Long-Term Surveillance of Antibiotic Prescriptions and the Prevalence of Antimicrobial Resistance in Non-Fermenting Gram-Negative Bacilli
Abstract
:1. Introduction
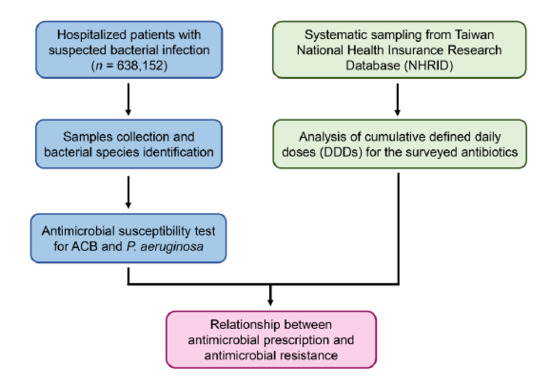
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Bacterial Cultures and Identifications
2.3. Antimicrobial Susceptibility Test
2.4. Data Source
2.5. Analysis of Antibiotic Prescription from the National Health Insurance Database (NHIRD)
2.6. Statistical Analysis
3. Results
3.1. Antimicrobial Susceptibility of ACB and P. aeruginosa
3.2. Relationship between Antimicrobial Prescription and Antimicrobial Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Edmond, M.B.; Pfaller, M.A.; Jones, R.N.; Wenzel, R.P.; Seifert, H. Nosocomial bloodstream infections caused by acinetobacter species in united states hospitals: Clinical features, molecular epidemiology, and antimicrobial susceptibility. Clin. Infect. Dis. 2000, 31, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Souli, M.; Galani, I.; Giamarellou, H. Emergence of extensively drug-resistant and pandrug-resistant gram-negative bacilli in europe. Eurosurveillance 2008, 13, 19045. [Google Scholar] [PubMed]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic pollution in the environment: From microbial ecology to public policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.Y.; Lauderdale, T.L.; Wang, J.T.; Chang, S.C. Carbapenem-resistant pseudomonas aeruginosa in Taiwan: Prevalence, risk factors, and impact on outcome of infections. J. Microbiol. Immunol. Infect. 2016, 49, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Maragakis, L.L.; Perl, T.M. Acinetobacter baumannii: Epidemiology, antimicrobial resistance, and treatment options. Clin. Infect. Dis. 2008, 46, 1254–1263. [Google Scholar] [CrossRef] [Green Version]
- Obritsch, M.D.; Fish, D.N.; MacLaren, R.; Jung, R. National surveillance of antimicrobial resistance in pseudomonas aeruginosa isolates obtained from intensive care unit patients from 1993 to 2002. Antimicrob. Agents Chemother. 2004, 48, 4606–4610. [Google Scholar] [CrossRef] [Green Version]
- Cornaglia, G.; Mazzariol, A.; Lauretti, L.; Rossolini, G.M.; Fontana, R. Hospital outbreak of carbapenem-resistant pseudomonas aeruginosa producing vim-1, a novel transferable metallo-beta-lactamase. Clin. Infect Dis. 2000, 31, 1119–1125. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.L.; Tang, C.H.; Hsu, Y.M.; Wan, L.; Chang, Y.F.; Lin, C.T.; Tseng, Y.R.; Lin, Y.J.; Sheu, J.J.; Lin, C.W.; et al. Nosocomial outbreak of infection with multidrug-resistant acinetobacter baumannii in a medical center in Taiwan. Infect. Control Hosp. Epidemiol. 2009, 30, 34–38. [Google Scholar] [CrossRef]
- Ho, C.M.; Ho, M.W.; Chi, C.Y.; Lin, C.D.; Lin, C.W.; Tseng, S.P.; Teng, L.J.; Chang, H.Y.; Chang, H.L.; Chang, Y.F.; et al. Repeated colonization by multi-drug-resistant acinetobacter calcoaceticus-a. Baumannii complex and changes in antimicrobial susceptibilities in surgical intensive care units. Surg. Infect. (Larchmt) 2013, 14, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olesen, S.W.; Barnett, M.L.; MacFadden, D.R.; Brownstein, J.S.; Hernandez-Diaz, S.; Lipsitch, M.; Grad, Y.H. The distribution of antibiotic use and its association with antibiotic resistance. Elife 2018, 7, e39435. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance 2014. Available online: https://www.who.int/drugresistance/documents/surveillancereport/en/ (accessed on 12 March 2020).
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 9 March 2020).
