Scientific Prospects for Cannabis-Microbiome Research to Ensure Quality and Safety of Products
Abstract
:1. Introduction
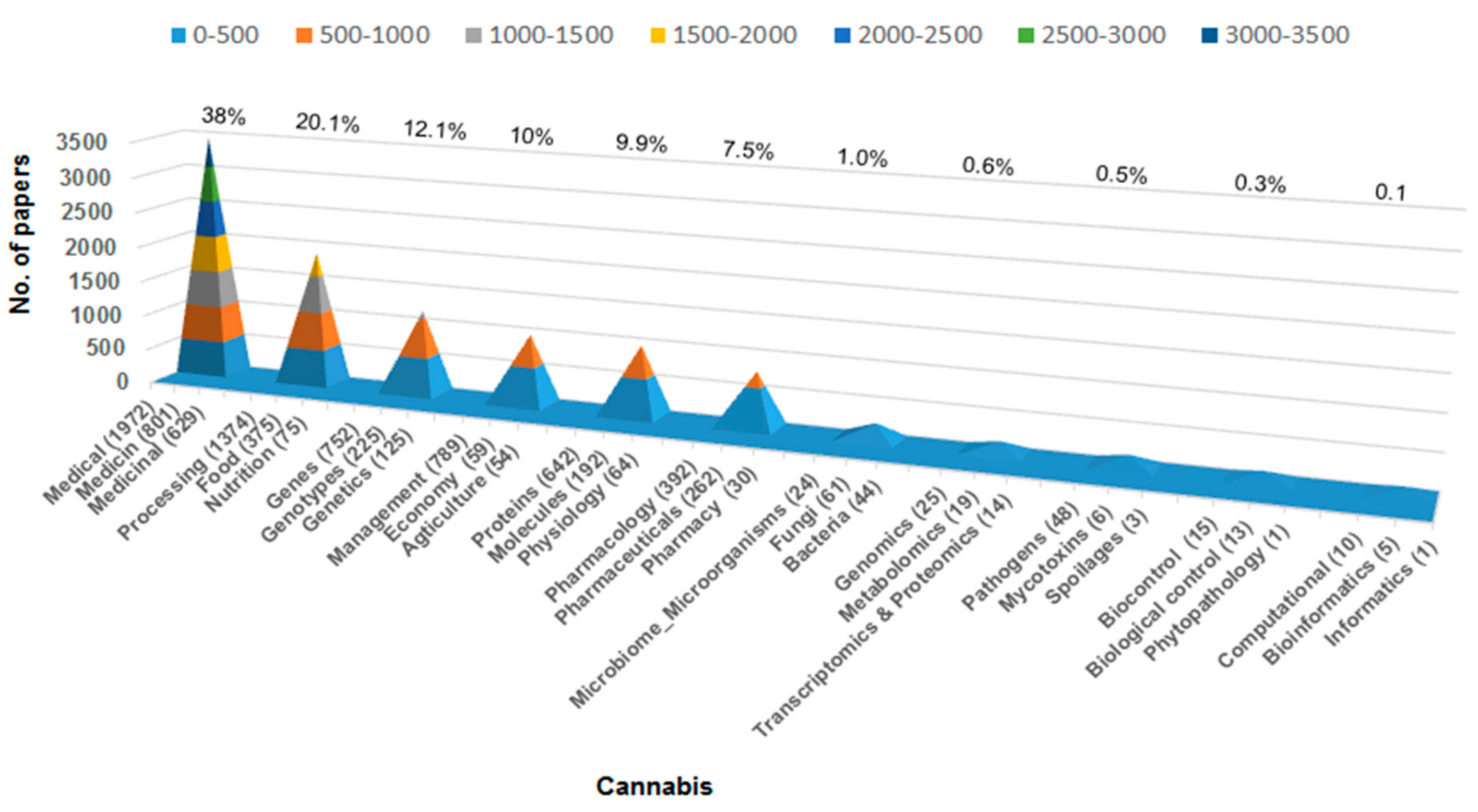
2. Why Advance Cannabis Science?
3. Where are the Opportunities for Cannabis Science?
4. What are the Major Risks Associated with Cannabis?
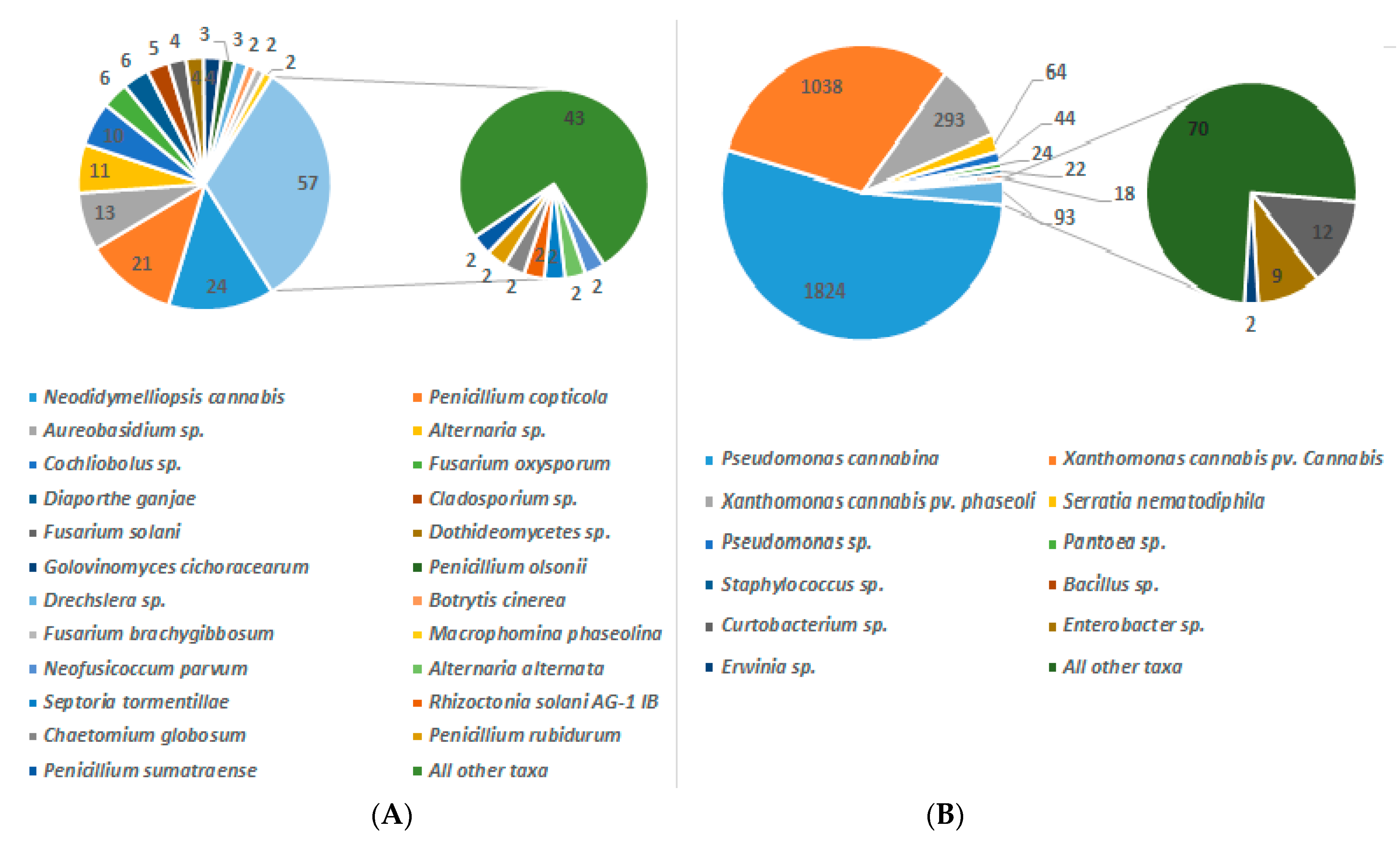
5. What are the Sources of Host Infection and Diminished Quality Versus Safety of Cannabis Products?
6. Indoor Cultivation and Storage of Cannabis are Propitious to Fungal and Bacterial Contamination
7. Risks and Effects of Consumption of Contaminated Cannabis
8. Where do the Scientific Solutions Lie?
9. What are The Future Technological Advancements?
10. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Potters, G. Systems biology of the cell. Nat. Educ. 2010, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Rashid, A.; Hasan, O.; Siddique, U.; Tahar, S. Formal reasoning about systems biology using theorem proving. PLoS ONE 2017, 12, e0180179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, N.; Hattori, C.; Cheema, J.; Donarski, J.; Charlton, A.; Dickinson, M.; Venditti, G.; Kaló, P.; Szabó, Z.; Kiss, G.B.; et al. NMR metabolomics defining genetic variation in pea seed metabolites. Front. Plant Sci. 2018, 9, 1022. [Google Scholar] [CrossRef]
- Durán, P.; Thiergart, T.; Oter, R.G.; Agler, M.; Kemen, E.; Schulze-Lefert, P.; Hacquard, S. Microbial inter Kingdom interactions in roots promote Arabidopsis survival. Cell 2018, 175, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, D.S.; Yourstone, S.; Mieczkowski, P.; Jones, C.D.; Dangl, J.L. Practical innovations for high-throughput amplicon sequencing. Nat. Meth. 2013, 10, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Ver Loren van Themaat, E.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef]
- Schlaeppi, K.; Dombrowski, N.; Garrido Oter, R.; Ver Loren van Themaat, E.; Schulze-Lefert, P. Quantitative divergence of the bacterial root microbiota in Arabidopsis thaliana relatives. Proc. Natl. Acad. Sci. USA 2014, 111, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Facchini, P.J.; De Luca, V. Opium poppy and Madagascar periwinkle: Model non-model systems to investigate alkaloid biosynthesis in plants. Plant J. 2008, 54, 763–784. [Google Scholar] [CrossRef]
- Gurkok, T.; Ozhuner, E.; Parmaksiz, I.; Özcan, S.; Turktas, M.; Ipek, A.; Demirtas, I.; Okay, S.; Unver, T. Functional Characterization of 4′OMT and 7OMT Genes in BIA biosynthesis. Front. Plant Sci. 2016, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Labanca, F.P.; Owesna, J.; Milellaa, L. Papaver somniferum L. taxonomy, uses and new insight in poppy alkaloid pathways. Phytochem. Rev. 2018, 17, 853–871. [Google Scholar] [CrossRef]
- Marciano, M.A.; Panicker, S.X.; Liddil, G.D.; Lindgren, D.; Sweder, K.S. Development of a method to extract opium poppy (Papaver somniferum L.) DNA from heroin. Sci. Rep. 2018, 8, 2590. [Google Scholar] [CrossRef] [PubMed]
