Uptake of Inorganic and Organic Nitrogen Sources by Dinophysis acuminata and D. acuta
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Conditions
2.2. Antibiotic Treatments
2.3. Background Nutrient Concentrations
2.4. N15-Radiolabeled Stock Solutions
2.5. Nitrogen Uptake by Dinophysis acuminata and D. acuta in Well-Fed (Experiment 1) and Starved (Experiment 2) Conditions
2.5.1. Experiment 1
2.5.2. Experiment 2
2.6. Nitrogen Isotope Analysis and Uptake Estimates
2.7. Reference Transcriptome of D. acuminata and Other Dinoflagellates
3. Results
3.1. Effect of Antibiotics in Dinophysis Cultures
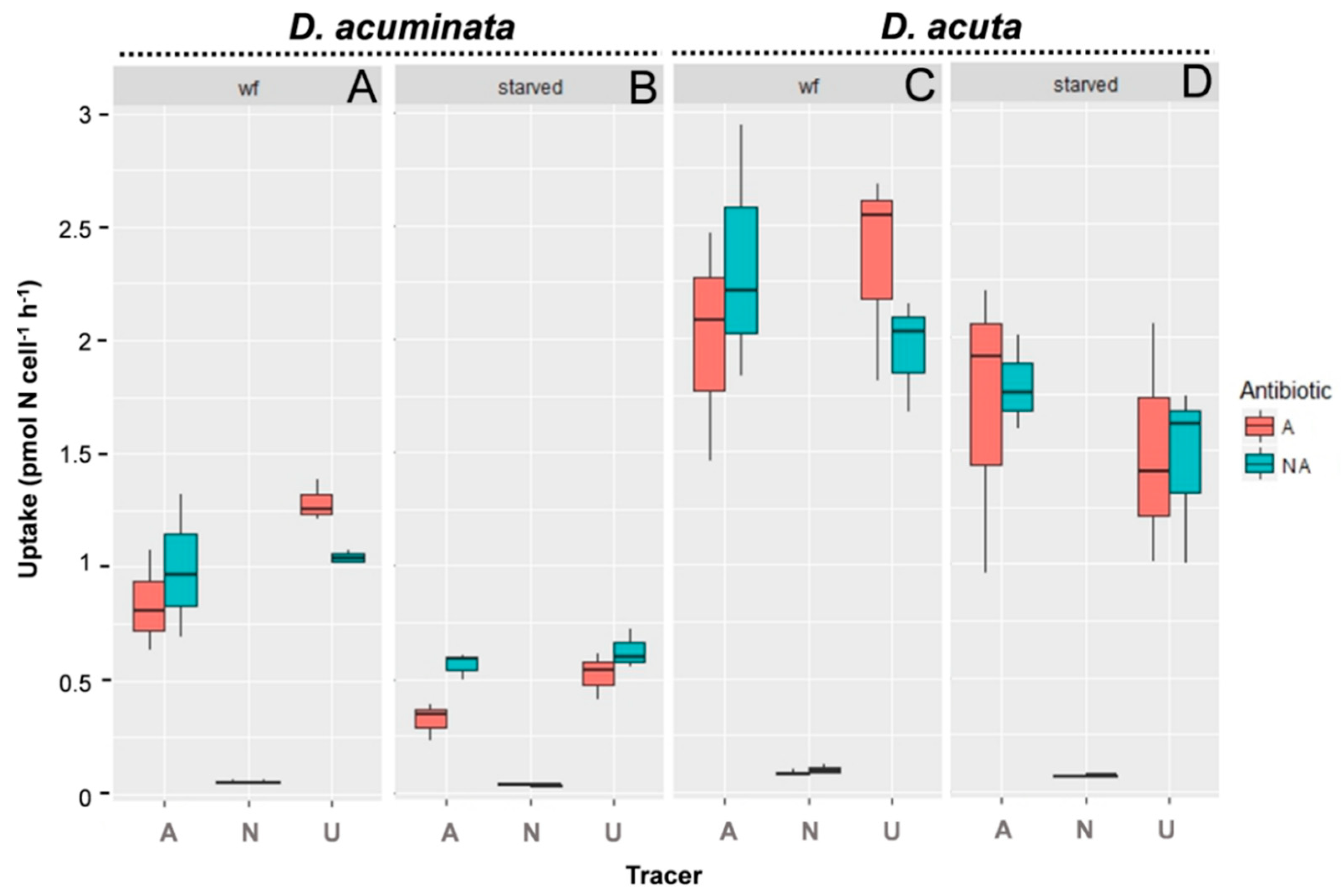
3.2. Uptake of Different N Sources by D. acuminata and D. acuta
3.3. Reference Transcriptome of D. acuminata
4. Discussion
4.1. Preferences and Uptake of Nitrogenous Compounds by Dinophysis Species
4.2. Uptake Rates of Well-Fed versus Starved Cells of Dinophysis
4.3. Why D. acuminata and D. acuta Do Not Take Up Nitrate?
4.4. Ecological Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Margalef, R. Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanol Acta 1978, 1, 493–509. [Google Scholar]
- Glibert, P.M. Margalef revisited: A new phytoplankton mandala incorporating twelve dimensions, including nutritional physiology. Harmful Algae 2016, 55, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A.; Maberly, S.C. Phytoplankton Nutrition and Related Mixotrophy; Lickens, G.E., Ed.; Encyclopedia of Inland Waters; Elsevier: Oxford, UK, 2009; pp. 192–196. [Google Scholar]
- Flynn, K.J.; Stoecker, D.K.; Mitra, A.; Raven, J.A.; Glibert, P.M.; Hansen, P.J.; Granéli, E.; Burkholder, J.M. Misuse of the phytoplankton–zooplankton dichotomy: The need to assign organisms as mixotrophs within plankton functional types. J. Plankt. Res. 2013, 35, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Stoecker, D.K. Mixotrophy in dinoflagellates. J. Eukaryot. Microbiol. 1999, 46, 397–401. [Google Scholar] [CrossRef]
- Tittel, J.; Bissinger, V.; Zippel, B.; Gaedke, U.; Bell, E.; Lorke, A.; Kamjunke, N. Mixotrophs combine resource use to outcompete specialists: Implications for aquatic food webs. PNAS 2003, 100, 12776–12781. [Google Scholar] [CrossRef] [Green Version]
- Mitra, A.; Flynn, K.J.; Tillmann, U.; Raven, J.A.; Caron, D.; Stoecker, D.K.; Not, F.; Hansen, P.J.; Hallegraeff, G.; Sanders, R.; et al. Defining planktonic protist functional groups on mechanisms for energy and nutrient acquisition: incorporation of diverse mixotrophic strategies. Protist 2016, 167, 106–120. [Google Scholar] [CrossRef]
- Stoecker, D.K.; Hansen, P.J.; Caron, D.A.; Mitra, A. Mixotrophy in the marine plankton. Ann. Rev. Mar. Sci. 2017, 9, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Sanders, R.W. Mixotrophic protists in marine and freshwater ecosystems. J. Protozool. 1991, 38, 76–81. [Google Scholar] [CrossRef]
- Hansen, P.J. The role of photosynthesis and food uptake for the growth of marine mixotrophic dinoflagellates. J. Eukaryot. Microb. 2011, 58, 203–214. [Google Scholar] [CrossRef]
