A Rotational Slurry Bioreactor Accelerates Biodegradation of A-Fuel in Oil-Contaminated Soil Even under Low Temperature Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Preparation of Model Slurry
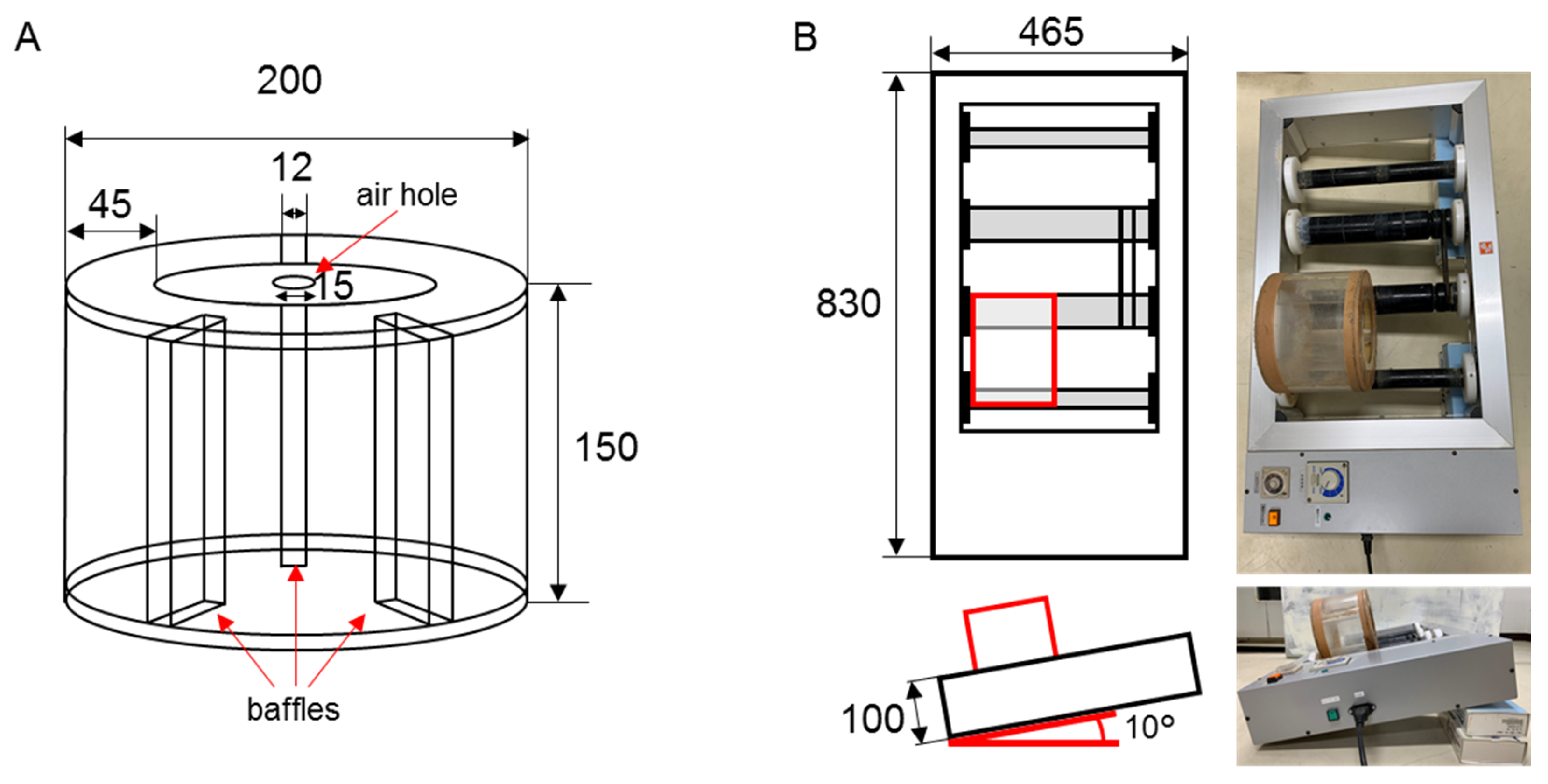
2.3. A Rotational Slurry Bioreactor
2.4. Biodegradative Assays in the Rotational Slurry Bioreactor
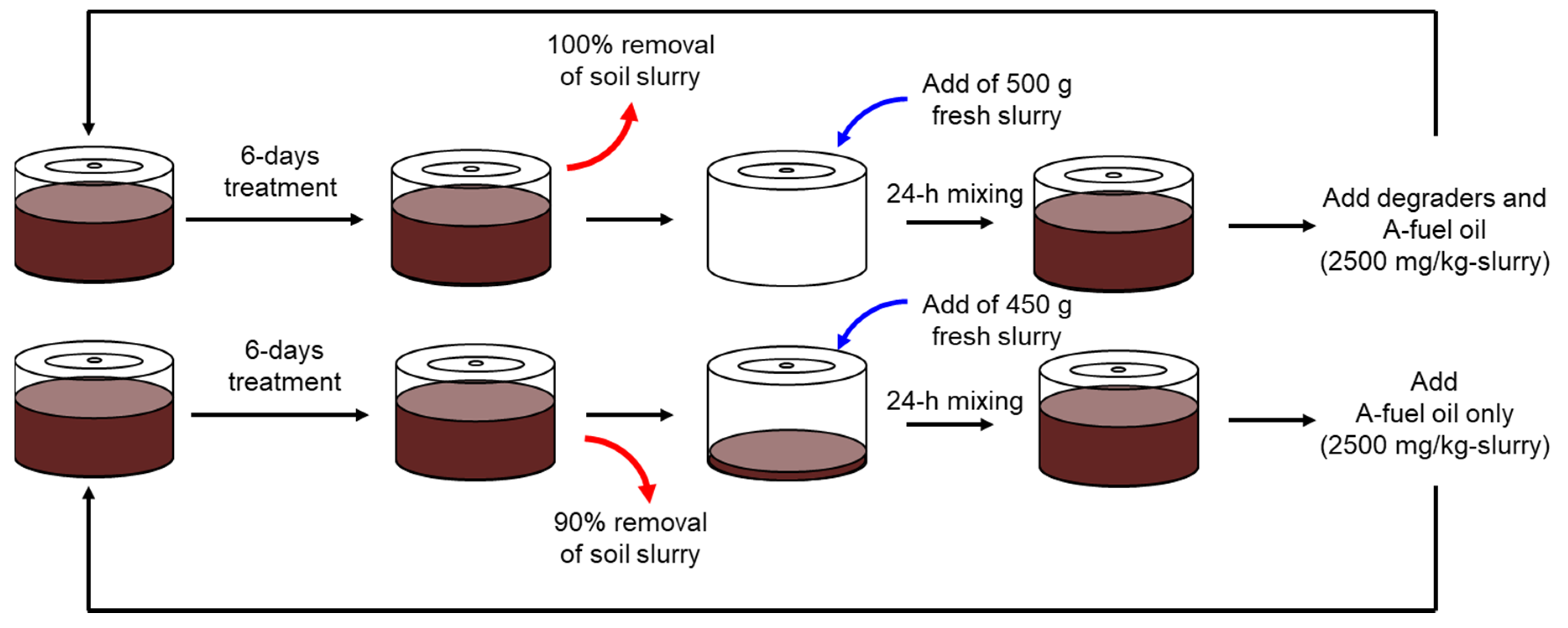
2.5. Semi-Batch Degradative Assays in the Rotational Slurry Bioreactor
3. Results
3.1. Biodegradation of A-Fuel Oil was Much Faster in a Rotational Slurry Bioreactor
3.2. Semi-Batch Degradative Assays in the Rotational Slurry Bioreactor
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bejarano, A.C.; Michel, J. Oil spills and their impacts on sand beach invertebrate communities: A literature review. Environ. Pollut. 2016, 218, 709–722. [Google Scholar] [CrossRef]
- Ivshina, I.B.; Kuyukina, M.S.; Krivoruchko, A.V.; Elkin, A.A.; Makarov, S.O.; Cunningham, C.J.; Peshkur, T.A.; Atlas, R.M.; Philp, J.C. Oil spill problems and sustainable response strategies through new technologies. Environ. Sci. Proc. Imp. 2015, 17, 1201–1219. [Google Scholar] [CrossRef]
- Limbergen, H.V.; Top, E.M.; Verstraete, W. Bioaugmentation in activated sludge: Current features and future perspectives. Appl. Microb. Biotechnol. 1998, 50, 16–23. [Google Scholar] [CrossRef]
- Szulc, A.; Ambrożewicz, D.; Sydow, M.; Ławniczak, Ł.; Piotrowska-Cyplik, A.; Marecik, R.; Chrzanowski, Ł. The influence of bioaugmentation and biosurfactant addition on bioremediation efficiency of diesel-oil contaminated soil: Feasibility during field studies. J. Environ. Manag. 2014, 132, 121–128. [Google Scholar] [CrossRef]
- Khan, S.; Afzal, M.; Iqbal, S.; Khan, Q.M. Plant–bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere 2013, 90, 1317–1332. [Google Scholar] [CrossRef]
- Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Microb. Res. 2010, 165, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Kostka, J.E. Hydrocarbon-degrading microbial communities are site specific, and their activity is limited by synergies in temperature and nutrient availability in surface ocean waters. Appl. Environ. Microbiol. 2019, 85, e00443-19. [Google Scholar] [CrossRef] [PubMed]
