Digestive Ability, Physiological Characteristics, and Rumen Bacterial Community of Holstein Finishing Steers in Response to Three Nutrient Density Diets as Fattening Phases Advanced
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experiment Design and Sample Collection
2.3. DNA Extraction and Volatile Fatty Acids (VFA) Determination
2.4. PCR Amplification, Sequencing, and Analysis
2.5. Chemical Analysis and Apparent Digestibility Calculation
2.6. Statistical Analysis
3. Results
3.1. Temperature-Humidity Index, Nutrient Intake, and Apparent Digestibility
3.2. Plasma Metabolic Parameters
3.3. Rumen Fermentation Characteristics
3.4. Rumen Bacterial Community
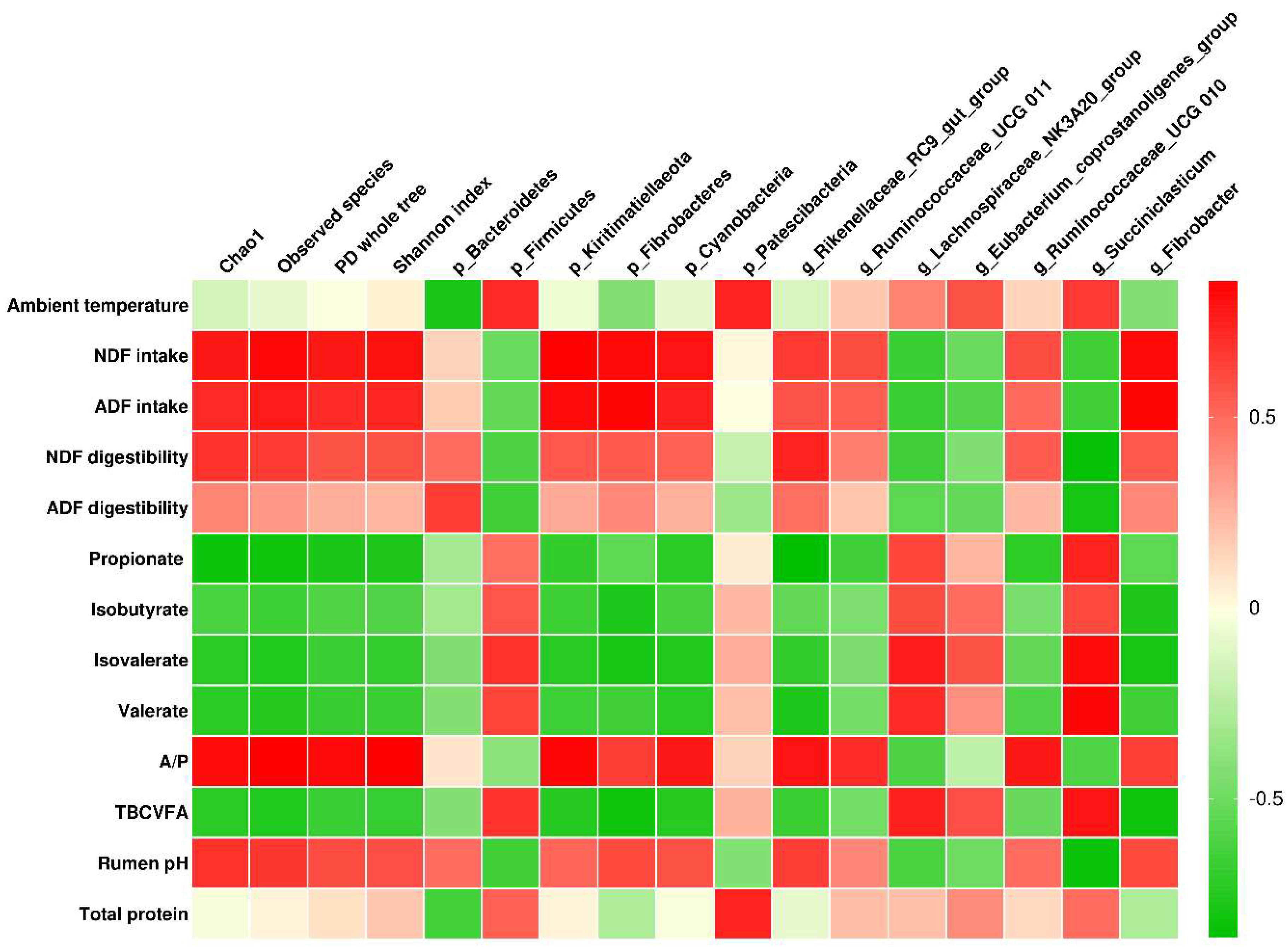
3.5. Correlation Analysis
4. Discussion
4.1. Digestive and Physiological Characteristics
4.2. Rumen Bacterial Community Profiles
4.3. Correlations between Ambient Temperature, Physiological and Digestive Characteristics, and Ruminal Bacterial Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McGovern, E.; Kenny, D.A.; McCabe, M.S.; Fitzsimons, C.; McGee, M.; Kelly, A.K.; Waters, S.M. 16S rRNA sequencing reveals relationship between potent cellulolytic genera and feed efficiency in the rumen of bulls. Front. Microbiol. 2018, 9, 1842. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, M.; Xue, C.; Zhu, W.; Mao, S. Characterization and comparison of the temporal dynamics of ruminal bacterial microbiota colonizing rice straw and alfalfa hay within ruminants. J. Dairy Sci. 2016, 99, 9668–9681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, S.C.; Purvis, H.T.; Najar, F.Z.; Sukharnikov, L.O.; Krehbiel, C.R.; Nagaraja, T.G.; Roe, B.A.; DeSilva, U. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl. Environ. Microbiol. 2010, 76, 7482–7490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weimer, P.J.; Cox, M.S.; de Paula, T.V.; Lin, M.; Hall, M.B.; Suen, G. Transient changes in milk production efficiency and bacterial community composition resulting from near-total exchange of ruminal contents between high- and low-efficiency Holstein cows. J. Dairy Sci. 2017, 100, 7165–7182. [Google Scholar] [CrossRef]
- Jami, E.; Israel, A.; Kotser, A.; Mizrahi, I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wu, H.; Liu, S.J.; Chai, S.T.; Meng, Q.X.; Zhou, Z.M. Dynamic alterations in yak rumen bacteria community and metabolome characteristics in response to feed type. Front. Microbiol. 2019, 10, 1116. [Google Scholar] [CrossRef] [Green Version]
- Plaizier, J.C.; Li, S.C.; Danscher, A.M.; Derakshani, H.; Andersen, P.H.; Khafipour, E. Changes in microbiota in rumen digesta and feces due to a grain-based subacute ruminal acidosis (SARA) challenge. Microb. Ecol. 2017, 74, 485–495. [Google Scholar] [CrossRef]
- Qiu, Q.; Gao, C.; Gao, Z.; Muhammad Aziz ur, R.; He, Y.; Cao, B.; Su, H. Temporal dynamics in rumen bacterial community composition of finishing steers during an adaptation period of three months. Microorganisms 2019, 7, 410. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Q.; Zhu, Y.; Qiu, X.; Gao, C.; Wang, J.; Wang, H.; He, Y.; Muhammad Aziz ur, R.; Cao, B.; Su, H. Dynamic variations in fecal bacterial community and fermentation profile of Holstein steers in response to three stepwise density diets. Animals 2019, 9, 560. [Google Scholar] [CrossRef] [Green Version]
