Gao, X. Quantitative assessment of the associations between CD28 T>C polymorphism (rs3116496) and cancer risk. Tumor Biol. 2014, 35, 9195–9200. [Google Scholar] [CrossRef] [PubMed]
- Elghzaly, A.A.; Metwally, S.S.; El-Chennawi, F.A.; Elgayaar, M.A.; Mosaad, Y.M.; El-Toraby, E.E.; Hegab, M.M.; Ibrahim, S.M. IRF5, PTPN22, CD28, IL2RA, KIF5A, BLK and TNFAIP3 genes polymorphisms and lupus susceptibility in a cohort from the Egypt Delta; relation to other ethnic groups. Hum. Immunol. 2015, 76, 525–531. [Google Scholar] [CrossRef]
- Niknam, A.; Karimi, M.H.; Geramizadeh, B.; Roozbeh, J.; Yaghobi, R.; Salehipour, M. Polymorphisms of the costimulatory genes CTLA-4, CD28, PD-1, and ICOS and outcome of kidney transplants in Iranian patients. Exp. Clin. Transpl. 2017, 15, 295–305. [Google Scholar]
- Cassiano, G.C.; Furini, A.A.; Capobianco, M.P.; Storti-Melo, L.M.; Cunha, M.G.; Kano, F.S.; Carvalho, L.H.; Soares, I.S.; Santos, S.E.; Póvoa, M.M.; et al. Polymorphisms in B cell co-stimulatory genes are associated with IgG antibody responses against blood-stage proteins of Plasmodium vivax. PLoS ONE 2016, 11, e0149581. [Google Scholar] [CrossRef]
- Leoratti, F.M.; Farias, L.; Alves, F.P.; Suarez-Mútis, M.C.; Coura, J.R.; Kalil, J.; Camargo, E.P.; Moraes, S.L.; Ramasawmy, R. Variants in the toll-like receptor signaling pathway and clinical outcomes of malaria. J. Infect. Dis. 2008, 198, 772–780. [Google Scholar] [CrossRef]
- Koukouikila-Koussounda, F.; Ntoumi, F.; Ndounga, M.; Tong, H.V.; Abena, A.A.; Velavan, T.P. Genetic evidence of regulatory gene variants of the STAT6, IL10R and FOXP3 locus as a susceptibility factor in uncomplicated malaria and parasitaemia in Congolese children. Malar. J. 2013, 12, 9. [Google Scholar] [CrossRef][Green Version]
- Amoako-Sakyi, D.; Adukpo, S.; Kusi, K.A.; Dodoo, D.; Ofori, M.F.; Adjei, G.O.; Edoh, D.E.; Asmah, R.H.; Brown, C.; Adu, B.; et al. A STAT6 intronic single nucleotide polymorphism is associated with clinical malaria in Ghanaian children. Genet. Epigenet. 2016, 8, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Trape, J.F. Rapid evaluation of malaria parasite density and standardization of thick smear examination for epidemiological investigations. Trans. R. Soc. Trop. Med. Hyg. 1985, 79, 181–184. [Google Scholar] [CrossRef]
- Solé, X.; Guinó, E.; Valls, J.; Iniesta, R.; Moreno, V. SNPStats: A web tool for the analysis of association studies. Bioinformatics 2006, 22, 1928–1929. [Google Scholar] [CrossRef] [PubMed]
- Adedokun, S.A.; Seamans, B.N.; Cox, N.T.; Liou, G.; Ojurongbe, O.; Thomas, B.N. Interleukin-4 and STAT-6 polymorphisms but not interleukin 10 or 13 are essential for regulating schistosomiasis and disease burden in south-western Nigeria. Infect. Genet. Evol. 2018, 65, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Seka-Seka, J.; Brouh, Y.; Yapo-Crézoit, A.C.; Atseye, N.H. The role of serum immunoglobulin E in the pathogenesis of Plasmodium falciparum malaria in Ivorian children. Scand. J. Immunol. 2004, 59, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Park, K.T.; ElNaggar, M.M.; Abdellrazeq, G.S.; Bannantine, J.P.; Mack, V.; Fry, L.M.; Davis, W.C. Phenotype and function of CD209+ bovine blood dendritic cells, monocyte-derived-dendritic cells and monocyte-derived macrophages. PLoS ONE 2016, 11, e0165247. [Google Scholar] [CrossRef]