- Vašek, J.; Cílová, D.; Melounová, M.; Svoboda, P.; Vejl, P.; Štikarová, R.; Vostrý, L.; Kuchtová, P.; Ovesná, J. New EST-SSR markers for individual genotyping of opium poppy cultivars (Papaver somniferum L.). Plants 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staley, C.; Ferrieri, A.P.; Tfaily, M.M.; Cui, Y.; Chu, R.K.; Wang, P.; Shaw, J.B.; Ansong, C.K.; Brewer, H.; Norbeck, A.D.; et al. Diurnal cycling of rhizosphere bacterial communities is associated with shifts in carbon metabolism. Microbiome 2017, 5, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-Q.; Zhang, A.-H.; Miao, J.-H.; Sun, H.; Yan, G.-L.; Wu, F.-F. Gut microbiota as important modulator of metabolism in health and disease. RSC Adv. 2019, 8, 42380. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, C.R.; Copeland, J.; Wang, P.W.; Guttman, S.D.; Kotanen, P.M.; Johnson, M.T.J. Assembly and ecological function of the root microbiome across angiosperm plant species. Proc. Natl. Acad. Sci. USA 2018, 115, 1157–1165. [Google Scholar] [CrossRef] [Green Version]
- Bartoli, C.; Frachon, L.; Barret, M.; Rigal, M.; Huard-Chauveau, C.; Mayjonade, B.; Zanchetta, C.; Bouchez, O.; Roby, D.; Carrère, S.; et al. In situ relationships between microbiota and potential pathobiota in Arabidopsis thaliana. ISMEJ 2018, 12, 2024–2038. [Google Scholar] [CrossRef] [Green Version]
- Gopal, M.; Gupta, A.; Thomas, G.V. Bespoke microbiome therapy to manage plant diseases. Front. Microbiol. 2013, 4, 355. [Google Scholar] [CrossRef] [Green Version]
- Gopal, M.; Gupta, A. Microbiome selection could spur next-generation plant breeding strategies. Front. Microbiol. 2016, 7, 1971. [Google Scholar] [CrossRef] [Green Version]
- Parfrey, L.W.; Moreau, C.S.; Russel, J.A. Introduction: The host-associated microbiome: Pattern, process and function. Mol. Ecol. 2018, 27, 1749–1765. [Google Scholar] [CrossRef]
- Winston, M.E.; Hampton-Marcell, J.; Zarraonaindia, I.; Owens, S.M.; Moreau, C.S.; Gilbert, J.A.; Hartsel, J.; Kennedy, S.J.; Gibbons, S.M. Understanding cultivar-specificity and soil determinants of the Cannabis microbiome. PLoS ONE 2014, 9, e99641. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Ver Loren van Themaat, E.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordenstein, S.R.; Theis, K.R. Host biology in light of the microbiome: Ten principles of holobionts and hologenomes. PLoS Biol. 2015, 13, e1002226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, J.-C.; Marchesi, J.R.; Mougel, C.; Selosse, M.-A. Host-microbiota interactions: From holobiont theory to analysis. Microbiome 2019, 7, 5. [Google Scholar] [CrossRef]