- Resende, J.A.; Godon, J.J.; Bonnafous, A.; Arcuri, P.B.; Silva, V.L.; Otenio, M.H.; Diniz, C.G. Seasonal variation on microbial community and methane production during anaerobic digestion of cattle manure in Brazil. Microb. Ecol. 2016, 71, 735–746. [Google Scholar] [CrossRef]
- Yadav, B.; Singh, G.; Wankar, A.; Dutta, N.; Chaturvedi, V.B.; Verma, M.R. Effect of simulated heat stress on digestibility, methane emission and metabolic adaptability in crossbred cattle. Asian-Australas. J. Anim. Sci. 2016, 29, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.J.; He, Y.; Xia, C.Q.; Rahman, M.A.U.; Qiu, Q.H.; Shao, T.Q.; Liang, Y.X.; Ji, L.B.; Wang, H.B.; Cao, B.H. Effects of replacing Leymus chinensis with whole-crop wheat hay on Holstein bull apparent digestibility, plasma parameters, rumen fermentation, and microbiota. Sci. Rep. 2017, 7, 2114. [Google Scholar] [CrossRef] [PubMed]
- NASEM. Nutrient Requirements of Beef Cattle, 8th ed.; The National Academies Press: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Paz, H.A.; Anderson, C.L.; Muller, M.J.; Kononoff, P.J.; Fernando, S.C. Rumen bacterial community composition in Holstein and Jersey cows is different under same dietary condition and is not affected by sampling method. Front. Microbiol. 2016, 7, 1206. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and invitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Arlington, VA, USA, 2000. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Vitali, A.; Segnalini, M.; Bertocchi, L.; Bernabucci, U.; Nardone, A.; Lacetera, N. Seasonal pattern of mortality and relationships between mortality and temperature-humidity index in dairy cows. J. Dairy Sci. 2009, 92, 3781–3790. [Google Scholar] [CrossRef] [Green Version]
- Walsh, K.; O’Kiely, P.; Taweel, H.Z.; McGee, M.; Moloney, A.P.; Boland, T.M. Intake, digestibility and rumen characteristics in cattle offered whole-crop wheat or barley silages of contrasting grain to straw ratios. Anim. Feed Sci. Technol. 2009, 148, 192–213. [Google Scholar] [CrossRef]
- Menezes, A.C.B.; Valadares, S.C.; Silva, L.; Pacheco, M.V.C.; Pereira, J.M.V.; Rotta, P.P.; Zanetti, D.; Detmann, E.; Silva, F.A.S.; Godoi, L.A.; et al. Does a reduction in dietary crude protein content affect performance nutrient requirements, nitrogen losses, and methane emissions in finishing Nellore bulls? Agric. Ecosyst. Environ. 2016, 223, 239–249. [Google Scholar] [CrossRef]