- Ribicic, D.; McFarlin, K.M.; Netzer, R.; Brakstad, O.G.; Winkler, A.; Throne-Holst, M.; Størseth, T.R. Oil type and temperature dependent biodegradation dynamics—Combining chemical and microbial community data through multivariate analysis. BMC Microbiol. 2018, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Whyte, L.G.; Bourbonniere, L.; Greer, C.W. Biodegradation of petroleum hydrocarbons by psychrotrophic Pseudomonas strains possessing both alkane (alk) and naphthalene (nah) catabolic pathways. Appl. Environ. Microbiol. 1997, 63, 3719–3723. [Google Scholar] [CrossRef]
- Whyte, L.G.; Hawari, J.; Zhou, E.; Bourbonniere, L.; Inniss, W.E.; Greer, C.W. Biodegradation of variable-chain-length alkanes at low temperatures by a psychrotrophic Rhodococcus sp. Appl. Environ. Microbiol. 1998, 64, 2578–2584. [Google Scholar] [CrossRef]
- Whyte, L.G.; Slagman, S.J.; Pietrantonio, F.; Bourbonniere, L.; Koval, S.F.; Lawrence, J.R.; Inniss, W.E.; Greer, C.W. Physiological adaptations involved in alkane assimilation at a low temperature by Rhodococcus sp. strain Q15. Appl. Environ. Microbiol. 1999, 65, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Labbe, D.; Schinner, F.; Greer, C.W.; Whyte, L.G. Characterization of hydrocarbon-degrading microbial populations in contaminated and pristine Alpine soils. Appl. Environ. Microbiol. 2003, 69, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Schinner, F. Efficiency of indigenous and inoculated cold-adapted soil microorganisms for biodegradation of diesel oil in alpine soils. Appl. Environ. Microbiol. 1997, 63, 2660–2664. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Schinner, F. Bioremediation (natural attenuation and biostimulation) of diesel-oil-contaminated soil in an alpine glacier skiing area. Appl. Environ. Microbiol. 2001, 67, 3127–3133. [Google Scholar] [CrossRef]
