Niche Partitioning with Temperature among Heterocystous Cyanobacteria (Scytonema spp., Nostoc spp., and Tolypothrix spp.) from Biological Soil Crusts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enrichment Cultures
2.2. Experimental Organisms and Growth Conditions
2.3. Delineation of Temperature Range for Growth and Survival of Isolates
2.4. Influence of Diazotrophy on the Upper Temperature Limit for Growth
2.5. Heterocyst and Vegetative Cell Counts
2.6. Chlorophyll a Determination
2.7. Meta-analysis of Temperature Niches
3. Results
3.1. Encrichment Cultivation
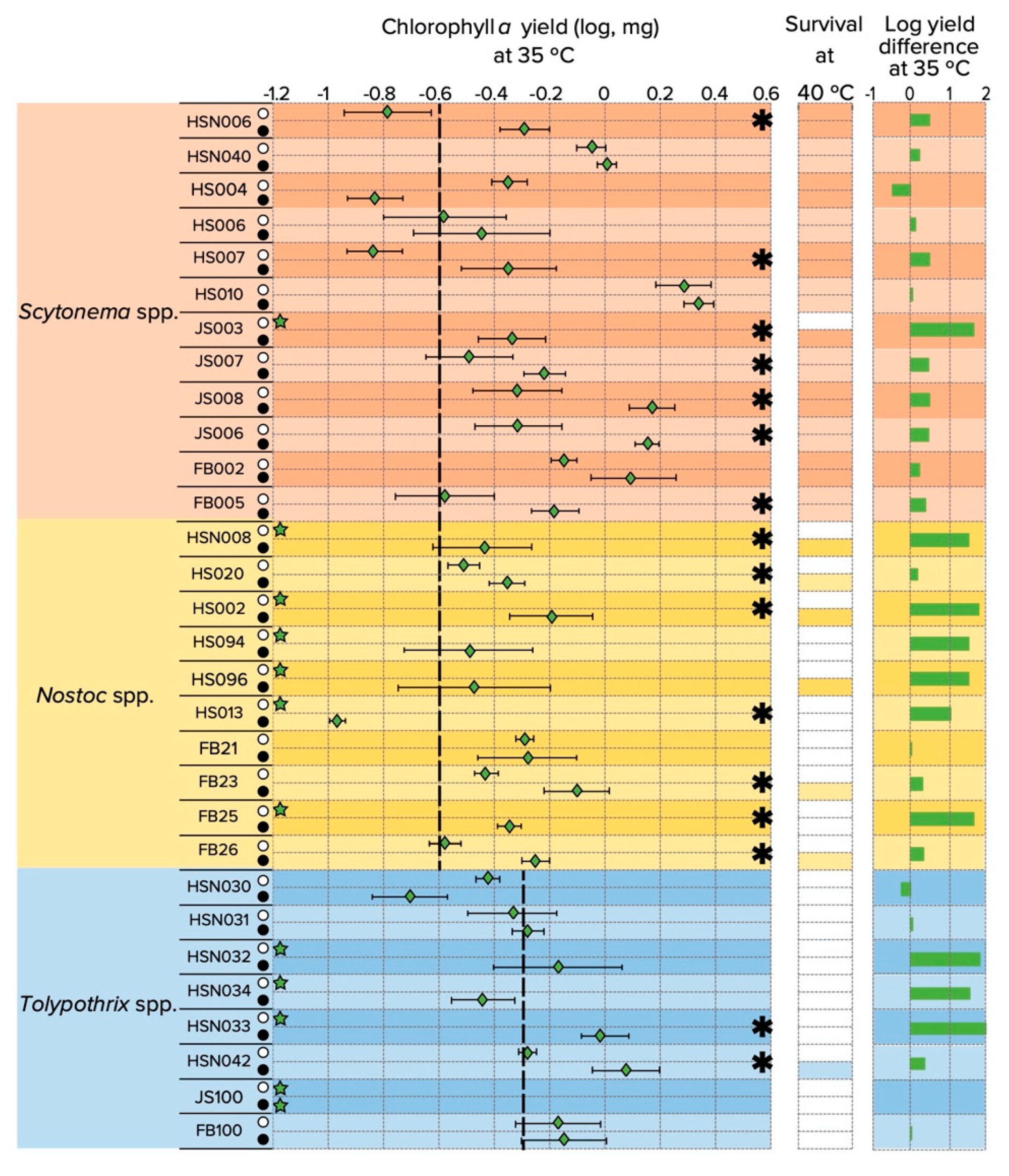
3.2. Temperature Range for Growth (or Survival) of Isolated Strains
3.3. Upper Temperature Limit for Growth and N2-Fixation
3.4. Heterocyst Frequency
3.5. Thermal Niche of Biocrust Heterocystous Cyanobacteria through Meta-Analyses of Molecular Surveys
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garcia-Pichel, F. Desert Environments: Biological Soil Crusts. In Encyclopedia of Environmental Microbiology 6 Volume; Bitton, G., Ed.; Set. Wiley-Interscience: New York, NY, USA, 2003; pp. 1019–1023. [Google Scholar]
- Weber, B.; Caldwell, M.M.; Jayne, B.; Bettina, W.; Büdel, B.; Belnap, J.; Weber, B.; Büdel, B. Biological Soil Crusts: An Organizing Principle in Drylands. In Biological Soil Crusts: An Organizing Principle in Drylands; Springer: Basel, Switzerland, 2016; pp. 3–14. ISBN 978-3-319-30212-6. [Google Scholar]
- Gaskin, S.; Gardner, R. The role of cryptogams in runoff and erosion control on Bariland in the Nepal middle hills of the Southern Himalaya. Earth Surf. Process. Landf. 2001, 26, 1303–1315. [Google Scholar] [CrossRef]
- Belnap, J.; Gillette, D.A. Disturbance of Biological Soil Crusts: Impacts on Potential Wind Erodibility of Sandy Desert Soils in Southeastern Utah. Land Degrad. Dev. 1997, 8, 355–362. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wang, H.L.; Wang, X.Q.; Yang, W.K.; Zhang, D.Y. The microstructure of microbiotic crust and its influence on wind erosion for a sandy soil surface in the Gurbantunggut Desert of Northwestern China. Geoderma 2006, 132, 441–449. [Google Scholar] [CrossRef]
- Couradeau, E.; Karaoz, U.; Lim, H.C.; Nunes da Rocha, U.; Northen, T.; Brodie, E.; Garcia-Pichel, F. Bacteria increase arid-land soil surface temperature through the production of sunscreens. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verrecchia, E.; Yair, A.; Kidron, G.J.; Verrecchia, K. Physical properties of the psammophile cryptogamic crust and their consequences to the water regime of sandy softs, north-western Negev Desert, Israel. J. Arid Environ. 1995, 427–437. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, E.; Cantón, Y.; Chamizo, S.; Afana, A.; Solé-Benet, A. Effects of biological soil crusts on surface roughness and implications for runoff and erosion. Geomorphology 2012, 145–146, 81–89. [Google Scholar] [CrossRef]