- Tjardes, K.E.; Buskirk, D.D.; Allen, M.S.; Tempelman, R.J.; Bourquin, L.D.; Rust, S.R. Neutral detergent fiber concentration in corn silage influences dry matter intake, diet digestibility, and performance of Angus and Holstein steers. J. Anim. Sci. 2002, 80, 841–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohri, M.; Sharifi, K.; Eidi, S. Hematology and serum biochemistry of Holstein dairy calves: Age related changes and comparison with blood composition in adults. Res. Vet. Sci. 2007, 83, 30–39. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yu, Z.T.; Qiu, Q.H.; Shao, T.Q.; Niu, W.J.; Xia, C.Q.; Wang, H.B.; Su, H.W.; Cao, B.H. Effects of dietary protein levels and calcium salts of long-chain fatty acids on nitrogen mobilization, rumen microbiota and plasma fatty acid composition in Holstein bulls. Anim. Feed Sci. Technol. 2018, 246, 1–10. [Google Scholar] [CrossRef]
- Pereira, D.H.; Pereira, O.G.; da Silva, B.C.; Leao, M.I.; Valadares, S.D.; Chizzotti, F.H.M.; Garcia, R. Intake and total and partial digestibility of nutrients, ruminal pH and ammonia concentration and microbial efficiency in beef cattle fed with diets containing sorghum (Sorghum bicolor (L.) Moench) silage and concentrate in different ratios. Livest. Sci. 2007, 107, 53–61. [Google Scholar] [CrossRef]
- Maekawa, M.; Beauchemin, K.A.; Christensen, D.A. Effect of concentrate level and feeding management on chewing activities, saliva production, and ruminal pH of lactating dairy cows. J. Dairy Sci. 2002, 85, 1165–1175. [Google Scholar] [CrossRef]
- Xia, C.Q.; Muhammad, A.U.R.; Niu, W.J.; Shao, T.Q.; Qiu, Q.H.; Su, H.W.; Cao, B.H. Effects of dietary forage to concentrate ratio and wildrye length on nutrient intake, digestibility, plasma metabolites, ruminal fermentation and fecal microflora of male Chinese Holstein calves. J. Integr. Agric. 2018, 17, 415–427. [Google Scholar] [CrossRef]
- Yang, C.M.J. Response of forage fiber degradation by ruminal microorganisms to branched-chain volatile fatty acids, amino acids, and dipeptides. J. Dairy Sci. 2002, 85, 1183–1190. [Google Scholar] [CrossRef]
- Owens, D.; McGee, M.; Boland, T.; O’Kiely, P. Intake, rumen fermentation and nutrient flow to the omasum in beef cattle fed grass silage fortified with sucrose and/or supplemented with concentrate. Anim. Feed Sci. Technol. 2008, 144, 23–43. [Google Scholar] [CrossRef]
- Tucker, C.M.; Cadotte, M.W.; Carvalho, S.B.; Davies, T.J.; Ferrier, S.; Fritz, S.A.; Grenyer, R.; Helmus, M.R.; Jin, L.S.; Mooers, A.O.; et al. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biol. Rev. 2017, 92, 698–715. [Google Scholar] [CrossRef]
- Hook, S.E.; Steele, M.A.; Northwood, K.S.; Dijkstra, J.; France, J.; Wright, A.D.G.; McBride, B.W. Impact of subacute ruminal acidosis (SARA) adaptation and recovery on the density and diversity of bacteria in the rumen of dairy cows. FEMS Microbiol. Ecol. 2011, 78, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; He, Y.; Li, H.; Wu, F.; Qiu, Q.; Niu, W.; Gao, Z.; Su, H.; Cao, B. Rumen fermentation, intramuscular fat fatty acid profiles and related rumen bacterial populations of Holstein bulls fed diets with different energy levels. Appl. Microbiol. Biotechnol. 2019, 103, 4931–4942. [Google Scholar] [CrossRef] [PubMed]