- Carvalho, W.F.; Granéli, E. Contribution of phagotrophy versus autotrophy to Prymnesium parvum growth under nitrogen and phosphorus sufficiency and deficiency. Harmful Algae 2010, 9, 105–115. [Google Scholar] [CrossRef]
- Hackett, J.D.; Maranda, L.; Yoon, H.S.; Bhattacharya, D. Phylogenetic evidence for the cryptophyte origin of the plastid of Dinophysis (Dinophysiales, Dinophyceae). J. Phycol. 2003, 39, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Park, M.G.; Kim, S.; Kim, H.S.; Myung, G.; Kang, Y.G.; Yih, W. First successful culture of the marine dinoflagellate Dinophysis acuminata. Aquat. Microb. Ecol. 2006, 45, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Hansen, P.J.; Nielsen, L.T.; Johnson, M.; Berge, T.; Flynn, K.J. Acquired phototrophy in Mesodinium and Dinophysis–A review of cellular organization, prey selectivity, nutrient uptake and bioenergetics. Harmful Algae 2013, 28, 126–139. [Google Scholar] [CrossRef]
- Seeyave, S.; Probyn, T.A.; Pitcher, G.C.; Lucas, M.I.; Purdie, D.A. Nitrogen nutrition in assemblages dominated by Pseudo-nitzschia spp., Alexandrium catenella and Dinophysis acuminata off the west coast of South Africa. Mar. Ecol. Prog. Ser. 2009, 379, 91–107. [Google Scholar] [CrossRef] [Green Version]
- Seeyave, S.; Probyn, T.; Álvarez-Salgado, X.A.; Figueiras, F.G.; Purdie, D.A.; Barton, E.D.; Lucas, M. Nitrogen uptake of phytoplankton assemblages under contrasting upwelling and downwelling conditions: The Ría de Vigo, NW Iberia. Estuar Coast Shelf Sci. 2013, 124, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hattenrath-Lehmann, T.K.; Marcoval, M.A.; Mittlesdorf, H.; Goleski, J.A.; Wang, Z.; Haynes, B.; Morton, S.L.; Gobler, C.J. Nitrogenous nutrients promote the growth and toxicity of Dinophysis acuminata during estuarine bloom events. PLoS ONE 2015, 10, e0124148. [Google Scholar] [CrossRef]
- Tong, M.; Smith, J.L.; Kulis, D.M.; Anderson, D.M. Role of dissolved nitrate and phosphate in isolates of Mesodinium rubrum and toxin-producing Dinophysis acuminata. Aquat. Microb. Ecol. 2015, 75, 169–185. [Google Scholar] [CrossRef] [Green Version]
- Hattenrath-Lehmann, T.; Gobler, C.J. The contribution of inorganic and organic nutrients to the growth of a North American isolate of the mixotrophic dinoflagellate, Dinophysis acuminata. Limnol. Oceanogr. 2015, 60, 1588–1603. [Google Scholar] [CrossRef]
- Yasumoto, T.; Oshima, Y.; Yamaguchi, M. Occurrence of a new type of shellfish poisoning in the Tohoku district. Bull Jpn. Soc. Sci. Fish 1978, 44, 1249–1255. [Google Scholar] [CrossRef]
- Reguera, B.; Riobó, P.; Rodríguez, F.; Díaz, P.A.; Pizarro, G.; Paz, B.; Franco, J.M.; Blanco, J. Dinophysis toxins: Causative organisms, distribution and fate in shellfish. Mar. Drugs 2014, 12, 394–461. [Google Scholar] [CrossRef]
- Yasumoto, T.; Murata, M.; Oshima, Y.; Sano, M.; Matsumoto, G.; Clardy, J. Diarrhetic shellfish toxins. Tetrahedron 1985, 41, 1019–1025. [Google Scholar] [CrossRef]