- Faist, A.M.; Herrick, J.E.; Belnap, J.; Zee, J.W.V.; Barger, N.N. Biological soil crust and disturbance controls on surface hydrology in a semi-Arid ecosystem. Ecosphere 2017, 8. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Wojciechowski, M.F. The evolution of a capacity to build supra-cellular ropes enabled filamentous cyanobacteria to colonize highly erodible substrates. PLoS ONE 2009, 4, e7801. [Google Scholar] [CrossRef]
- Nunes da Rocha, U.; Cadillo-Quiroz, H.; Karaoz, U.; Rajeev, L.; Klitgord, N.; Dunn, S.; Truong, V.; Buenrostro, M.; Bowen, B.P.; Garcia-Pichel, F.; et al. Isolation of a significant fraction of non-phototroph diversity from a desert biological soil crust. Front. Microbiol. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Nagy, M.L.; Pérez, A.; Garcia-Pichel, F. The prokaryotic diversity of biological soil crusts in the Sonoran Desert (Organ Pipe Cactus National Monument, AZ). FEMS Microbiol. Ecol. 2005, 54, 233–245. [Google Scholar] [CrossRef] [Green Version]
- Soule, T.; Anderson, I.J.; Johnson, S.L.; Bates, S.T.; Garcia-Pichel, F. Archaeal populations in biological soil crusts from arid lands in North America. Soil Biol. Biochem. 2009, 41, 2069–2074. [Google Scholar] [CrossRef]
- Bates, S.T.; Nash, T.H.; Garcia-Pichel, F. Patterns of diversity for fungal assemblages of biological soil crusts from the southwestern United States. Mycologia 2012, 104, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, L.; Büdel, B. Ecological Determinants of Species Composition of Biological Soil Cruts on a Landscape Scale.pdf. In Biological Soil Cruts: Structure, Function, and Managment; Belnap, J., Lange, O.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 203–213. [Google Scholar]
- Johnson, S.L.; Budinoff, C.R.; Belnap, J.; Garcia-Pichel, F. Relevance of ammonium oxidation within biological soil crust communities. Environ. Microbiol. 2005, 7, 1–12. [Google Scholar] [CrossRef]
- Pepe-Ranney, C.; Koechli, C.; Potrafka, R.; Andam, C.; Eggleston, E.; Garcia-Pichel, F.; Buckley, D.H. Non-cyanobacterial diazotrophs dominate dinitrogen fixation in biological soil crusts during early crust formation. ISME J. 2015, 10, 287–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couradeau, E.; Giraldo-Silva, A.; De Martini, F.; Garcia-Pichel, F. Spatial segregation of the biological soil crust microbiome around its foundational cyanobacterium, Microcoleus vaginatus, and the formation of a nitrogen-fixing cyanosphere. Microbiome 2019, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeager, C.M.; Kornosky, J.L.; Morgan, R.E.; Cain, E.C.; Garcia-Pichel, F.; Housman, D.C.; Belnap, J.; Kuske, C.R. Three distinct clades of cultured heterocystous cyanobacteria constitute the dominant N2-fixing members of biological soil crusts of the Colorado Plateau, USA. FEMS Microbiol. Ecol. 2007, 60, 85–97. