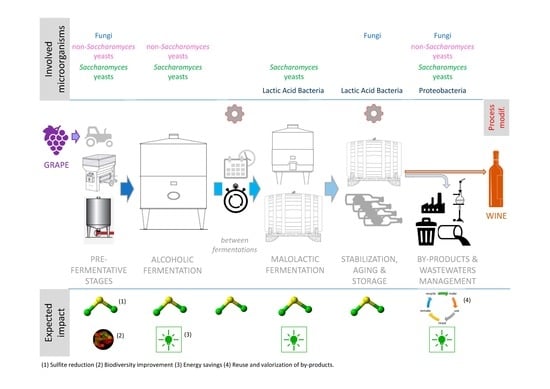
Microbial Resources as a Tool for Enhancing Sustainability in Winemaking
Abstract
1. Introduction
2. Improving Sustainability in Pre-Fermentative Stages
2.1. Non-Saccharomyces Yeasts Producing Antimicrobial Compounds
2.2. Non-Saccharomyces Yeasts Exerting Indirect Bioprotective Effect
3. Managing Alcoholic Fermentation for Sustainability
3.1. Control of Microbial Spoilage during Alcoholic Fermentation
3.2. Energy Savings Associated with Alcoholic Fermentation
4. Improving Sustainability between Alcoholic and Malolactic Fermentation
5. Managing Malolactic Fermentation for Sustainability
5.1. Control of Microbial Spoilage during Malolactic Fermentation
5.2. Energy Savings Associated with Malolactic Fermentation
6. Sustainable Procedures in Post-Fermentation, Stabilization, Aging, and Storage
7. Sustainable Management of By-Products and Wastewater
7.1. Microbial Valorization of Solid Co-Products
7.2. Microbial Treatment of Wastewater and Solid Residues
8. Conclusions
List of abbreviations
| ADY | Active dry yeast |
| AF | Alcoholic fermentation |
| BA | Biogenic amine |
| COD | Chemical oxygen demand |
| GHG | Greenhouse gas |
| LAB | Lactic acid bacteria |
| MLF | Malolactic fermentation |
| MFC | Microbial fuel cells |
| OIV | International Organisation of Vine and Wine |
| PCM | Pre-fermentative cold maceration |
| VOC | Volatile organic compound |
Funding
Conflicts of Interest
References
- Trioli, G.; Sacchi, A.; Corbo, C.; Trevisan, M. Environmental impact of vinegrowing and winemaking inputs: An european survey. Internet J. Viticult. Enol. 2015, 7, 2. [Google Scholar]
- De Matos, C.T.; Garcia, J.C.; Aurambout, J.-P.; Manfredi, S. Environmental sustainability assessment of bioeconomy products and processes—Progress report 1. Eur. Comm. Rep. EUR 27356 EN 2015. [Google Scholar] [CrossRef]
- Pomarici, E.; Vecchio, R. Will sustainability shape the future wine market? Wine Econ. Policy 2019, 8, 1–4. [Google Scholar] [CrossRef]
- Santini, C.; Cavicchi, A.; Casini, L. Sustainability in the wine industry: Key questions and research trends a. Agric. Food Econ. 2013, 1, 9. [Google Scholar] [CrossRef]
- Bonamente, E.; Scrucca, F.; Rinaldi, S.; Merico, M.C.; Asdrubali, F.; Lamastra, L. Environmental impact of an Italian wine bottle: Carbon and water footprint assessment. Sci. Total Environ. 2016, 560–561, 274–283. [Google Scholar] [CrossRef] [PubMed]
- REGULATION (EC) No 1221/2009 on the Voluntary Participation by Organisations in a Community Eco-Management and Audit Scheme (EMAS). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=OJ:L:2009:342:FULL&from=EN (accessed on 4 December 2019).