- Spring, S.; Bunk, B.; Sproer, C.; Schumann, P.; Rohde, M.; Tindall, B.J.; Klenk, H.P. Characterization of the first cultured representative of Verrucomicrobia subdivision 5 indicates the proposal of a novel phylum. ISME J. 2016, 10, 2801–2816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purushe, J.; Fouts, D.E.; Morrison, M.; White, B.A.; Mackie, R.I.; Coutinho, P.M.; Henrissat, B.; Nelson, K.E. Comparative genome analysis of prevotella ruminicola and prevotella bryantii: Insights into their environmental niche. Microb. Ecol. 2010, 60, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Pitta, D.W.; Pinchak, W.E.; Dowd, S.E.; Osterstock, J.; Gontcharova, V.; Youn, E.; Dorton, K.; Yoon, I.; Min, B.R.; Fulford, J.D.; et al. Rumen bacterial diversity dynamics associated with changing from bermudagrass hay to grazed winter wheat diets. Microb. Ecol. 2010, 59, 511–522. [Google Scholar] [CrossRef]
- Herrmann, M.; Wegner, C.E.; Taubert, M.; Geesink, P.; Lehmann, K.; Yan, L.J.; Lehmann, R.; Totsche, K.U.; Kusel, K. Predominance of Cand. Patescibacteria in groundwater is caused by their preferential mobilization from soils and flourishing under oligotrophic conditions. Front. Microbiol. 2019, 10, 1407. [Google Scholar] [CrossRef] [Green Version]
- Rauprich, A.B.E.; Hammon, H.M.; Blum, J.W. Effects of feeding colostrum and a formula with nutrient contents as colostrum on metabolic and endocrine traits in neonatal calves. Biol. Neonate 2000, 78, 53–64. [Google Scholar] [CrossRef]
- Reis, W.L.S.; Detmann, E.; Batista, E.D.; Rufino, L.M.A.; Gomes, D.I.; Bento, C.B.P.; Mantovani, H.C.; Valadares, S.C. Effects of ruminal and post-ruminal protein supplementation in cattle fed tropical forages on insoluble fiber degradation, activity of fibrolytic enzymes, and the ruminal microbial community profile. Anim. Feed Sci. Technol. 2016, 218, 1–16. [Google Scholar] [CrossRef]
- Vangylswyk, N.O. Succiniclasticum Ruminis Gen-Nov, Sp-Nov, a ruminal bacterium converting succinate to propionate as the sole energy-yielding mechanism. Int. J. Syst. Bacteriol. 1995, 45, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Bainbridge, M.L.; Saldinger, L.K.; Barlow, J.W.; Alvez, J.P.; Roman, J.; Kraft, J. Alteration of rumen bacteria and protozoa through grazing regime as a tool to enhance the bioactive fatty acid content of bovine milk. Front. Microbiol. 2018, 9, 904. [Google Scholar] [CrossRef]
| Item 1 | Phase 1 | Phase 2 | Phase 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| H | C | L | H | C | L | H | C | L | |
| Ingredients, g/kg of DM | |||||||||
| Corn grain | 529 | 414 | 299 | 588 | 468 | 348 | 620 | 501 | 382 |
| Wheat grain | 73.2 | 57.3 | 41.4 | 71.7 | 57.1 | 42.5 | 87.8 | 70.9 | 54.0 |
| Soybean meal | 94.1 | 73.7 | 53.2 | 93.7 | 74.6 | 55.5 | 89.7 | 72.5 | 55.3 |
| Leymus chinensis | 282 | 438 | 594 | 216 | 376 | 536 | 169 | 329 | 489 |
| Limestone | 7.20 | 5.60 | 4.10 | 7.80 | 6.20 | 4.60 | 8.30 | 6.70 | 5.10 |
| NaHCO3 | 0.00 | 0.00 | 0.00 | 7.80 | 6.20 | 4.60 | 8.30 | 6.70 | 5.10 |
| NaCl | 7.10 | 5.60 | 4.00 | 7.80 | 6.20 | 4.60 | 8.30 | 6.70 | 5.10 |
| Premix 2 | 7.20 | 5.60 | 4.10 | 7.80 | 6.20 | 4.60 | 8.30 | 6.70 | 5.10 |