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.L.; Neuer, S.; Garcia-Pichel, F. Export of nitrogenous compounds due to incomplete cycling within biological soil crusts of arid lands. Environ. Microbiol. 2007, 9, 680–689. [Google Scholar] [CrossRef]
- Strauss, S.L.; Day, T.A.; Garcia-Pichel, F. Nitrogen cycling in desert biological soil crusts across biogeographic regions in the Southwestern United States. Biogeochemistry 2012, 108, 171–182. [Google Scholar] [CrossRef]
- Thiet, R.K.; Boerner, R.E.J.; Nagy, M.; Jardine, R. The effect of biological soil crusts on throughput of rainwater and N into Lake Michigan sand dune soils. Plant Soil 2005, 278, 235–251. [Google Scholar] [CrossRef]
- Thomazo, C.; Couradeau, E.; Garcia-Pichel, F. Possible nitrogen fertilization of the early Earth Ocean by microbial continental ecosystems. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Beraldi-Campesi, H.; Hartnett, H.E.; Anbar, A.; Gordon, G.W.; Garcia-Pichel, F. Effect of biological soil crusts on soil elemental concentrations: Implications for biogeochemistry and as traceable biosignatures of ancient life on land. Geobiology 2009, 7, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Prăvălie, R. Drylands extent and environmental issues. A global approach. Earth Sci. Rev. 2016, 161, 259–278. [Google Scholar] [CrossRef]
- Seager, R.; Vecchi, G.A. Greenhouse warming and the 21st century hydroclimate of southwestern North America. Proc. Natl. Acad. Sci. USA 2010, 107, 21277–21282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrie, M.D.; Collins, S.L.; Litvak, M.E. The ecological role of small rainfall events in a desert grassland. Ecohydrology 2015, 8, 1614–1622. [Google Scholar] [CrossRef]
- Petrie, M.D.; Collins, S.L.; Gutzler, D.S.; Moore, D.M. Regional trends and local variability in monsoon precipitation in the northern Chihuahuan Desert, USA. J. Arid Environ. 2014, 103, 63–70. [Google Scholar] [CrossRef]
- Elbert, W.; Weber, B.; Burrows, S.; Steinkamp, J.; Büdel, B.; Andreae, M.O.; Pöschl, U. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 2012, 5, 459–462. [Google Scholar] [CrossRef]
- Barger, N.N.; Castle, S.C.; Dean, G.N. Denitrification from nitrogen-fixing biologically crusted soils in a cool desert environment, southeast Utah, USA. Ecol. Process. 2013, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Smith, H.; Giraldo Silva, A.; Belnap, J.; Garcia-Pichel, F. Differential Responses of Dinitrogen Fixation, Diazotrophic Cyanobacteria and Ammonia Oxidation Reveal a Potential Warming-Induced Imbalance of the N-Cycle in Biological Soil Crusts. PLoS ONE 2016, 11, e0164932. [Google Scholar] [CrossRef]