- Merli, R.; Preziosi, M.; Acampora, A. Sustainability experiences in the wine sector: Toward the development of an international indicators system. J. Clean. Prod. 2018, 172, 3791–3805. [Google Scholar] [CrossRef]
- Berbegal, C.; Fragasso, M.; Russo, P.; Bimbo, F.; Grieco, F.; Spano, G.; Capozzi, V. Climate Changes and Food Quality: The Potential of Microbial Activities as Mitigating Strategies in the Wine Sector. Fermentation 2019, 5, 85. [Google Scholar] [CrossRef]
- Castrillo, D.; Rabuñal, E.; Neira, N.; Blanco, P. Oenological potential of non-Saccharomyces yeasts to mitigate effects of climate change in winemaking: Impact on aroma and sensory profiles of Treixadura wines. FEMS Yeast Res. 2019, 19, foz065. [Google Scholar] [CrossRef]
- Dequin, S.; Escudier, J.-L.; Bely, M.; Noble, J.; Albertin, W.; Masneuf-Pomarède, I.; Marullo, P.; Salmon, J.-M.; Sablayrolles, J.M. How to adapt winemaking practices to modified grape composition under climate change conditions. OENO One 2017, 51, 205–214. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology, the Microbiology of Wine and Vinifications; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 1. [Google Scholar]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Pretorius, I.S. Microbial formation and modification of flavor and off-flavor compounds in wine. In Biology of Microorganisms on Grapes, in Must and in Wine; Springer: Berlin/Heidelberg, Germany, 2009; pp. 209–231. [Google Scholar]
- Malfeito-Ferreira, M. Yeasts and wine off-flavours: A technological perspective. Annals Microbiol. 2011, 61, 95–102. [Google Scholar] [CrossRef]
- Mas, A. Microbial challenges in sustainable winemaking. OENOVITI Int. Netw. 2018, 25, 38. [Google Scholar]
- Simonin, S.; Alexandre, H.; Nikolantonaki, M.; Coelho, C.; Tourdot-Maréchal, R. Inoculation of Torulaspora delbrueckii as a bio-protection agent in winemaking. Food Res. Int. 2018, 107, 451–461. [Google Scholar] [CrossRef]
- Berbegal, C.; Spano, G.; Fragasso, M.; Grieco, F.; Russo, P.; Capozzi, V. Starter cultures as biocontrol strategy to prevent Brettanomyces bruxellensis proliferation in wine. Appl. Microbiol. Biotechnol. 2018, 102, 569–576. [Google Scholar] [CrossRef]
- Rubio-Bretón, P.; Gonzalo-Diago, A.; Iribarren, M.; Garde-Cerdán, T.; Pérez-Álvarez, E.P. Bioprotection as a tool to free additives winemaking: Effect on sensorial, anthocyanic and aromatic profile of young red wines. LWT 2018, 98, 458–464. [Google Scholar] [CrossRef]
- OIV Oenology Resolutions—OIV/OENO 462/2014. Available online: http://www.oiv.int/en/technical-standards-and-documents/resolutions-of-the-oiv/oenology-resolutions (accessed on 29 October 2016).