- Ho, J.; Tambyah, P.A.; Paterson, D.L. Multiresistant gram-negative infections: A global perspective. Curr. Opin. Infect. Dis. 2010, 23, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Liang, C.C.; Chang, R.; Kuo, C.M.; Hung, C.H.; Liao, T.N.; Liao, C.S. Detection and colonization of multidrug resistant organisms in a regional teaching hospital of Taiwan. Int. J. Environ. Res. Public Health 2019, 16, 1104. [Google Scholar] [CrossRef] [Green Version]
- Teo, J.Q.; Cai, Y.; Lim, T.P.; Tan, T.T.; Kwa, A.L. Carbapenem resistance in gram-negative bacteria: The not-so-little problem in the little red dot. Microorganisms 2016, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Chiang, T.L. Taiwan’s 1995 health care reform. Health Policy 1997, 39, 225–239. [Google Scholar] [CrossRef]
- Ho, M. Taiwan seeks to solve its resistance problems. Science 2001, 291, 2550–2551. [Google Scholar] [CrossRef]
- Ho, M.; Hsiung, C.A.; Yu, H.T.; Chi, C.L.; Chang, H.J. Changes before and after a policy to restrict antimicrobial usage in upper respiratory infections in Taiwan. Int. J. Antimicrob. Agents 2004, 23, 438–445. [Google Scholar] [CrossRef]
- Poon, S.K.; Lai, C.H.; Chang, C.S.; Lin, W.Y.; Chang, Y.C.; Wang, H.J.; Lin, P.H.; Lin, H.J.; Wang, W.C. Prevalence of antimicrobial resistance in helicobacter pylori isolates in Taiwan in relation to consumption of antimicrobial agents. Int. J. Antimicrob. Agents 2009, 34, 162–165. [Google Scholar] [CrossRef]
- Hsueh, P.R. Decreasing rates of resistance to penicillin, but not erythromycin, in streptococcus pneumoniae after introduction of a policy to restrict antibiotic usage in Taiwan. Clin. Microbiol. Infect. 2005, 11, 925–927. [Google Scholar] [CrossRef] [Green Version]
- Ho, M.; Hsiung, C.A.; Yu, H.T.; Chi, C.L.; Yin, H.C.; Chang, H.J. Antimicrobial usage in ambulatory patients with respiratory infections in Taiwan, 2001. J. Formos Med. Assoc. 2004, 103, 96–103. [Google Scholar] [PubMed]
- Tsai, C.T.; Chi, C.Y.; Ho, C.M.; Lin, P.C.; Chou, C.H.; Wang, J.H.; Wang, J.H.; Lin, H.C.; Tien, N.; Lin, K.H.; et al. Correlation of virulence genes to clinical manifestations and outcome in patients with streptococcus dysgalactiae subspecies equisimilis bacteremia. J. Microbiol. Immunol. Infect. 2014, 47, 462–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, M100-S28. 2018. Available online: http://file.qums.ac.ir/repository/mmrc/CLSI-2018-M100-S28.pdf (accessed on 12 March 2020).
- Falagas, M.; Koletsi, P.; Bliziotis, I. The diversity of definitions of multidrug-resistant (mdr) and pandrug-resistant (pdr). J. Med. Microbiol. 2007, 55, 1619–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Classification of Diseases, 9th Revision, Clinical Modification. In Practice Management Information Corporation, 6th ed.; 2004. Available online: https://www.cdc.gov/nchs/icd/icd9cm.htm (accessed on 12 March 2020).
- Chen, Y.A.; Lin, Y.J.; Lin, C.L.; Lin, H.J.; Wu, H.S.; Hsu, H.Y.; Sun, Y.C.; Wu, H.Y.; Lai, C.H.; Kao, C.H. Simvastatin therapy for drug repositioning to reduce the risk of prostate cancer mortality in patients with hyperlipidemia. Front. Pharmacol. 2018, 9, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juyal, D.; Shamanth, A.S.; Pal, S.; Sharma, M.K.; Prakash, R.; Sharma, N. The prevalence of inducible clindamycin resistance among staphylococci in a tertiary care hospital—A study from the garhwal hills of uttarakhand, india. J. Clin. Diagn. Res. 2013, 7, 61–65. [Google Scholar] [CrossRef]
- Vijaya, D.; Kamala; Bavani, S.; Veena, M. Prevalence of nonfermenters in clinical specimens. Indian J. Med. Sci. 2000, 54, 87–91. [Google Scholar]
- Baruah, F.K.; Hussain, A.N.; Kausalya; Grover, R.K. Antibiotic resistance profile of non-fermenting gram-negative bacilli isolated from the blood cultures of cancer patients. J. Glob. Infect. Dis. 2015, 7, 46–47. [Google Scholar]
- Grewal, U.S.; Bakshi, R.; Walia, G.; Shah, P.R. Antibiotic susceptibility profiles of non-fermenting gram-negative bacilli at a tertiary care hospital in patiala, india. Niger. Postgrad. Med. J. 2017, 24, 121–125. [Google Scholar] [CrossRef]
- Gajdacs, M.; Burian, K.; Terhes, G. Resistance levels and epidemiology of non-fermenting gram-negative bacteria in urinary tract infections of inpatients and outpatients (renfuti): A 10-year epidemiological snapshot. Antibiotics 2019, 8, 143. [Google Scholar] [CrossRef] [Green Version]