- Muñoz-Martín, M.Á.; Becerra-Absalón, I.; Perona, E.; Fernández-Valbuena, L.; Garcia-Pichel, F.; Mateo, P. Cyanobacterial biocrust diversity in Mediterranean ecosystems along a latitudinal and climatic gradient. New Phytol. 2018, 221, 123–141. [Google Scholar] [CrossRef] [Green Version]
- Becerra-Absalón, I.; Muñoz-Martín, M.Á.; Montejano, G.; Mateo, P. Differences in the Cyanobacterial Community Composition of Biocrusts From the Drylands of Central Mexico. Are There Endemic Species? Front. Microbiol. 2019, 10, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Pichel, F.; Loza, V.; Marusenko, Y.; Mateo, P.; Potrafka, R.M. Temperature Drives the Continental-Scale Distribution of Key Microbes in Topsoil Communities. Science 2013, 340, 1574–1577. [Google Scholar] [CrossRef] [PubMed]
- Cable, J.M.; Huxman, T.E. Precipitation pulse size effects on Sonoran Desert soil microbial crusts. Oecologia 2004, 141, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Knapp, A.K.; Beier, C.; Briske, D.D.; Classen, A.T.; Luo, Y.; Reichstein, M.; Smith, M.D.; Smith, S.D.; Bell, J.E.; Fay, P.A.; et al. Consequences of More Extreme Precipitation Regimes for Terrestrial Ecosystems. Bioscience 2008, 58, 811–821. [Google Scholar] [CrossRef]
- Sala, O.E.; Lauenroth, W.K. Small Rainfall Events: An Ecological Role in Semiarid Regions. Oecologia 1982, 53, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Silva, A.; Nelson, C.; Barger, N.; Garcia-Pichel, F. Nursing biocrusts: Isolation, cultivation and fitness test of indigenous cyanobacteria. Restor. Ecol. 2019, 27, 793–803. [Google Scholar] [CrossRef]
- Allen, M.M.; Stanier, R. Growth and Division of Some Unicellular Blue-green Algae. J. Gen. Microbiol. 1968, 51, 199–202. [Google Scholar] [CrossRef] [Green Version]
- Rippka, R.; Deruelles, J.; Waterbury, J.B. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Clarke, K.; Gorley, R. Primer v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Anderson, M.; Gorley, R.N.; Clarke, K.R. PERMANOVA + for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Nübel, U.; Muyzer, G.; Garcia-pichel, F.; Muyzer, G. PCR primers to amplify 16S rRNA genes from cyanobacteria PCR Primers To Amplify 16S rRNA Genes from Cyanobacteria. Microbiology 1997, 63, 3327–3332. [Google Scholar]