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKernan, K.; Spangler, J.; Zhang, L.; Tadigotla, V.; Helbert, Y.; Foss, T.; Smith, D. Cannabis microbiome sequencing reveals several mycotoxic fungi native to dispensary grade Cannabis flowers. F1000Research 2015, 4, 1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liew, W.-P.-P.; Mohd-Redzwan, S. Mycotoxin: Its impact on gut health and microbiota. Front. Cell. Infect. Microbiol. 2018, 8, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punja, Z. Flower and foliage-infecting pathogens of marijuana (Cannabis sativa L.) plants. Can. J. Plant Pathol. 2018, 40, 514–527. [Google Scholar] [CrossRef] [Green Version]
- Fulthorpe, R.; MacIvor, J.S.; Jia, P.; Yasui, S.-L.E. The green roof microbiome: Improving plant survival for ecosystem service delivery. Front. Ecol. Evol. 2018, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Scott, M.; Rani, M.; Samsatly, J.; Charron, J.B.; Jabaji, S. Endophytes of industrial hemp (Cannabis sativa L.) cultivars: Identification of culturable bacteria and fungi in leaves, petioles, and seeds. Can. J. Microbiol. 2018, 64, 664–680. [Google Scholar] [CrossRef] [Green Version]
- Dohlman, A.B.; Shen, X. Mapping the microbial interactome: Statistical and experimental approaches for microbiome network inference. Exp. Biol. Med. 2019, 244, 445–458. [Google Scholar] [CrossRef]
- Chye, Y.; Suoa, C.; Lorenzetti, V.; Batalla, A.; Cousijn, J.; Goudriaan, A.E.; Martin-Santos, R.; Whittle, S.; Solowij, N.; Yücel, M. Cortical surface morphology in long-term Cannabis users: A multi-site MRI study. Eur. Neuropsychopharmacol. 2019, 29, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Mudge, E.M.; Murch, S.J.; Brown, P.N. Chemometric analysis of cannabinoids: Chemotaxonomy and domestication syndrome. Sci. Rep. 2018, 8, 3090. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, K.; Kia, D.A.; Vandrovcova, J.; Hardy, J.; Wood, N.W.; Lewis, P.A.; Ferrari, R. Genome, transcriptome and proteome: The rise of omics data and their integration in biomedical sciences. Brief. Bioinform. 2018, 19, 286–302. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M.; McKernan, K.J. Contaminants of concern in Cannabis: Microbes, heavy metals and pesticides. Canabis Sativa L. Bot. Biotechnol. 2017, 22, 457–474. [Google Scholar] [CrossRef]
- Chakraborty, S.; Newton, A.C. Climate change, plant diseases and food security: An overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef] [Green Version]
- Walker, W. Fungus in Medical Marijuana Eyed as Possible Cause in California Man’s Death, UC Davis Medical Center. 2017. Available online: https://sanfrancisco.cbslocal.com/2017/02/06/medical-marijuana-fungus-death-uc-davis-medical-center/ (accessed on 20 February 2019).
- Kagen, S.L.; Kurup, V.P.; Sohnle, P.G.; Fink, J.N. Marijuana smoking and fungal sensitization. J. Allergy Clin. Immunol. 1983, 71, 389–393. [Google Scholar] [CrossRef]