- Lawrence, J.; Loreal, H.; Toyofuku, H.; Hess, P.; Iddya, K.; Ababouch, L. Assessment and Management of Biotoxin Risks in Bivalve Molluscs; FAO Fisheries and Aquaculture Technical Paper (551); FAO: Rome, Italy, 2011; p. 337. [Google Scholar]
- O’Mahony, M. EU regulatory risk management of marine biotoxins in the marine bivalve mollusc food-chain. Toxins 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Reguera, B.; Bravo, I.; Mariño, J.; Campos-Loriz, M.J.; Fraga, S.; Carbonell, A. Trends in the occurrence of Dinophysis spp in Galician coastal waters. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: Amsterdam, Netherlands, 1993; pp. 559–564. [Google Scholar]
- Díaz, P.A.; Ruiz-Villarreal, M.; Velo-Suárez, L.; Ramilo, I.; Gentien, P.; Lunven, M.; Fernand, L.; Raine, R.; Reguera, B. Tidal and wind-event variability and the distribution of two groups of Pseudo-nitzschia species in an upwelling-influenced Ría. Deep Sea Res Part II: Top. Stud. Oceanogr. 2014, 101, 163–179. [Google Scholar] [CrossRef] [Green Version]
- Escalera, L.; Reguera, B.; Moita, T.; Pazos, Y.; Cerejo, M.; Cabanas, J.M.; Ruiz-Villarreal, M. Bloom dynamics of Dinophysis acuta in an upwelling system: In situ growth versus transport. Harmful Algae 2010, 9, 312–322. [Google Scholar] [CrossRef]
- Ruiz-Villarreal, M.; García-García, L.M.; Cobas, M.; Díaz, P.A.; Reguera, B. Modelling the hydrodynamic conditions associated with Dinophysis blooms in Galicia (NW Spain). Harmful Algae 2016, 53, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Reguera, B.; Bravo, I.; Fraga, S. Autoecology and some life history stages of Dinophysis acuta Ehrenberg. J. Plankton. Res. 1995, 17, 999–1015. [Google Scholar] [CrossRef]
- Díaz, P.A.; Ruiz-Villarreal, M.; Pazos, Y.; Moita, T.; Reguera, B. Climate variability and Dinophysis acuta blooms in an upwelling system. Harmful Algae 2016, 53, 145–159. [Google Scholar] [CrossRef]
- Ríos, A.F.; Fraga, F.; Figueiras, F.G.; Pérez, F.F. New and regenerated production in relation to the proliferations of diatoms and dinoflagellates in natural conditions. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard, E., Gentien, P., Marcaillou, C., Eds.; Technique et Documentation-Lavoisier, Intercept Ltd.: Paris, France, 1995; pp. 663–668. [Google Scholar]
- Doval, M.D.; López, A.; Madriñán, M. Spatio-temporal variability of inorganic and organic nutrients in five Galician rias (NW Spain). Sci. Mar. 2013, 77S1, 15e24. [Google Scholar] [CrossRef] [Green Version]
- Doval, M.D.; López, A.; Madriñán, M. Temporal variation and trends of inorganic nutrients in the coastal upwelling of the NW Spain (Atlantic Galician rías). J. Sea. Res. 2016, 108, 19–29. [Google Scholar] [CrossRef]
- García-Portela, M.; Reguera, B.; Sibat, M.; Altenburger, A.; Rodríguez, F.; Hess, P. Metabolomic profiles of Dinophysis acuminata and Dinophysis acuta using non-targeted high-resolution mass spectrometry: effect of nutritional status and prey. Mar. Drugs 2018b, 16, 143. [Google Scholar] [CrossRef] [Green Version]
- García-Portela, M.; Reguera, B.; d’Alcalà, M.R.; Rodríguez, F.; Montresor, M. Effects of small-scale turbulence on two species of Dinophysis. Harmful Algae 2019, 89, 101654. [Google Scholar] [CrossRef] [PubMed]