- Castle, S.C.; Morrison, C.D.; Barger, N.N. Extraction of chlorophyll a from biological soil crusts: A comparison of solvents for spectrophotometric determination. Soil Biol. Biochem. 2011, 43, 853–856. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Castenholz, R.W. Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment. J. Phycol. 1991, 409, 395–409. [Google Scholar] [CrossRef]
- Velasco Ayuso, S.; Giraldo Silva, A.; Nelson, C.; Barger, N.N.; Garcia-pichel, F. Microbial Nursery Production of High- Quality Biological Soil Crust Biomass for Restoration of Degraded Dryland Soils. Appl. Environ. Microbiol. 2017, 83, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, V.M.C.; Machado de Lima, N.M.; Roush, D.; Rudgers, J.; Collins, S.L.; Garcia-Pichel, F. Exposure to predicted precipitation patterns decreases population size and alters community structure of cyanobacteria in biological soil crusts from the Chihuahuan Desert. Environ. Microbiol. 2018, 20, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Bethany, J.; Giraldo-Silva, A.; Nelson, C.; Barger, N.N.; Garcia-Pichel, F. Optimizing the Production of Nursery-Based Biological Soil Crusts for Restoration of Arid Land Soils. Appl. Environ. Microbiol. 2019, 85, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira-Grez, B.; Tam, K.; Cross, A.T.; Yong, J.W.H.; Kumaresan, D.; Nevill, P.; Farrell, M.; Whiteley, A.S. The Bacterial Microbiome Associated With Arid Biocrusts and the Biogeochemical Influence of Biocrusts Upon the Underlying Soil. Front. Microbiol. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, L.; Loewen-Schneider, K.; Maier, S.; Büdel, B. Cyanobacterial diversity of western European biological soil crusts along a latitudinal gradient. FEMS Microbiol. Ecol. 2016, 92, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Kong, W.; Nan, W.; Zhang, Y. Bacterial diversity and community along the succession of biological soil crusts in the Gurbantunggut Desert, Northern China. J. Basic Microbiol. 2016, 56, 670–679. [Google Scholar] [CrossRef]
- Machado-de-Lima, N.M.; Fernandes, V.M.C.; Roush, D.; Velasco Ayuso, S.; Rigonato, J.; Garcia-Pichel, F.; Zanini Branco, L.H. The Compositionally Distinct Cyanobacterial Biocrusts From Brazilian Savanna and Their Environmental Drivers of Community Diversity. Front. Microbiol. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High resolution sample inference from amplicon data. bioRxiv 2015, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-thorughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability Article Fast Track. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2-Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Xu, S. Bayesian Naïve Bayes classifiers to text classification. J. Inf. Sci. 2016, 44, 48–59. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, S.A.; Stamatakis, A. Aligning short reads to reference alignments and trees. Bioinformatics 2011, 27, 2068–2075. [Google Scholar] [CrossRef] [Green Version]