- Ruchlemer, R.; Amit-Kohn, M.; Raveh, D.; Hanuš, L. Inhaled medicinal Cannabis and the immunocompromised patient. Supportive Care Cancer 2015, 23, 819–822. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars-grin.gov/fungaldatabases/ (accessed on 12 January 2019).
- Hosseini-Nasabnia, Z.; Van Rees, K.; Vujanovic, V. Preventing unwanted spread of invasive fungal species in willow (Salix spp.) plantations. J. Plant Pathol. 2016, 38, 325–337. [Google Scholar] [CrossRef]
- Hua, L.; Yong, C.; Zhanquan, Z.; Boqiang, L.; Guozheng, Q.; Shiping, T. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf. 2018, 3, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Dalmais, B.; Schumacher, J.; Moraga, J.; Le Pêcheur, P.; Tudzynski, B.; Collado, I.G.; Vlaud, M. The Botrytis cinerea phytotoxin botcinic acid requires two polyketide synthases for production and has a redundant role in virulence with botrydial. Mol. Plant Pathol. 2011, 12, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Temme, N.; Oeser, B.; Massaroli, M.; Heller, J.; Simon, A.; Collado, I.G.; Vlaud, M.; Tudzynski, B. BcAtf1, a global regulator, controls various differentiation processes and phytotoxin production in Botrytis cinerea. Mol. Plant Pathol. 2012, 13, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Kusch, S.; Panstruga, R. mlo-based resistance: An apparently universal “weapon” to defeat powdery mildew disease. Mol. Plant Microbe Interact. 2017, 30, 179–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punja, Z.K.; Collyer, D.; Scott, C.; Lung, S.; Holmes, J.; Sutton, D. Pathogens and molds affecting production and quality of Cannabis sativa L. Front. Plant Sci. 2019, 10, 1120. [Google Scholar] [CrossRef] [Green Version]
- Thompson, G.R.; Tuscano, J.M.; Dennis, M.; Singapuri, A.; Libertini, S.; Gaudino, R.A.; Torres, A.; Delisle, A.M.P.; Gillece, J.D.; Schupp, J.M.; et al. A microbiome assessment of medical marijuana. Clin. Microbiol. Infect. 2017, 23, 269–270. [Google Scholar] [CrossRef] [Green Version]
- Pauly, J.L.; Paszkiewicz, G. Cigarette smoke, Bacteria, mold, microbial toxins, and chronic lung inflammation. J. Oncol. 2011, 819129. [Google Scholar] [CrossRef] [Green Version]
- Hazekamp, A. Evaluating the effects of gamma-irradiation for decontamination of medicinal Cannabis. Front. Pharmacol. 2016, 7, 108. [Google Scholar] [CrossRef] [Green Version]
- Backes, M.; Weil, A. Use of Medicinal Cannabis. In Cannabis Pharmacy: The Practical Guide to Medical Marijuana; Black Dog & Leventhal Publ.: New York, NY, USA, 2014; p. 320. [Google Scholar]
- Bennet, J.W.; Klich, M. Mycotoxins. Clinic. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [Green Version]
- Balendres, M.A.O.; Karlovsky, P.; Cumagun, C.J.R. Mycotoxigenic fungi and mycotoxins in agricultural crop commodities in the Philippines. Foods 2019, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Llamas, R.; Hart, D.R.; Schneider, N.S. Allergic bronchopulmonary aspergillosis associated with smoking moldy marihuana. Chest 1978, 73, 871–872. [Google Scholar] [CrossRef] [Green Version]