- McGuillicudy, D.J., Jr.; Glibert, P.M.; Berdalet, E.; Edwards, C.; Franks, P.; Ross, O. GEOHAB Modelling, Linking Observations to Predictions. Available online: http://hab.ioc-unesco.org/index.php?option=com_oe&task=viewDocumentRecord&docID=6659 (accessed on 17 December 2019).
- Moita, T.; Pazos, Y.; Rocha, C.; Nolasco, R.; Oliveira, P.B. Toward predicting Dinophysis blooms off NW Iberia: A decade of events. Harmful Algae 2016, 53, 17–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillard, R.R.L.; Hargraves, P.E. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar] [CrossRef]
- Álvarez-Salgado, X.A.; Doval, M.D.; Pérez, F.F. Dissolved organic matter in shelf waters off the Ría de Vigo (NW Iberian upwelling system). J. Mar. Syst. 1999, 18, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Hansen, H.P.; Grasshoff, K. Automated chemical analysis. In Methods of Seawater Analysis, 2nd ed.; Grasshoff, K., Ehrhardt, M., Kremling, K., Eds.; Verl. Chemie: Weinheim, Germany, 1983; pp. 347–379. [Google Scholar]
- Goeyens, L.; Semeneh, M.; Elskens, M.; Shopova, D.; Baumann, M.E.M.; Dehairs, F. Phytoplankton nutrient utilisation and nutrient signature in the Southern Ocean. J. Mar.Syst. 1998, 17, 143–157. [Google Scholar] [CrossRef]
- UNESCO. Protocol for the Joint Global Ocean Flux Study (JGOFS) Core Measurements. (IOC Manuals and Guides No. 29); UNESCO-IOC: Paris, France, 1994; pp. 130–135. [Google Scholar]
- Dugdale, R.C.; Goering, J.J. Uptake of new and regenerated forms of nitrogen in primary productivity 1. Limnol. Oceanogr. 1967, 12, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Keeling, P.J.; Burki, F.; Wilcox, H.M.; Allam, B.; Allen, E.E.; Amaral-Zettler, L.A.; Armbrust, E.V.; Archibald, J.M.; Bharti, A.K.; Bell, C.J. The Marine Microbial Eukaryote Transcriptome Sequencing Project (MMETSP): Illuminating the functional diversity of eukaryotic life in the oceans through transcriptome sequencing. PLoS Biol. 2014, 12, e1001889. [Google Scholar] [CrossRef] [Green Version]
- Le Gac, M.; Metegnier, G.; Chomérat, N.; Malestroit, P.; Quéré, J.; Bouchez, O.; Siano, R.; Destombe, C.; Guillou, R.; Chapelle, A. Evolutionary processes and cellular functions underlying divergence in Alexandrium minutum. Mol. Ecol. 2016, 25, 5129–5143. [Google Scholar] [CrossRef] [Green Version]
- Collos, Y.; Harrison, P.J. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar. Poll. Bull. 2014, 80, 8–23. [Google Scholar] [CrossRef]
- Baek, S.H.; Shimode, S.; Han, M.S.; Kikuchi, T. Growth of dinoflagellates, Ceratium furca and Ceratium fusus in Sagami Bay, Japan: the role of nutrients. Harmful algae 2008, 7, 729–739. [Google Scholar] [CrossRef]
- García-Portela, M.; Riobó, P.; Reguera, B.; Garrido, J.L.; Blanco, J.; Rodríguez, F. Comparative ecophysiology of Dinophysis acuminata and D. acuta (DINOPHYCEAE, DINOPHYSIALES): Effect of light intensity and quality on growth, cellular toxin content, and photosynthesis. J. Phycol. 2018a, 54, 899–917. [Google Scholar]