| Nutrient composition 3, g/kg of DM | |||||||||
| ME, Mcal/kg | 2.71 | 2.53 | 2.35 | 2.77 | 2.58 | 2.40 | 2.82 | 2.64 | 2.46 |
| MP, g/kg | 82.9 | 73.9 | 64.8 | 83.9 | 75.2 | 66.4 | 84.3 | 76.0 | 67.7 |
| ME/MP, Mcal/g | 0.033 | 0.034 | 0.036 | 0.033 | 0.034 | 0.036 | 0.034 | 0.035 | 0.036 |
| NDF | 277 | 371 | 464 | 236 | 332 | 428 | 208 | 304 | 400 |
| ADF | 146 | 202 | 258 | 121 | 179 | 237 | 104 | 162 | 219 |
| Ether extract | 34.6 | 32.5 | 30.5 | 35.6 | 33.4 | 31.3 | 36.1 | 34.0 | 31.9 |
| Main fatty acid profile 4 (mg/g feed, DM) | |||||||||
| C16:0 | 3.87 | 3.53 | 3.19 | 3.33 | 3.12 | 2.92 | 4.06 | 3.73 | 3.39 |
| C18:1n9c | 3.64 | 2.92 | 2.21 | 3.25 | 2.66 | 2.07 | 4.25 | 3.50 | 2.75 |
| C18:2n6 | 4.27 | 3.47 | 2.67 | 3.37 | 2.80 | 2.24 | 3.55 | 2.98 | 2.41 |
| SFA | 6.01 | 5.61 | 5.20 | 4.85 | 4.71 | 4.56 | 5.88 | 5.55 | 5.21 |
| UFA | 8.75 | 7.53 | 6.31 | 7.33 | 6.48 | 5.62 | 8.48 | 7.46 | 6.43 |
| Item 1 | Phase 1 | Phase 2 | Phase 3 | SEM 2 | p-Value 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | C | L | H | C | L | H | C | L | Phase | Diet | P × D | ||
| Nutrient intake, kg/d | |||||||||||||
| Dry matter | 10.7 | 11.3 | 10.6 | 13.5 | 13.4 | 12.9 | 11.9 | 12.5 | 12.8 | 0.41 | <0.001 | 0.491 | 0.401 |
| OM | 10.2 | 10.7 | 10.0 | 12.4 | 12.4 | 12.0 | 11.3 | 11.8 | 12.1 | 0.39 | <0.001 | 0.489 | 0.445 |
| Crude protein | 1.41 | 1.33 | 1.10 | 1.69 | 1.47 | 1.21 | 1.41 | 1.30 | 1.16 | 0.05 | <0.001 | <0.001 | 0.168 |
| Ether extract | 0.40 | 0.39 | 0.34 | 0.51 | 0.47 | 0.41 | 0.45 | 0.44 | 0.42 | 0.02 | <0.001 | <0.001 | 0.448 |
| NDF | 2.92 | 4.11 | 4.79 | 3.03 | 4.28 | 5.29 | 2.16 | 3.31 | 4.44 | 0.11 | <0.001 | <0.001 | 0.231 |
| ADF | 1.50 | 2.20 | 2.63 | 1.53 | 2.28 | 2.90 | 1.00 | 1.63 | 2.25 | 0.06 | <0.001 | <0.001 | 0.242 |
| Apparent digestibility of nutrient, % | |||||||||||||
| Dry matter | 69.8 | 68.6 | 68.2 | 71.0 | 65.1 | 66.5 | 78.9 | 75.5 | 74.1 | 1.42 | <0.001 | 0.004 | 0.477 |
| OM | 71.4 | 70.8 | 70.5 | 72.4 | 66.8 | 68.7 | 80.5 | 77.5 | 76.5 | 1.45 | <0.001 | 0.019 | 0.469 |
| Crude protein | 71.5 | 67.8 | 67.9 | 70.4 | 62.6 | 61.6 | 76.7 | 72.2 | 69.5 | 1.79 | <0.001 | <0.001 | 0.574 |
| Ether extract | 71.8 bcd | 69.9 cd | 64.0 d | 69.7 cd | 66.4 d | 71.5 cd | 84.9 a | 79.7 ab | 75.5 bc | 1.73 | <0.001 | 0.003 | 0.006 |
| NDF | 64.7 | 68.7 | 74.3 | 55.0 | 60.8 | 67.5 | 59.4 | 64.2 | 66.3 | 1.65 | <0.001 | <0.001 | 0.486 |
| ADF | 65.2 bc | 68.2 ab | 73.6 a | 55.2 e | 58.1 de | 62.4 bcd | 56.0 de | 61.0 cde | 57.3 de | 1.46 | <0.001 | <0.001 | 0.023 |
| Item 1 | Phase 1 | Phase 2 | Phase 3 | SEM 2 | p – Value 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | C | L | H | C | L | H | C | L | Phase | Diet | P × D | ||
| Albumin, g/L | 34.03 | 33.70 | 32.89 | 33.70 | 34.62 | 33.98 | 35.85 | 34.59 | 35.26 | 0.44 | 0.001 | 0.476 | 0.139 |
| ALT, U/L | 20.6 | 20.8 | 24.1 | 20.2 | 24.4 | 25.8 | 23.5 | 19.7 | 25.7 | 1.19 | 0.232 | 0.005 | 0.091 |
| AST, U/L | 47.2 | 45.8 | 41.9 | 50.1 | 53.0 | 47.0 | 46.6 | 48.6 | 47.6 | 3.24 | 0.093 | 0.206 | 0.253 |