- Ocampo, T.L.; Rans, T.S. Cannabis sativa: The unconventional “weed” allergen. Ann. Allergy Asthma Immunol. 2014, 3, 187–192. [Google Scholar] [CrossRef]
- Georggiett, O.C.; Muiño, J.C.; Montrull, H.; Brizuela, N.; Avalos, S.; Gómez, R.M. Relationship between lung cancer and aflatoxin B1. Rev. Fac. Cien. Med. Univ. Nac. Cordoba 2000, 57, 95–107. [Google Scholar] [PubMed]
- Smith, L.E.; Prendergast, J.A.; Turner, P.C.; Humphrey, J.H.; Stoltzfus, R.J. Aflatoxin exposure during pregnancy, maternal anemia, and adverse birth outcomes. Am. J. Trop. Med. Hyg. 2017, 96, 770–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, K.; McHenry, M.A.; Cats-Baril, W.; Grace, T. Cannabis testing for Public Safety—Best Practices for Vermont Analytical Laboratories, State of Vermont. PhytoScience Inst. 2016, 1, 1–34. [Google Scholar]
- Scott, P.M. Effects of food processing on mycotoxins. J. Food Prot. 1984, 47, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, M.D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
- Mowgli, H.; Vyas, J.M.; Steinbach, W.; McPartland, J. Microbiological Safety Testing of Cannabis. 2015. Available online: https://extractionmagazine.com/wp-content/uploads/2018/07/Microbiological-Safety-Testing-of-Cannabis.pdf (accessed on 3 February 2019).
- Taylor, D.R.; Fergusson, D.M.; Milne, B.J.; Horwood, L.J.; Moffitt, T.E.; Sears, M.R.; Poulton, R. A longitudinal study of the effects of tobacco and cannabis exposure on lung function in young adults. Addiction 2002, 97, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Kerremans, J.J.; Voss, A.; Meis, J.F.G.M. Fungal contamination of tobacco and marijuana. JAMA 2000, 284, 2875. [Google Scholar] [CrossRef]
- Yang, J.; Yang, J.; Duan, Y.; Gu, W.; Gong, X.; Zhe, W.; Su, C.; Zhang, K.Q. Bacterial diversities on unaged and aging flue-cured tobacco leaves estimated by 16S rRNAsequence analysis. Appl. Microbiol. Biotechnol. 2010, 88, 553–562. [Google Scholar] [CrossRef]
- Olmedo, P.; Goessler, W.; Tanda, S.; Grau-Perez, M.; Jarmul, S.; Aherrera, A.; Chen, R.; Hilpert, M.; Cohen, J.E.; Navas-Acien, A.; et al. Metal Concentrations in e-Cigarette Liquid and Aerosol Samples: The Contribution of Metallic Coils. Environ. Health Perspect. 2018, 126, 027010. [Google Scholar] [CrossRef]
- Seltenrich, N. Cannabis contaminants: Regulating solvents, microbes, and metals in legal weed. Environ. Health Perspect. 2019, 127, 8. [Google Scholar] [CrossRef] [PubMed]
- Cescon, D.W.; Page, A.V.; Richardson, S.; Moore, M.J.; Boerner, S.; Gold, W.L. Invasive pulmonary aspergillosis associated with marijuana use in a man with colorectal cancer. J. Clin. Oncol. 2018, 26, 2214–2215. [Google Scholar] [CrossRef]