- Syrett, P.J. The assimilation of ammonia and nitrate by nitrogen-starved cells of Chlorella vulgaris. 11. The assimilation of large quantities of nitrogen. Physiol. Plant. 1956, 9, 19. [Google Scholar] [CrossRef]
- Collos, Y. Transient situations in nitrate assimilation by marine diatoms. 1. Changes in uptake parameters during nitrogen starvation 1. Limnol. Oceanogr. 1980, 25, 1075–1081. [Google Scholar] [CrossRef]
- Kim, S.; Kang, Y.G.; Kim, H.S.; Yih, W.; Coats, D.W.; Park, M.G. Growth and grazing responses of the mixotrophic dinoflagellate Dinophysis acuminata as functions of light intensity and prey concentration. Aquat. Microb. Ecol. 2008, 51, 301–310. [Google Scholar] [CrossRef]
- Johnson, M.D.; Beaudoin, D.J.; Laza-Martínez, A.; Dyhrman, S.T.; Fensin, E.; Lin, S.; Merculief, A.; Nagai, S.; Pompeu, M.; Setälä, O. The genetic diversity of Mesodinium and associated Cryptophytes. Front. Microbiol. 2016, 7, 2017. [Google Scholar] [CrossRef]
- Minnhagen, S.; Kim, M.; Salomon, P.; Yih, W.; Granéli, E.; Park, M.G. Active uptake of kleptoplastids by Dinophysis caudata from its ciliate prey Myrionecta rubra. Aquat. Microb. Ecol. 2011, 62, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Fritz, L.; Stringher, C.G.; Colepicolo, P. Immunolocalization of nitrate reductase in the marine dinoflagellate gonyaulax polyedra (pyrrophyta) 1. J. Phycol. 1996, 32, 632–637. [Google Scholar] [CrossRef]
- Park, M.G.; Park, J.S.; Kim, M.; Yih, W. Plastid dynamics during survival of Dinophysis caudata without its ciliate prey. J. Phycol. 2008, 44, 1154–1163. [Google Scholar] [CrossRef]
- Rusterholz, P.M.; Hansen, P.J.; Daugbjerg, N. Evolutionary transition towards permanent chloroplasts?‒Division of kleptochloroplasts in starved cells of two species of Dinophysis (Dinophyceae). PloS ONE 2017, 12, e0177512. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Luque, E.; Chamizo-Ampudia, A.; Llamas, A.; Galvan, A.; Fernandez, E. Understanding nitrate assimilation and its regulation in microalgae. Front. Plant. Sci. 2015, 6, 899. [Google Scholar] [CrossRef] [Green Version]
- Collos, Y.; Berges, J.A. Nitrogen metabolism in phytoplankton. In Encyclopedia of Life Support Systems; Duarte, C.M., Ed.; EOLSS Publishers: Paris, France, 2003. [Google Scholar]
- Raven, J.A. A cost-benefit analysis of photon absorbtion by photosynthetic unicells. New Phytol. 1984, 98, 593–625. [Google Scholar] [CrossRef]
- Chang, F.H.; McClean, M. Growth responses of Alexandrium minutum (Dinophyceae) as a function of three different nitrogen sources and irradiance. N. Z. J. Mar. Freshw Res. 1997, 31, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Leong, S.C.Y.; Taguchi, S. Response of the dinoflagellate Alexandrium tamarense to a range of nitrogen sources and concentrations: growth rate, chemical carbon and nitrogen, and pigments. Hydrobiologia 2004, 515, 215–224. [Google Scholar] [CrossRef]
- Lee, Y.S. Utilization of various nitrogen, phosphorus, and selenium compounds by Cochlodinium polykrikoides. J. Environ. Biol. 2008, 29, 799–804. [Google Scholar] [PubMed]