- Tseng, S.H.; Lee, C.M.; Lin, T.Y.; Chang, S.C.; Chuang, Y.C.; Yen, M.Y.; Hwang, K.P.; Leu, H.S.; Yen, C.C.; Chang, F.Y. Combating antimicrobial resistance: Antimicrobial stewardship program in Taiwan. J. Microbiol. Immunol. Infect. 2012, 45, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; He, Q.; Wang, Z.; Wei, M.; Yang, Z.; Du, Y.; Wu, C.; He, J. Influence of antimicrobial consumption on gram-negative bacteria in inpatients receiving antimicrobial resistance therapy from 2008-2013 at a tertiary hospital in Shanghai, China. Am. J. Infect. Control 2015, 43, 358–364. [Google Scholar] [CrossRef]
- Zou, Y.M.; Ma, Y.; Liu, J.H.; Shi, J.; Fan, T.; Shan, Y.Y.; Yao, H.P.; Dong, Y.L. Trends and correlation of antibacterial usage and bacterial resistance: Time series analysis for antibacterial stewardship in a chinese teaching hospital (2009–2013). Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.Y.; Kwa, A.L.; Lye, D.C.; Chlebicki, M.P.; Tan, T.Y.; Ling, M.L.; Wong, S.Y.; Goh, L.G. Reducing antimicrobial resistance through appropriate antibiotic usage in Singapore. Singap. Med. J. 2008, 49, 749–755. [Google Scholar] [PubMed]
- Cook, P.P.; Gooch, M. Long-term effects of an antimicrobial stewardship programme at a tertiary-care teaching hospital. Int. J. Antimicrob. Agents 2015, 45, 262–267. [Google Scholar] [CrossRef] [PubMed]
- File, T.M., Jr.; Srinivasan, A.; Bartlett, J.G. Antimicrobial stewardship: Importance for patient and public health. Clin. Infect. Dis. 2014, 59 (Suppl. S3), S93–S96. [Google Scholar] [CrossRef] [Green Version]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an antibiotic stewardship program: Guidelines by the infectious diseases society of america and the society for healthcare epidemiology of america. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.Y.; Lin, T.Y.; Huang, C.T.; Deng, S.T.; Wu, T.L.; Leu, H.S.; Chiu, C.H. Implementation and outcomes of a hospital-wide computerised antimicrobial stewardship programme in a large medical centre in Taiwan. Int. J. Antimicrob. Agents 2011, 38, 486–492. [Google Scholar] [CrossRef]
- Tan, R.; Liu, J.; Li, M.; Huang, J.; Sun, J.; Qu, H. Epidemiology and antimicrobial resistance among commonly encountered bacteria associated with infections and colonization in intensive care units in a university-affiliated hospital in shanghai. J. Microbiol. Immunol. Infect. 2014, 47, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Lin, L.C.; Chang, Y.J.; Chen, Y.M.; Chang, C.Y.; Huang, C.C. Infection control programs and antibiotic control programs to limit transmission of multi-drug resistant acinetobacter baumannii infections: Evolution of old problems and new challenges for institutes. Int. J. Environ. Res. Public Health 2015, 12, 8871–8882. [Google Scholar] [CrossRef]
- Lin, C.J.; Liao, W.C.; Lin, H.J.; Hsu, Y.M.; Lin, C.L.; Chen, Y.A.; Feng, C.L.; Chen, C.J.; Kao, M.C.; Lai, C.H.; et al. Statins attenuate helicobacter pylori caga translocation and reduce incidence of gastric cancer: In vitro and population-based case-control studies. PLoS ONE 2016, 11, e0146432. [Google Scholar]
- Lin, C.J.; Liao, W.C.; Chen, Y.A.; Lin, H.J.; Feng, C.L.; Lin, C.L.; Lin, Y.J.; Kao, M.C.; Huang, M.Z.; Lai, C.H.; et al. Statin therapy is associated with reduced risk of peptic ulcer disease in the Taiwanese population. Front. Pharmacol. 2017, 8, 210. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, C.-H.; Lai, Y.-R.; Chi, C.-Y.; Ho, M.-W.; Chen, C.-L.; Liao, W.-C.; Ho, C.-M.; Chen, Y.-A.; Chen, C.-Y.; Lin, Y.-T.; et al. Long-Term Surveillance of Antibiotic Prescriptions and the Prevalence of Antimicrobial Resistance in Non-Fermenting Gram-Negative Bacilli. Microorganisms 2020, 8, 397. https://doi.org/10.3390/microorganisms8030397
Chou C-H, Lai Y-R, Chi C-Y, Ho M-W, Chen C-L, Liao W-C, Ho C-M, Chen Y-A, Chen C-Y, Lin Y-T, et al. Long-Term Surveillance of Antibiotic Prescriptions and the Prevalence of Antimicrobial Resistance in Non-Fermenting Gram-Negative Bacilli. Microorganisms. 2020; 8(3):397. https://doi.org/10.3390/microorganisms8030397
Chicago/Turabian StyleChou, Chia-Huei, Yi-Ru Lai, Chih-Yu Chi, Mao-Wang Ho, Chao-Ling Chen, Wei-Chih Liao, Cheng-Mao Ho, Yu-An Chen, Chih-Yu Chen, Yu-Tzu Lin, and et al. 2020. "Long-Term Surveillance of Antibiotic Prescriptions and the Prevalence of Antimicrobial Resistance in Non-Fermenting Gram-Negative Bacilli" Microorganisms 8, no. 3: 397. https://doi.org/10.3390/microorganisms8030397