- Gargani, Y.; Bishop, P.; Denning, D.W. Too mouldy joints-Marijuana and chronic pulmonary aspergillosis. Mediterr. J. Hematol. Infect. Dis. 2011, 3, e2011005. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, S.; Yacoub, A.; Cheema, A.; Rihana, N.; Russo, R.; Velez, A.P.; Nanjappa, S.; Sandin, R.L.; Bohra, C.; Gajanan, G.; et al. Marijuana smoking in patients with leukemia. Cancer Control 2016, 23, 278–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, T.; Henkle, J.; Prakash, V. Pulmonary mucormycosis associated with medicinal marijuana. Respir. Med. Case Rep. 2019, 26, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Ungerleider, J.T.; Andrysiak, T.; Tashkin, D.P.; Gale, R.P. Contamination of marihuana cigarettes with pathogenic bacteria-possible source of infection in cancer patients. Cancer Treat Rep. 1982, 66, 589–591. [Google Scholar]
- Hamadeh, R.; Ardehali, A.; Locksley, R.M.; York, M.K. Fatal aspergillosis associated with smoking contaminated marijuana, in a marrow transplant recipient. Chest 1988, 94, 432–433. [Google Scholar] [CrossRef] [Green Version]
- Kagen, S.L. Aspergillus: An inhalable contaminant of marihuana. N. Engl. J. Med. 1981, 304, 483–484. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, B.B.; Hedrick, R.; Vanle, B.C.; Becker, C.A.; Nguyen, C.; Underhill, D.M.; Morgan, M.A.; Kopple, J.D.; Danovitch, I.; Hak, W.W. Cryptococcal meningitis in a daily Cannabis smoker without evidence of immunodeficiency. BMJ Case Rep. 2018. [Google Scholar] [CrossRef]
- Schwartz, I.S. Fungal sinusitis and marijuana. JAMA 1987, 257, 2914–2915. [Google Scholar] [CrossRef]
- Szyper-Kravits, M.; Lang, R.; Manor, Y.; Lahav, M. Early invasive pulmonary aspergillosis in a leukemia patient linked to aspergillus contaminated marijuana smoking. Leuk. Lymphoma 2001, 42, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- DeVillaer, M. Cannabis Law Reform in Canada: Pretense & Perils; The Peter Boris Centre for Addictions Research McMaster University: Hamilton, ON, Canada, 2017; p. 110. [Google Scholar]
- Schier, J.G.; Meiman, J.G.; Layden, J.; Mikosz, C.A.; VanFrank, B.; King, B.A.; Salvatore, P.P.; Weissman, D.N.; Thomas, J.; Melstrom, P.C.; et al. Severe pulmonary disease associated with electronic-cigarette-product use—Interim guidance. MMWR Morb. Mortal Wkly Rep. 2019, 68, 787–790. [Google Scholar] [CrossRef] [PubMed]
- McInnis, O.A.; Plecas, D. Clearing the Smoke on Cannabis: Respiratory Effects of Cannabis Smoking; Canadian Centre on Substance Abuse: Ottawa, ON, Canada, 2016; ISBN 978-1-77178-310-1. [Google Scholar]
- Becker, W.J.; Nagarkatti, M.; Nagarkatti, P.S. Δ9-tetrahydrocannabinol (THC) activation of cannabinoid receptors induces unique changes in the murine gut microbiome and associated induction of myeloid-derived suppressor cells and Th17 cells. J. Immunol. Available online: https://www.jimmunol.org/content/198/1_supplement/218.11 (accessed on 1 May 2017).