- Wisecaver, J.H.; Hackett, J.D. Transcriptome analysis reveals nuclear-encoded proteins for the maintenance of temporary plastids in the dinoflagellate Dinophysis acuminata. BMC Genom. 2010, 11, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagenais-Bellefeuille, S.; Morse, D. Putting the N in dinoflagellates. Front Microbiol. 2013, 4, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Camacho, A.; González, R.; Fuentes, J. Mussel culture in Galicia (NW Spain). Aquaculture 1991, 94, 263–278. [Google Scholar] [CrossRef]
- Figueiras, F.G.; Wyatt, T.; Alvarez-Salgado, X.A.; Jenkinson, I.R. Advection, diffusion and patch development in the Rias Baixas. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard, E., Gentien, P., Marcaillou, C., Eds.; Technique et Documentation-Lavoisier, Intercept Ltd.: Paris, France, 1995; pp. 579–584. [Google Scholar]
- Álvarez-Salgado, X.A.; Labarta, U.; Fernández-Reiriz, M.J.; Figueiras, F.G.; Rosón, G.; Piedracoba, S.; Filgueira, R.; Cabanas, J.M. Renewal time and the impact of harmful algal blooms on the extensive mussel raft culture of the Iberian coastal upwelling system (SW Europe). Harmful Algae 2008, 7, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Salgado, X.A.; Rosón, G.; Pérez, F.F.; Figueiras, F.G.; Pazos, Y. Nitrogen cycling in an estuarine upwelling system, the Ría de Arousa (NW, Spain). I. Short-time-scale patterns of hydrodynamic and biogeochemical circulation. Mar. Ecol. Prog. Ser. 1996, 135, 259–273. [Google Scholar] [CrossRef] [Green Version]
- Blanco, J.; Correa, J.; Muñíz, S.; Mariño, C.; Martín, H.; Arévalo, A. Evaluación del impacto de los métodos y niveles utilizados para el control de toxinas en el mejillón. Revista Galega dos Recursos Mariños (Art. Inf. Tecn.) 2013, 3, 1–55. [Google Scholar]
- Figueiras, F.G.; Ríos, A.F. Phytoplankton succession, red tides and hydrographic regime in the Rías Bajas of Galicia. In Toxic Phytoplankton Blooms in the Sea; Smayda, J., Shimizu, Y., Eds.; Elsevier: New York, NY, USA, 1993; pp. 239–244. [Google Scholar]
- Álvarez-Salgado, X.A.; Gago, J.; Míguez, B.M.; Gilcoto, M.; Pérez, F.F. Surface waters of the NW Iberian margin: Upwelling on the shelf versus outwelling of upwelled waters from the Rías Baixas. Estuar. Coast Shelf Sci. 2000, 51, 821–837. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, E.; Ibañez, F.; Figueiras, F.G. Effect of meteorological and hydrographic disturbances on the microplankton community structure in the Ría de Vigo (NW Spain). Mar. Ecol. Prog. Ser. 2000, 203, 23–45. [Google Scholar] [CrossRef]
- Tilstone, G.H.; Miguez, B.M.; Figueiras, F.G.; Fermín, E.G. Diatom dynamics in a coastal ecosystem affected by upwelling: Coupling between species succession, circulation and biogeochemical processes. Mar. Ecol. Prog. Ser. 2000, 205, 23–41. [Google Scholar] [CrossRef] [Green Version]
- Velo-Suárez, L.; González-Gil, S.; Gentien, P.; Lunven, M.; Bechemin, C.; Fernand, L.; Raine, R.; Reguera, B. Thin layers of Pseudonitzschia spp. and the fate of Dinophysis acuminata during an upwelling-downwelling cycle in a Galician Ría. Limnol. Oceanogr. 2008, 53, 1816. [Google Scholar] [CrossRef]