- Taylor, A.; Rudd, C.E. Glycogen synthase kinase 3 inactivation compensates for the lack of CD28 in the priming of CD8+ cytotoxic T-cells: Implications for anti-PD-1 immunotherapy. Front. Immunol. 2017, 8, 1653. [Google Scholar] [CrossRef]
- Singh, S.K.; Larsson, M.; Schön, T.; Stendahl, O.; Blomgran, R. HIV interferes with the dendritic cell-T cell axis of macrophage activation by shifting Mycobacterium tuberculosis-specific CD4 T cells into a dysfunctional phenotype. J. Immunol. 2019, 202, 816–826. [Google Scholar] [CrossRef]
- Duetsch, G.; Illig, T.; Loesgen, S.; Rohde, K.; Klopp, N.; Herbon, N.; Gohlke, H.; Altmueller, J.; Wjst, M. STAT6 as an asthma candidate gene: Polymorphism-screening, association and haplotype analysis in a Caucasian subpair study. Hum. Mol. Genet. 2002, 11, 613–621. [Google Scholar] [CrossRef]
- Vannberg, F.O.; Chapman, S.J.; Khor, C.C.; Tosh, K.; Floyd, S.; Jackson-Sillah, D.; Crampin, A.; Sichali, L.; Bah, B.; Gustafson, P.; et al. CD209 genetic polymorphism and tuberculosis disease. PLoS ONE 2008, 3, e1388. [Google Scholar] [CrossRef]
- Colmenares, M.; Puig-Kroger, A.; Pello, O.M.; Corbi, A.L.; Rivas, L. Dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN, CD209), a C-type surface lectin in human DCs, is a receptor for Leishmania amastigotes. J. Biol. Chem. 2002, 277, 36766–36769. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Hashemi, M.; Taheri, M.; Pesarakli, H.; Eskandari-Nasab, E.; Bahari, G. CD209 promoter -n336 A/G (rs4804803) polymorphism is associated with susceptibility to pulmonary tuberculosis in Zahedan, southeast Iran. J. Microbiol. Immunol. Infect. 2014, 47, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.C. Protection afforded by sickle cell trait against sub-tertian malarial infection. Br. Med. J. 1954, 1, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.E.; Luiz, R.S.; Karina, R.B.B.; Cláudia, A.Z.; Ana Paula, F.S.; José, M.A.; Maria, R.D.L. Role of CD28 in polyclonal and specific T and B cell responses required for protection against blood stage malaria. J. Immunol. 2005, 174, 790–799. [Google Scholar]
- Gause, W.C.; Mitro, V.; Via, C.; Linsley, P.; Urban, J.F.; Greenwald, R.J. Do effector and memory T helper cells also need B7 ligand costimulatory signals? J. Immunol. 1997, 159, 1055–1058. [Google Scholar] [PubMed]
- Gause, W.C.; Chen, S.J.; Greenwald, R.J.; Halvorson, M.J.; Lu, P.; Zhou, X.D.; Morris, S.C.; Lee, K.P.; June, C.H.; Finkelman, F.D.; et al. CD28 dependence of T cell differentiation to IL-4 production varies with the particular type 2 immune response. J. Immunol. 1997, 158, 4082–4087. [Google Scholar] [PubMed]
- Teutsch, S.M.; Booth, D.R.; Bennetts, B.H.; Heard, R.N.; Stewart, G.J. Association of common T cell activation gene polymorphisms with multiple sclerosis in Australian patients. J. Neuroimmunol. 2004, 148, 218–230. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Q.; Shen, L.; Liu, Y.; Xu, F.; Li, D.; Fu, Z.; Yuan, W.; Pang, D.; Li, D. Investigation of CD28 gene polymorphisms in patients with sporadic breast cancer in a Chinese Han population in Northeast China. PLoS ONE 2012, 7, e48031. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).