- Sarafian, T.A.; Habib, N.; Oldham, M.; Seeram, N.; Lee, R.-P.; Lin, L.; Tashkin, D.P.; Roth, M.D. Inhaled marijuana smoke disrupts mitochondrial energetics in pulmonary epithelial cells in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L1202–L1209. [Google Scholar] [CrossRef] [PubMed]
- Shay, A.H.; Choi, R.; Whittaker, K.; Salehi, K.; Kitchen, C.M.; Tashkin, D.P.; Roth, D.; Baldwin, G.C. Impairment of antimicrobial activity and nitric oxide production in alveolar macrophages from smokers of marijuana and cocaine. J. Infect. Dis. 2003, 187, 700–704. [Google Scholar] [CrossRef] [Green Version]
- Tashkin, D.P. Smoked marijuana as a cause of lung injury. Monaldi Arch. Chest Dis. 2005, 63, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.N.; Wachsmuth, I.K.; Shangkuan, Y.H.; Schmidt, E.V.; Barrett, T.J.; Schrader, J.S.; Scherach, C.S.; McGee, H.B.; Feldman, R.A.; Brenner, D.J. Salmonellosis associated with marijuana—A multistate outbreak traced by plasmid fingerprinting. N. Engl. J. Med. 1982, 306, 1249–1253. [Google Scholar] [CrossRef]
- Baldwin, G.C.; Tashkin, D.P.; Buckley, D.M.; Park, A.N.; Dubinett, S.M.; Roth, M.D. Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am. J. Respir. Crit. Care Med. 1997, 156, 1606–1613. [Google Scholar] [CrossRef]
- Fligiel, S.E.; Roth, M.D.; Kleerup, E.C.; Barsky, S.H.; Simmons, M.S.; Tashkin, D.P. Tracheobronchial histopathology in habitual smokers of cocaine, marijuana, and/or tobacco. Chest 1997, 112, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Moody, M.M.; Wharton, R.C.; Schnaper, N.; Schimpff, S.C. Do water pipes prevent transmission of fungi from contaminated marijuana? N. Engl. J. Med. 1982, 306, 1492–1493. [Google Scholar] [CrossRef]
- Burns, M. Cannabis use in the immunocompromised increasing rates of Pulmonary Aspergillus. Sch. Physician Assist. Stud. 2019, 676, 1–22. [Google Scholar]
- Bilodeau, S.E.; Wu, B.-S.; Rufyikiri, A.-S.; MacPherson, S.; Lefsrud, M. An update on plant photobiology and implications for Cannabis production. Front. Plant Sci. 2019, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Schwinghamer, T.; Rosenbaum, P.; McCarty, V.; Bilodeau, S.E.; Lyu, D.; Ahmed, M.B.; Robinson, G.; Lefsrud, M.; Wilkins, O.; et al. Closing the yield gap for Cannabis: A meta-analysis of factors determining cannabis yield. Front. Plant Sci. 2019, 10, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshannaq, A.; Yu, J.-H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Holmes, M.; Vyas, J.M.; Steinbach, W.; McPartland, J. Microbiological Safety Testing of Cannabis. 2017. Available online: https://www.analyticalcannabis.com/white-papers/microbiological-safety-testing-of-cannabis-288152 (accessed on 20 February 2019).
- McPartland, J.M.; Clarke, R.C.; Watson, D.P. Cannabis Diseases and Pests: Management with an Emphasis on Organic and Biological Control: An Advanced Treatise; Cornell University: Ithaca, NY, USA, 2015; p. 225. [Google Scholar]
- Kim, S.-H.; Vujanovic, V. Relationship between mycoparasites lifestyles and biocontrol behaviors against Fusarium spp. and mycotoxins production. Appl. Microbiol. Biotechnol. 2016, 100, 5257–5272. [Google Scholar] [CrossRef]
- Kuhn, D.M.; Ghannoum, M.A. Indoor mold, toxigenic fungi, and Stachybotrys chartarum: Infectious disease perspective. Clin. Microbiol. Rev. 2013, 16, 144–172. [Google Scholar] [CrossRef] [Green Version]
- McNeil, J.N.; Cotnoir, P.A.; Leroux, T.; Laprade, R.; Schwartz, J.L. A Canadian national survey on the public perception of biological control. BioControl 2010, 55, 445–454. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, J.R.; Zhang, G.Z.; Cai, L.; Crous, P.W. Resolving the Phoma enigma. Stud. Mycol. 2015, 82, 137–217. [Google Scholar] [CrossRef] [Green Version]
- Deardorff, D.; Wadsworth, K. What’s Wrong with My Marijuana Plant? In A Cannabis Grower’s Visual Guide to Easy Diagnosis and Organic Remedies; Ten Speed Press: Aberkeley, CA, USA, 2017; p. 175. [Google Scholar]
- Manceau, C.; Lydon, J.; Kong, H.; Vinatzer, B.A.; Fischer-Le Saux, M. Pseudomonas cannabina pv. cannabina pv. nov., and Pseudomonas cannabina pv. alisalensis (Cintas Koike and Bull, 2000) comb. nov., are members of the emended species Pseudomonas cannabina (ex Sutic & Dowson 1959) Gardan, Shafik, Belouin, Brosch, Grimont & Grimont 1999. Syst. Appl. Microbiol. 2010, 33, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Gomila, M.; Busquets, A.; Mulet, M.; García-Valdés, E.; Lalucat, J. Clarification of taxonomic status within the Pseudomonas syringae species group based on a phylogenomic analysis. Front. Microbiol. 2017, 8, 2422. [Google Scholar] [CrossRef] [Green Version]