- Brakstad, O.G.; Bonaunet, K. Biodegradation of petroleum hydrocarbons in seawater at low temperatures (0-5 degrees C) and bacterial communities associated with degradation. Biodegradation 2006, 17, 71–82. [Google Scholar] [CrossRef]
- Shintani, M.; Sugiyama, K.; Sakurai, T.; Yamada, K.; Kimbara, K. Biodegradation of A-fuel oil in soil samples with bacterial mixtures of Rhodococcus and Gordonia strains under low temperature conditions. J. Biosci. Bioeng. 2019, 127, 197–200. [Google Scholar] [CrossRef]
- Semrany, S.; Favier, L.; Djelal, H.; Taha, S.; Amrane, A. Bioaugmentation: Possible solution in the treatment of bio-refractory organic compounds (Bio-ROCs). Biochem. Eng. J. 2012, 69, 75–86. [Google Scholar] [CrossRef]
- Shintani, M.; Matsui, K.; Takemura, T.; Yamane, H.; Nojiri, H. Behavior of the IncP-7 carbazole-degradative plasmid pCAR1 in artificial environmental samples. Appl. Microbiol. Biotechnol. 2008, 80, 485–497. [Google Scholar] [CrossRef]
- Fenlon, K.A.; Jones, K.C.; Semple, K.T. The effect of soil:water ratios on the induction of isoproturon, cypermethrin and diazinon mineralisation. Chemosphere 2011, 82, 163–168. [Google Scholar] [CrossRef]
- Chang, J.I.; Tsai, J.J.; Wu, K.H. Thermophilic composting of food waste. Bioresour. Technol. 2006, 97, 116–122. [Google Scholar] [CrossRef]
- Kalamdhad, A.S.; Singh, Y.K.; Ali, M.; Khwairakpam, M.; Kazmi, A.A. Rotary drum composting of vegetable waste and tree leaves. Bioresour. Technol. 2009, 100, 6442–6450. [Google Scholar] [CrossRef] [PubMed]
- Alkoaik, F.N. Integrating aeration and rotation processes to accelerate composting of agricultural residues. PLoS ONE 2019, 14, e0220343. [Google Scholar] [CrossRef] [PubMed]
- Robles-González, I.V.; Fava, F.; Poggi-Varaldo, H.M. A review on slurry bioreactors for bioremediation of soils and sediments. Microb. Cell Fact. 2008, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Kimbara, K.; Hashimoto, T.; Fukuda, M.; Koana, T.; Takagi, M.; Oishi, M.; Yano, K. Cloning and sequencing of two tandem genes involved in degradation of 2,3-dihydroxybiphenyl to benzoic acid in the polychlorinated biphenyl-degrading soil bacterium Pseudomonas sp. strain KKS102. J. Bacteriol. 1989, 171, 2740–2747. [Google Scholar] [CrossRef] [PubMed]