| AST/ALT | 2.36 | 2.24 | 1.68 | 2.49 | 2.21 | 1.81 | 2.16 | 2.55 | 1.86 | 0.21 | 0.621 | 0.075 | 0.208 |
| Cholesterol, mmol/L | 2.75 | 2.84 | 2.46 | 2.89 | 3.18 | 2.75 | 2.28 | 2.38 | 2.48 | 0.12 | <0.001 | 0.211 | 0.072 |
| Glucose, mmol/L | 4.27 a | 4.13 ab | 3.95 abc | 3.74 bcd | 3.78 bcd | 3.49 d | 3.63 cd | 3.50 d | 3.86 bcd | 0.09 | <0.001 | 0.369 | 0.007 |
| Total protein, g/L | 58.6 | 56.2 | 53.7 | 58.7 | 61.4 | 60.2 | 62.5 | 65.8 | 68.0 | 1.77 | <0.001 | 0.701 | 0.116 |
| Triglyceride, mmol/L | 0.20 b | 0.22 ab | 0.29 a | 0.21 ab | 0.24 ab | 0.24 ab | 0.24 ab | 0.23 ab | 0.24 ab | 0.02 | 0.663 | 0.002 | 0.018 |
| Urea N, mmol/L | 4.07 a | 3.52 abc | 2.27 d | 3.77 ab | 3.61 abc | 2.59 d | 2.82 cd | 3.04 bcd | 2.78 cd | 0.18 | 0.008 | <0.001 | 0.001 |
| Item 1 | Phase 1 | Phase 2 | Phase 3 | SEM 2 | p – Value 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | C | L | H | C | L | H | C | L | Phase | Diet | P × D | ||
| NH3-N, mg/dL | 4.41 | 3.66 | 2.56 | 4.65 | 4.23 | 3.04 | 5.01 | 4.31 | 3.16 | 1.156 | 0.679 | 0.313 | 0.998 |
| Rumen pH | 6.56 abc | 6.65 ab | 6.78 a | 6.26 de | 6.33 cde | 6.71 ab | 5.86 f | 6.25 e | 6.52 bcd | 0.054 | <0.001 | <0.001 | 0.002 |
| Acetate, mM | 39.1 | 46.5 | 48.3 | 46.8 | 53.2 | 56.5 | 47.0 | 50.8 | 56.1 | 1.570 | <0.001 | <0.001 | 0.620 |
| Propionate, mM | 13.5 b | 12.0 b | 10.3 b | 18.8 a | 13.7 b | 12.1 b | 21.0 a | 13.8 b | 10.7 b | 0.738 | <0.001 | <0.001 | <0.001 |
| Isobutyrate, mM | 0.64 bc | 0.65 bc | 0.52 d | 0.62 cd | 0.66 bc | 0.52 d | 0.81 a | 0.74 ab | 0.57 cd | 0.022 | <0.001 | <0.001 | 0.013 |
| Butyrate, mM | 7.23 cde | 6.62 de | 6.29 e | 9.03 abc | 10.0 a | 8.20 abcd | 9.67 ab | 8.90 abc | 7.79 bcde | 0.401 | <0.001 | 0.035 | 0.030 |
| Isovalerate, mM | 0.92 cde | 0.88 cde | 0.60 e | 1.16 c | 1.09 cd | 0.62 e | 2.17 a | 1.67 b | 0.78 de | 0.076 | <0.001 | <0.001 | <0.001 |
| Valerate, mM | 0.78 cde | 0.68 ef | 0.58 f | 0.98 b | 0.85 bcd | 0.70 def | 1.40 a | 0.90 bc | 0.66 ef | 0.033 | <0.001 | <0.001 | <0.001 |
| TVFA, mM | 62.2 b | 67.3 b | 66.7 b | 77.4 a | 79.6 a | 78.6 a | 82.1 a | 76.8 a | 76.5 a | 1.861 | <0.001 | 0.937 | 0.008 |
| A/P | 2.98 de | 3.92 bc | 4.71 ab | 2.58 e | 3.93 bc | 4.67 ab | 2.41 e | 3.81 cd | 5.38 a | 0.171 | 0.380 | <0.001 | 0.007 |
| TBCVFA, mM | 1.56 cde | 1.53 cdef | 1.12 f | 1.78 c | 1.74 cd | 1.14 ef | 2.98 a | 2.41 b | 1.35 def | 0.087 | <0.001 | <0.001 | <0.001 |
| Item 1 | Phase 1 | Phase 2 | Phase 3 | SEM 2 | p – Value 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | C | L | H | C | L | H | C | L | Phase | Diet | P × D | ||
| Chao1 | 1852 | 2221 | 2407 | 1685 | 2116 | 2288 | 1601 | 1970 | 2425 | 90.78 | 0.053 | <0.001 | 0.265 |
| Observed species | 1195 | 1440 | 1586 | 1112 | 1428 | 1567 | 1049 | 1305 | 1660 | 53.80 | 0.179 | <0.001 | 0.064 |
| PD whole tree | 100.0 | 117.4 | 124.8 | 100.1 | 117.6 | 123.1 | 96.25 | 110.6 | 132.5 | 3.654 | 0.929 | <0.001 | 0.093 |
| Shannon index | 7.456 d | 8.084 bc | 8.323 abc | 7.385 d | 8.167 bc | 8.445 ab | 7.364 d | 7.858 cd | 8.718 a | 0.113 | 0.855 | <0.001 | 0.029 |