- Sarris, P.F.; Trantas, E.A.; Baltrus, D.A.; Bull, C.T.; Wechter, W.P.; Yan, S.; Ververidis, F.; Almeida, N.F.; Jones, C.D.; Dangl, J.L.; et al. Comparative genomics of multiple strains of Pseudomonas cannabina pv. alisalensis, a potential model pathogen of both monocots and dicots. PLoS ONE 2013, 8, e59366. [Google Scholar] [CrossRef] [PubMed]
- McKernan, K.; Spangler, J.; Helbert, Y.; Lynch, R.C.; Devitt-Lee, A.; Zhang, L.; Orphe, W.; Warner, J.; Foss, T.; Hudalla, C.J.; et al. Metagenomic analysis of medicinal Cannabis samples; pathogenic bacteria, toxigenic fungi, and beneficial microbes grow in culture-based yeast and mold tests. F1000Research 2016, 5, 2471. [Google Scholar] [CrossRef] [PubMed]
- Szarka, D.; Tymon, L.; Amsden, B.; Dixon, E.; Judy, J.; Gauthier, N. First report of powdery mildew caused by Golovinomyces spadiceus on industrial hemp (Cannabis sativa) in Kentucky. Plant Dis. 2019, 103, 1773. [Google Scholar] [CrossRef]
- Doyle, V.P.; Tonry, H.T.; Amsden, B.; Beale, J.; Dixon, E.; Li, H.; Szarka, D.; Gauthier, N.W. First report of Cercospora cf flagellaris on industrial hemp (Cannabis saliva) in Kentucky. Plant Dis. 2019, 103, 1784. [Google Scholar] [CrossRef]
- D’Argeno, V. The high-throughput analyses era: Are we ready for the data struggle? High Throughput 2018, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; Glavina del Rio, T.; et al. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis sativa—From plant genome to humans. Plant Sci. 2019, 284, 67–72. [Google Scholar] [CrossRef]
- Iseppi, R.; Brighenti, V.; Licata, M.; Lambertini, A.; Sabia, C.; Messi, P.; Pellati, B.; Benvenuti, S. Chemical characterization and evaluation of the antibacterial activity of essential oils from fibre-type Cannabis sativa L. (Hemp). Molecules 2019, 24, 2302. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.; Jyoti, K.; Patnaik, A.; Singh, A.; Chauhand, S.C. Spectroscopic, microscopic characterization of Cannabis sativa leaf extract mediated silver nanoparticles and their synergistic effect with antibiotics against human pathogen. AEJ 2018, 57, 3043–3051. [Google Scholar] [CrossRef]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Mezrioui, N.; Setzer, W.; Abbad, A.; Hassani, L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Ind. Crop. Prod. 2019, 137, 396–400. [Google Scholar] [CrossRef]
- Luo, X.; Reiter, M.A.; d’Espaux, L.; Wong, J.; Denby, C.M.; Lechner, A.; Zhang, Y.; Grzybowski, A.T.; Harth, S.; Lin, W.; et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 2019, 567, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.Q.; Liu, G.N.; Ji, R.I.; Song, P.; Ren, L.J.; Huang, H.; Ji, X.J. CRISPR/Cas9-based genome editing of the filamentous fungi: The state of the art. Appl. Microbiol. Biotechnol. 2017, 101, 7435–7443. [Google Scholar] [CrossRef] [PubMed]
- Malla, M.A.; Dubey, A.; Kumar, A.; Yadav, S.; Hashem, A.; Abd_Allah, E.F. Exploring the human microbiome: The potential future role of next-generation sequencing in disease diagnosis and treatment. Front. Immunol. 2019, 9, 868. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vujanovic, V.; Korber, D.R.; Vujanovic, S.; Vujanovic, J.; Jabaji, S. Scientific Prospects for Cannabis-Microbiome Research to Ensure Quality and Safety of Products. Microorganisms 2020, 8, 290. https://doi.org/10.3390/microorganisms8020290
Vujanovic V, Korber DR, Vujanovic S, Vujanovic J, Jabaji S. Scientific Prospects for Cannabis-Microbiome Research to Ensure Quality and Safety of Products. Microorganisms. 2020; 8(2):290. https://doi.org/10.3390/microorganisms8020290
Chicago/Turabian StyleVujanovic, Vladimir, Darren R. Korber, Silva Vujanovic, Josko Vujanovic, and Suha Jabaji. 2020. "Scientific Prospects for Cannabis-Microbiome Research to Ensure Quality and Safety of Products" Microorganisms 8, no. 2: 290. https://doi.org/10.3390/microorganisms8020290
APA StyleVujanovic, V., Korber, D. R., Vujanovic, S., Vujanovic, J., & Jabaji, S. (2020). Scientific Prospects for Cannabis-Microbiome Research to Ensure Quality and Safety of Products. Microorganisms, 8(2), 290. https://doi.org/10.3390/microorganisms8020290