- Berger, S.A.; Krompass, D.; Stamatakis, A. Performance, accuracy, and web server for evolutionary placement of short sequence reads under maximum likelihood. Syst. Biol. 2011, 60, 291–302. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Bork, P. Interactive tree of life ( iTOL ) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, 242–245. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Rajeev, L.; Da Rocha, U.N.; Klitgord, N.; Luning, E.G.; Fortney, J.; Axen, S.D.; Shih, P.M.; Bouskill, N.J.; Bowen, B.P.; Kerfeld, C.A.; et al. Dynamic cyanobacterial response to hydration and dehydration in a desert biological soil crust. ISME J. 2013, 7, 2178–2191. [Google Scholar] [CrossRef] [Green Version]
- Yeager, C.M.; Kornosky, J.L.; Housman, D.C.; Grote, E.E.; Belnap, J.; Kuske, C.R. Diazotrophic Community Structure and Function in Two Successional Stages of Biological Soil Crusts from the Colorado Plateau and Chihuahuan Desert. Appl. Environ. Microbiol. 2004, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castenholz, R.W. Thermophilic blue-green algae and the thermal environment. Bacteriol. Rev. 1969, 33, 476–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nübel, U.; Garcia-Pichel, F.; Kuhl, M.; Muyzer, G. Spatial scale and diversity of benthic cyanobacteria and diatoms in a salina. In Molecular Ecology of Aquatic Communities. Developments in Hydrology; Zehr, J., Voytek, M.A., Eds.; Springer: Dordrecht, The Netherlands, 1999. [Google Scholar]
- Garcia-pichel, F.; Ku, M.; Nübel, U.; Muyzer, G. Salinity-dependent limitation of photosynthesis and oxygen exchange in microbial mats. J. Phycol. 1999, 35, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Rothrock, M.J.; Garcia-Pichel, F. Microbial diversity of benthic mats along a tidal desiccation gradient. Environ. Microbiol. 2005, 7, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.G.; Duggan, P.S. Heterocyst and akinete differentiation in cyanobacteria. New Phytol. 1999, 144, 3–33. [Google Scholar] [CrossRef]
- Hennecke, H.; Shanmugam, K.T. Temperature control of nitrogen fixation in Klebsiella pneumoniae. Arch. Microbiol. 1979, 123, 259–265. [Google Scholar] [CrossRef]
- Nierzwicki Bauer, S.A.; Balkwill, D.L.; Stevens, S.E. Heterocyst differentiation in the cyanobacterium Mastigocladus laminosus. J. Bacteriol. 1984, 157, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.R.; Castenholz, R.W.; Pedersen, D. Phylogeography of the thermophilic cyanobacterium Mastigocladus laminosus. Appl. Environ. Microbiol. 2007, 73, 4751–4759. [Google Scholar] [CrossRef] [Green Version]