| Phylum Name 1 | Phase 1 | Phase 2 | Phase 3 | SEM 2 | p - Value 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | C | L | H | C | L | H | C | L | Phase | Diet | P × D | ||
| Bacteroidetes | 72.15 | 66.52 | 67.66 | 61.21 | 61.14 | 63.55 | 56.70 | 58.49 | 58.82 | 1.73 | <0.001 | 0.482 | 0.269 |
| Firmicutes | 21.48 | 25.08 | 21.07 | 30.15 | 26.64 | 21.36 | 37.18 | 33.62 | 29.25 | 1.60 | <0.001 | 0.001 | 0.135 |
| Proteobacteria | 1.75 | 2.58 | 2.46 | 2.40 | 4.45 | 3.62 | 1.24 | 2.14 | 3.00 | 0.54 | 0.022 | 0.008 | 0.579 |
| Kiritimatiellaeota | 0.48 | 1.17 | 1.90 | 0.49 | 1.30 | 3.49 | 0.46 | 0.79 | 2.12 | 0.32 | 0.096 | <0.001 | 0.187 |
| Fibrobacteres | 0.86 | 1.12 | 2.39 | 1.04 | 1.51 | 3.01 | 0.38 | 0.45 | 1.27 | 0.20 | <0.001 | <0.001 | 0.122 |
| Spirochaetes | 0.64 | 0.85 | 1.07 | 0.79 | 0.96 | 1.27 | 0.80 | 0.66 | 0.90 | 0.12 | 0.101 | 0.037 | 0.431 |
| Cyanobacteria | 0.49 | 0.58 | 1.33 | 0.49 | 0.85 | 1.24 | 0.23 | 0.52 | 1.47 | 0.15 | 0.604 | <0.001 | 0.362 |
| Patescibacteria | 0.38 | 0.58 | 0.53 | 0.33 | 0.80 | 0.64 | 0.98 | 1.09 | 0.90 | 0.08 | <0.001 | 0.008 | 0.272 |
| Actinobacteria | 0.80 | 0.25 | 0.20 | 0.96 | 0.64 | 0.30 | 0.68 | 0.80 | 0.41 | 0.24 | 0.349 | 0.172 | 0.557 |
| Tenericutes | 0.41 | 0.66 | 0.59 | 0.44 | 0.66 | 0.52 | 0.28 | 0.42 | 0.72 | 0.08 | 0.426 | 0.013 | 0.078 |
| Genus Name 1 | Phase 1 | Phase 2 | Phase 3 | SEM 2 | p - Value 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | C | L | H | C | L | H | C | L | Phase | Diet | P × D | ||
| Prevotella | 38.93 | 31.53 | 29.48 | 31.20 | 30.57 | 28.56 | 28.20 | 25.78 | 22.53 | 2.49 | 0.005 | 0.042 | 0.651 |
| Uncultured rumen bacterium | 14.14 | 14.76 | 14.63 | 14.85 | 14.81 | 15.89 | 15.33 | 16.04 | 15.64 | 1.17 | 0.434 | 0.833 | 0.920 |
| Rikenellaceae RC9 gut group | 4.63 | 7.96 | 10.83 | 2.80 | 5.34 | 7.85 | 4.35 | 6.29 | 10.06 | 0.83 | 0.001 | <0.001 | 0.629 |
| Succiniclasticum | 3.60 | 2.96 | 1.74 | 6.68 | 4.25 | 2.75 | 8.74 | 5.98 | 3.94 | 0.48 | <0.001 | <0.001 | 0.111 |
| Prevotellaceae UCG-003 | 3.53 | 3.09 | 4.63 | 1.69 | 3.13 | 4.37 | 1.37 | 2.92 | 3.36 | 0.47 | 0.026 | 0.003 | 0.246 |
| Ruminococcaceae NK4A214 group | 1.54 | 2.15 | 1.37 | 2.58 | 1.91 | 1.61 | 3.64 | 3.11 | 2.27 | 0.23 | <0.001 | 0.003 | 0.069 |
| Prevotellaceae UCG-001 | 3.38 | 2.22 | 1.73 | 4.95 | 1.50 | 1.66 | 2.08 | 1.26 | 1.44 | 0.56 | 0.095 | 0.017 | 0.186 |
| Ruminococcus 2 | 2.16 | 1.71 | 0.79 | 2.50 | 1.70 | 0.72 | 1.89 | 2.38 | 0.85 | 0.36 | 0.771 | 0.003 | 0.412 |
| Fibrobacter | 0.86 | 1.12 | 2.38 | 1.04 | 1.51 | 3.01 | 0.38 | 0.45 | 1.26 | 0.20 | <0.001 | <0.001 | 0.117 |
| Christensenellaceae R-7_group | 0.82 | 1.27 | 1.01 | 0.82 | 1.00 | 1.18 | 1.71 | 1.76 | 1.81 | 0.16 | <0.001 | 0.247 | 0.538 |
| Ruminococcaceae UCG-011 | 0.56 | 1.28 | 1.17 | 0.60 | 1.10 | 1.51 | 0.41 | 1.55 | 1.90 | 0.19 | 0.109 | 0.001 | 0.099 |
| Lachnospiraceae NK3A20 group | 0.85 | 0.82 | 0.53 | 1.90 | 0.89 | 0.58 | 2.01 | 1.29 | 0.75 | 0.20 | 0.003 | 0.003 | 0.066 |