- Díaz, P.A.; Ruiz-Villarreal, M.; Mouriño-Carballido, B.; Fernández-Pena, C.; Riobó, P.; Reguera, B. Fine scale physical-biological interactions during a shift from relaxation to upwelling with a focus on Dinophysis acuminata and its potential ciliate prey. Progr. Oceanogr. 2019, 175, 309–327. [Google Scholar] [CrossRef]
- Hällfors, H.; Hajdu, S.; Kuosa, H.; Larsson, U. Vertical and temporal distribution of the dinoflagellates Dinophysis acuminata and D. norvegica in the Baltic Sea. Boreal. Environ. Res. 2011, 16, 121–135. [Google Scholar]


| Label | NO3− | NO2 | NH4+ | PO43− | SiO2 | CO(NH2)2 |
|---|---|---|---|---|---|---|
| 1 | 2.58 | 0.68 | 0.96 | 0.22 | 5.21 | 0.14 |
| 2 | 2.08 | 0.56 | 0.81 | 0.18 | 4.25 | 0.12 |
| 3 | 2.23 | 0.60 | 0.85 | 0.18 | 4.43 | 0.08 |
| 4 | 3.28 | 0.74 | 1.04 | 0.25 | 6.56 | 0.08 |
| 5 | 2.30 | 0.51 | 0.77 | 0.19 | 4.57 | 0.08 |
| 6 | 2.69 | 0.62 | 0.89 | 0.21 | 5.41 | 0.12 |
| Parameters | Statistical Test | D. acuminata | D. acuta | ||
|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | ||
| Tracer | 2w-ANOVA | 147.21 | 3 × 10−14* | 89.06 | 8 × 10−12* |
| Nutritional state | 2w-ANOVA | 76.56 | 24 × 10−9* | 8.63 | 7 × 10−3* |
| Antibiotic | 2w-ANOVA | 0.99 | 0.33 | 0.00 | 0.99 |
| Tracer:antibiotic | 2w-ANOVA | 3.73 | 0.04 | 0.93 | 0.41 |
| Tracer:nutritional state | 2w-ANOVA | 17.31 | 2 × 10−5* | 2.20 | 0.13 |
| Antibiotic:nutritional state | 2w-ANOVA | 2.86 | 10−1 | 0.02 | 0.89 |
| Nitrate-ammonium | TukeyHSD | 10−7* | 10−7* | ||
| Urea-ammonium | TukeyHSD | 3 × 10−3* | 0.64 | ||
| Urea-nitrate | TukeyHSD | 10−7* | 10−7* | ||
| No antibiotic:antibiotic | TukeyHSD | 0.33 | 0.99 | ||
| Urea: well-fed-starved cells | TukeyHSD | 3 × 10−7* | 0.05 | ||
| Ammonium: well-fed-starved cells | TukeyHSD | 1 × 10−5* | 0.41 | ||
| Nitrate: well-fed-starved cells | TukeyHSD | 0.99 | 0.99 | ||
| Protein Family | D. acuminata | Odd Ratio | p-Value |
|---|---|---|---|
| Nitrate reductase | 77 | 0.75 | 0.01 |
| Nitrite reductase | 13 | 0.65 | 0.14 |
| Nitrate transporter | 8 | 0.3 | 4 × 10−5 |
| Ammonium transporter | 15 | 0.57 | 0.03 |
| Urease | 42 | 0.95 | 0.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Portela, M.; Reguera, B.; Gago, J.; Le Gac, M.; Rodríguez, F. Uptake of Inorganic and Organic Nitrogen Sources by Dinophysis acuminata and D. acuta. Microorganisms 2020, 8, 187. https://doi.org/10.3390/microorganisms8020187
García-Portela M, Reguera B, Gago J, Le Gac M, Rodríguez F. Uptake of Inorganic and Organic Nitrogen Sources by Dinophysis acuminata and D. acuta. Microorganisms. 2020; 8(2):187. https://doi.org/10.3390/microorganisms8020187
Chicago/Turabian StyleGarcía-Portela, María, Beatriz Reguera, Jesús Gago, Mickael Le Gac, and Francisco Rodríguez. 2020. "Uptake of Inorganic and Organic Nitrogen Sources by Dinophysis acuminata and D. acuta" Microorganisms 8, no. 2: 187. https://doi.org/10.3390/microorganisms8020187