- United States. Division of Soil Survey. In Soil Survey Manual, 4th ed.; United States Department of Agriculture: Washington, DC, USA, 2017. [Google Scholar]
- Sambrook, J.; Russell, D. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Ma, X.-K.; Ding, N.; Peterson, E.C. Bioaugmentation of soil contaminated with high-level crude oil through inoculation with mixed cultures including Acremonium sp. Biodegradation 2015, 26, 259–269. [Google Scholar] [CrossRef]
- Liu, P.W.G.; Whang, L.M.; Chang, T.C.; Tseng, I.C.; Pan, P.T.; Cheng, S.S. Verification of necessity for bioaugmentation—lessons from two batch case studies for bioremediation of diesel-contaminated soils. J. Chem. Technol. Biotechnol. 2009, 84, 808–819. [Google Scholar] [CrossRef]
- Lin, T.-C.; Pan, P.-T.; Young, C.-C.; Chang, J.-S.; Chang, T.-C.; Cheng, S.-S. Evaluation of the optimal strategy for ex situ bioremediation of diesel oil-contaminated soil. Environ. Sci. Pollut. Res. 2011, 18, 1487–1496. [Google Scholar] [CrossRef]
- Varjani, S.; Upasani, V.N. Influence of abiotic factors, natural attenuation, bioaugmentation and nutrient supplementation on bioremediation of petroleum crude contaminated agricultural soil. J. Environ. Manag. 2019, 245, 358–366. [Google Scholar] [CrossRef]
- Bidja Abena, M.T.; Li, T.; Shah, M.N.; Zhong, W. Biodegradation of total petroleum hydrocarbons (TPH) in highly contaminated soils by natural attenuation and bioaugmentation. Chemosphere 2019, 234, 864–874. [Google Scholar] [CrossRef]
- Al-Mailem, D.M.; Kansour, M.K.; Radwan, S.S. Cross-bioaugmentation among four remote soil samples contaminated with oil exerted just inconsistent effects on oil-bioremediation. Front. Microbiol. 2019, 10, 2827. [Google Scholar] [CrossRef]