| Prevotellaceae UCG-004 | 1.36 | 1.22 | 1.01 | 0.69 | 1.05 | 1.27 | 0.47 | 0.74 | 0.90 | 0.14 | 0.009 | 0.104 | 0.112 |
| Succinivibrionaceae UCG-002 | 0.56 | 1.02 | 0.81 | 0.79 | 1.72 | 1.22 | 0.49 | 0.96 | 0.81 | 0.32 | 0.160 | 0.089 | 0.929 |
| Moryella | 0.99 | 0.87 | 0.38 | 0.91 | 1.30 | 0.55 | 1.18 | 1.24 | 0.85 | 0.15 | 0.054 | 0.003 | 0.484 |
| Ruminococcaceae UCG-014 | 0.61 | 0.90 | 0.93 | 0.92 | 1.18 | 0.56 | 0.93 | 1.12 | 0.88 | 0.14 | 0.302 | 0.087 | 0.103 |
| Eubacterium coprostanoligenes group | 0.72 | 0.80 | 0.62 | 0.96 | 0.69 | 0.55 | 1.32 | 1.17 | 1.00 | 0.09 | <0.001 | 0.025 | 0.440 |
| Veillonellaceae UCG-001 | 0.44 b | 0.66 b | 0.71 b | 0.61 b | 0.67 b | 0.76 b | 1.96 a | 1.01 ab | 0.96 b | 0.17 | 0.002 | 0.541 | 0.031 |
| Treponema 2 | 0.60 | 0.77 | 0.95 | 0.77 | 0.89 | 1.13 | 0.77 | 0.61 | 0.78 | 0.12 | 0.097 | 0.121 | 0.441 |
| Prevotellaceae NK3B31 group | 0.71 ab | 0.98 ab | 0.59 ab | 0.60 ab | 0.74 ab | 0.94 ab | 0.32 b | 0.70 ab | 1.27 a | 0.16 | 0.999 | 0.100 | 0.011 |
| Selenomonas 1 | 0.77 | 0.68 | 0.61 | 0.88 | 0.98 | 0.52 | 0.91 | 0.70 | 0.51 | 0.11 | 0.352 | 0.045 | 0.247 |
| Candidatus_Saccharimonas | 0.37 | 0.56 | 0.50 | 0.32 | 0.74 | 0.63 | 0.83 | 0.97 | 0.80 | 0.08 | <0.001 | 0.021 | 0.393 |
| Ruminococcus 1 | 0.46 | 0.66 | 0.69 | 0.74 | 0.69 | 0.66 | 0.36 | 0.45 | 0.72 | 0.08 | 0.031 | 0.063 | 0.072 |
| CAG-352 | 0.37 | 0.63 | 0.45 | 0.93 | 1.04 | 0.49 | 0.33 | 0.68 | 0.46 | 0.13 | 0.003 | 0.057 | 0.153 |
| Ruminococcaceae UCG-010 | 0.34 | 0.59 | 0.68 | 0.30 | 0.43 | 0.66 | 0.33 | 0.60 | 0.95 | 0.07 | 0.018 | <0.001 | 0.118 |
| Saccharofermentans | 0.56 abc | 0.68 ab | 0.60 abc | 0.37 cd | 0.40 cd | 0.41 bcd | 0.28 d | 0.56 abc | 0.70 a | 0.06 | 0.002 | 0.004 | 0.043 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Q.; Gao, C.; Aziz ur Rahman, M.; Cao, B.; Su, H. Digestive Ability, Physiological Characteristics, and Rumen Bacterial Community of Holstein Finishing Steers in Response to Three Nutrient Density Diets as Fattening Phases Advanced. Microorganisms 2020, 8, 335. https://doi.org/10.3390/microorganisms8030335
Qiu Q, Gao C, Aziz ur Rahman M, Cao B, Su H. Digestive Ability, Physiological Characteristics, and Rumen Bacterial Community of Holstein Finishing Steers in Response to Three Nutrient Density Diets as Fattening Phases Advanced. Microorganisms. 2020; 8(3):335. https://doi.org/10.3390/microorganisms8030335
Chicago/Turabian StyleQiu, Qinghua, Chaoyu Gao, Muhammad Aziz ur Rahman, Binghai Cao, and Huawei Su. 2020. "Digestive Ability, Physiological Characteristics, and Rumen Bacterial Community of Holstein Finishing Steers in Response to Three Nutrient Density Diets as Fattening Phases Advanced" Microorganisms 8, no. 3: 335. https://doi.org/10.3390/microorganisms8030335
APA StyleQiu, Q., Gao, C., Aziz ur Rahman, M., Cao, B., & Su, H. (2020). Digestive Ability, Physiological Characteristics, and Rumen Bacterial Community of Holstein Finishing Steers in Response to Three Nutrient Density Diets as Fattening Phases Advanced. Microorganisms, 8(3), 335. https://doi.org/10.3390/microorganisms8030335