- Bowker, M.A.; Antoninka, A.J. Rapid ex situ culture of N-fixing soil lichens and biocrusts is enhanced by complementarity. Plant Soil 2016, 408, 415–428. [Google Scholar] [CrossRef]
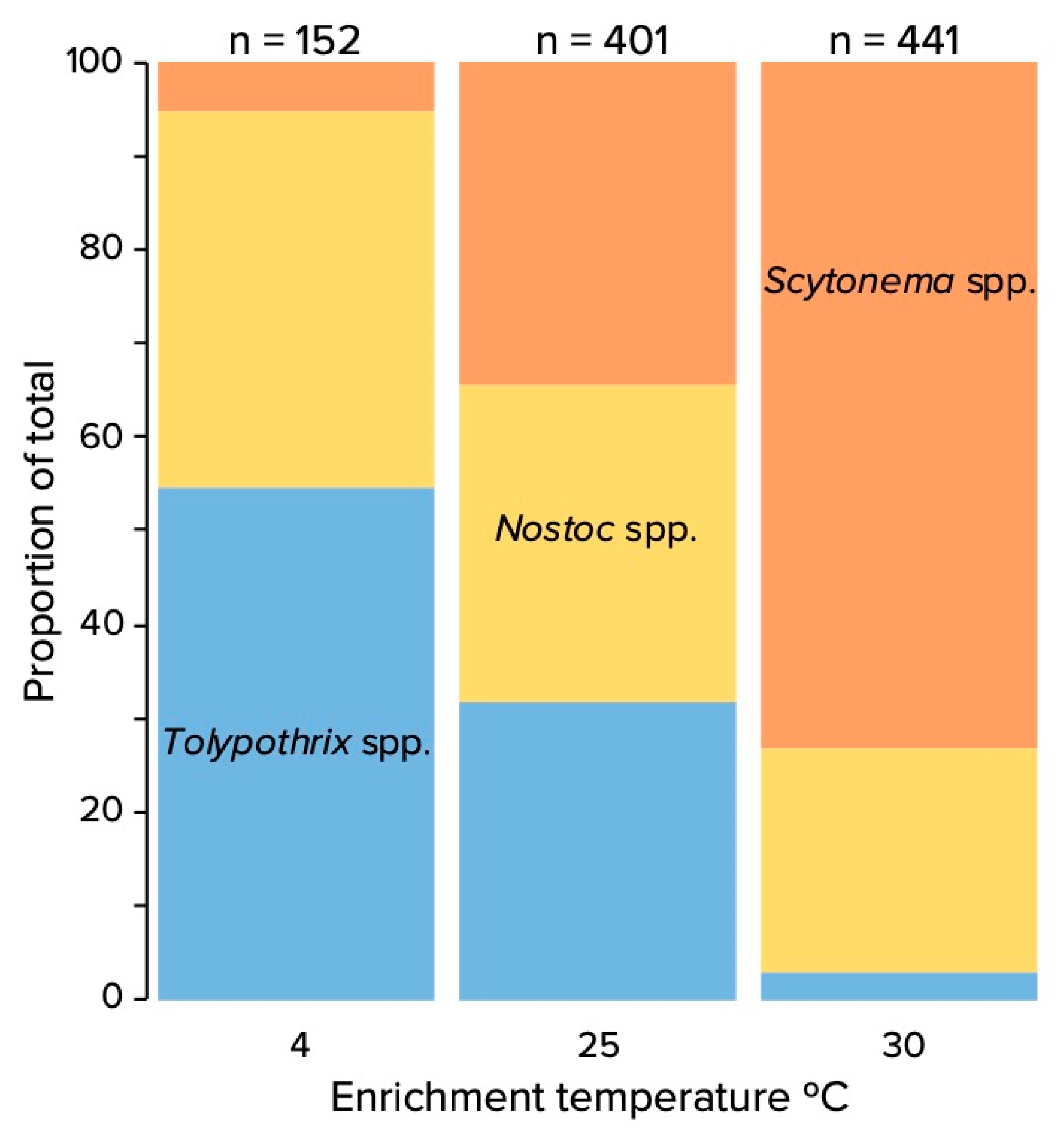


| Strain | Incubation Temperature °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 35 | 40 | |||||||
| H | VG | Ratio H:VG | H | VG | Ratio H:VG | H | VG | Ratio H:VG | |
| Nostoc spp. | 26 | 230 | 1:9 | 10 | 207 | 1:21 | 18 | 569 | 1:32 |
| 23 | 205 | 1:9 | 12 | 258 | 1:22 | 28 | 773 | 1:28 | |
| 32 | 206 | 1:6 | 11 | 223 | 1:20 | 21 | 642 | 1:31 | |
| 24 | 208 | 1:9 | 13 | 246 | 1:10 | 23 | 730 | 1:32 | |
| 26 | 207 | 1:8 | 14 | 236 | 1:17 | 26 | 701 | 1:27 | |
| 22 | 212 | 1:10 | - | - | - | 27 | 689 | 1:26 | |
| Tolypothrix spp. | 35 | 467 | 1:13 | 6 | 628 | 1:105 | 3 | 900 | 1:300 |
| 38 | 507 | 1:13 | 3 | 444 | 1:148 | 4 | 900 | 1:225 | |
| 40 | 538 | 1:13 | 2 | 250 | 1:125 | 2 | 900 | 1:450 | |
| 37 | 557 | 1:15 | 5 | 576 | 1:115 | 6 | 900 | 1:150 | |
| 43 | 613 | 1:14 | 6 | 553 | 1:92 | 4 | 900 | 1:225 | |
| 36 | 582 | 1:15 | 3 | 324 | 1:108 | 4 | 900 | 1:224 | |
| Scytonema spp. | 27 | 534 | 1:20 | 15 | 352 | 1:23 | 12 | 526 | 1:44 |
| 29 | 553 | 1:19 | 5 | 150 | 1:20 | 12 | 600 | 1:50 | |
| 32 | 669 | 1:21 | 7 | 225 | 1:32 | 10 | 550 | 1:55 | |
| 37 | 844 | 1:23 | 9 | 276 | 1:32 | 14 | 708 | 1:44 | |
| 18 | 308 | 1:17 | 8 | 242 | 1:30 | 16 | 641 | 1:50 | |
| 25 | 412 | 1:16 | 7 | 233 | 1:33 | 41 | 1087 | 1:55 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giraldo-Silva, A.; Fernandes, V.M.C.; Bethany, J.; Garcia-Pichel, F. Niche Partitioning with Temperature among Heterocystous Cyanobacteria (Scytonema spp., Nostoc spp., and Tolypothrix spp.) from Biological Soil Crusts. Microorganisms 2020, 8, 396. https://doi.org/10.3390/microorganisms8030396
Giraldo-Silva A, Fernandes VMC, Bethany J, Garcia-Pichel F. Niche Partitioning with Temperature among Heterocystous Cyanobacteria (Scytonema spp., Nostoc spp., and Tolypothrix spp.) from Biological Soil Crusts. Microorganisms. 2020; 8(3):396. https://doi.org/10.3390/microorganisms8030396
Chicago/Turabian StyleGiraldo-Silva, Ana, Vanessa M. C. Fernandes, Julie Bethany, and Ferran Garcia-Pichel. 2020. "Niche Partitioning with Temperature among Heterocystous Cyanobacteria (Scytonema spp., Nostoc spp., and Tolypothrix spp.) from Biological Soil Crusts" Microorganisms 8, no. 3: 396. https://doi.org/10.3390/microorganisms8030396
APA StyleGiraldo-Silva, A., Fernandes, V. M. C., Bethany, J., & Garcia-Pichel, F. (2020). Niche Partitioning with Temperature among Heterocystous Cyanobacteria (Scytonema spp., Nostoc spp., and Tolypothrix spp.) from Biological Soil Crusts. Microorganisms, 8(3), 396. https://doi.org/10.3390/microorganisms8030396