- Pino-Herrera, D.O.; Pechaud, Y.; Huguenot, D.; Esposito, G.; van Hullebusch, E.D.; Oturan, M.A. Removal mechanisms in aerobic slurry bioreactors for remediation of soils and sediments polluted with hydrophobic organic compounds: An overview. J. Hazard. Mater. 2017, 339, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.; Viraraghavan, T.; Srinivasan, A. Biological treatment processes for fish processing wastewater—A review. Bioresour. Technol. 2010, 101, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Maeng, J.H.; Sakai, Y.; Tani, Y.; Kato, N. Isolation and characterization of a novel oxygenase that catalyzes the first step of n-alkane oxidation in Acinetobacter sp. strain M-1. J. Bacteriol. 1996, 178, 3695–3700. [Google Scholar] [CrossRef] [PubMed]
- Gibu, N.; Kasai, D.; Ikawa, T.; Akiyama, E.; Fukuda, M. Characterization and transcriptional regulation of n-alkane hydroxylase gene cluster of Rhodococcus jostii RHA1. Microorganisms 2019, 7, 479. [Google Scholar] [CrossRef]



| Numbers of Carbon | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | |
| Concentration in 2500-mg A-fuel oil (mg) | 27 | 67 | 76 | 134 | 231 | 330 | 341 | 293 | 258 | 209 | 172 | 131 | 90 | 63 | 38 | 25 | 17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyoshi, Y.; Okada, J.; Urata, T.; Shintani, M.; Kimbara, K. A Rotational Slurry Bioreactor Accelerates Biodegradation of A-Fuel in Oil-Contaminated Soil Even under Low Temperature Conditions. Microorganisms 2020, 8, 291. https://doi.org/10.3390/microorganisms8020291
Miyoshi Y, Okada J, Urata T, Shintani M, Kimbara K. A Rotational Slurry Bioreactor Accelerates Biodegradation of A-Fuel in Oil-Contaminated Soil Even under Low Temperature Conditions. Microorganisms. 2020; 8(2):291. https://doi.org/10.3390/microorganisms8020291
Chicago/Turabian StyleMiyoshi, Yuna, Jo Okada, Tomotaka Urata, Masaki Shintani, and Kazuhide Kimbara. 2020. "A Rotational Slurry Bioreactor Accelerates Biodegradation of A-Fuel in Oil-Contaminated Soil Even under Low Temperature Conditions" Microorganisms 8, no. 2: 291. https://doi.org/10.3390/microorganisms8020291
APA StyleMiyoshi, Y., Okada, J., Urata, T., Shintani, M., & Kimbara, K. (2020). A Rotational Slurry Bioreactor Accelerates Biodegradation of A-Fuel in Oil-Contaminated Soil Even under Low Temperature Conditions. Microorganisms, 8(2), 291. https://doi.org/10.3390/microorganisms8020291