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its importance to wine aroma-a review. S. Afr. J. Enol. Viticult. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Aranda, A. Enological Repercussions of Non-Saccharomyces Species. Fermentation 2019, 5, 68. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and Future of Non-Saccharomyces Yeasts: From Spoilage Microorganisms to Biotechnological Tools for Improving Wine Aroma Complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef]
- Varela, C. The impact of non-Saccharomyces yeasts in the production of alcoholic beverages. Appl. Microbiol Biotechnol. 2016, 100, 9861–9874. [Google Scholar] [CrossRef]
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, M.R.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial Resources and Enological Significance: Opportunities and Benefits. Front. Microbiol. 2017, 8, 995. [Google Scholar] [CrossRef]
- Nardi, T.; Nadai, C.; Bovo, B.; Treu, L.; Campanaro, S.; Giacomini, A.; Corich, V. Yeast selection criteria for improvement of sulphite management in winemaking. In Proceedings of the Oeno2011-Actes de Colloques du 9 e Symposium International D’oenologie de Bordeaux, Bordeaux, France, 15–17 June 2012; pp. 445–450. [Google Scholar]
- Berbegal, C.; Garofalo, C.; Russo, P.; Pati, S.; Capozzi, V.; Spano, G. Use of autochthonous yeasts and bacteria in order to control Brettanomyces bruxellensis in wine. Fermentation 2017, 3, 65. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Escott, C.; del Fresno, J.M.; Bañuelos, M.A.; Suárez-Lepe, J.A. Applications of Metschnikowia pulcherrima in Wine Biotechnology. Fermentation 2019, 5, 63. [Google Scholar] [CrossRef]
- Oro, L.; Ciani, M.; Comitini, F. Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J. Appl. Microbiol. 2014, 116, 1209–1217. [Google Scholar] [CrossRef]
- Pawlikowska, E.; James, S.A.; Breierova, E.; Antolak, H.; Kregiel, D. Biocontrol capability of local Metschnikowia sp. isolates. Antonie Leeuwenhoek 2019, 112, 1425–1445. [Google Scholar] [CrossRef]
- Piano, S.; Neyrotti, V.; Migheli, Q.; Gullino, M.L. Biocontrol capability of Metschnikowia pulcherrima against Botrytis postharvest rot of apple. Postharvest Biol. Technol. 1997, 11, 131–140. [Google Scholar] [CrossRef]
- Saravanakumar, D.; Ciavorella, A.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Metschnikowia pulcherrima strain MACH1 outcompetes Botrytis cinerea, Alternaria alternata and Penicillium expansum in apples through iron depletion. Postharvest Biol. Technol. 2008, 49, 121–128. [Google Scholar] [CrossRef]
- Spadaro, D.; Vola, R.; Piano, S.; Gullino, M.L. Mechanisms of action and efficacy of four isolates of the yeast Metschnikowia pulcherrima active against postharvest pathogens on apples. Postharvest Biol. Technol. 2002, 24, 123–134. [Google Scholar] [CrossRef]
- Kinay, P.; Yildiz, M. The shelf life and effectiveness of granular formulations of Metschnikowia pulcherrima and Pichia guilliermondii yeast isolates that control postharvest decay of citrus fruit. Biol. Control 2008, 45, 433–440. [Google Scholar] [CrossRef]
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Sipiczki, M. Metschnikowia Strains Isolated from Botrytized Grapes Antagonize Fungal and Bacterial Growth by Iron Depletion. Appl. Environ. Microbiol. 2006, 72, 6716–6724. [Google Scholar] [CrossRef] [PubMed]
- Elmer, P.A.G.; Reglinski, T. Biosuppression of Botrytis cinerea in grapes. Plant Pathol. 2006, 55, 155–177. [Google Scholar] [CrossRef]
- Raspor, P.; Miklič-Milek, D.; Avbelj, M.; Čadež, N. Biocontrol of Grey Mould Disease on Grape Caused by Botrytis cinerea with Autochthonous Wine Yeasts. Food Technol. Biotechnol. 2010, 48, 336–343. [Google Scholar]
- Roudil, L.; Russo, P.; Berbegal, C.; Albertin, W.; Spano, G.; Capozzi, V. Non-Saccharomyces Commercial Starter Cultures: Scientific Trends, Recent Patents and Innovation in the Wine Sector. Recent Patents food Nutr. Agric. 2019, 10, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Benucci, I.; Luziatelli, F.; Cerreti, M.; Liburdi, K.; Nardi, T.; Vagnoli, P.; Ruzzi, M.; Esti, M. Pre-fermentative cold maceration in the presence of non-Saccharomyces strains: Effect on fermentation behaviour and volatile composition of a red wine. Aust. J. Grape Wine Res. 2017, 24, 135–281. [Google Scholar] [CrossRef]
- Benucci, I.; Cerreti, M.; Liburdi, K.; Nardi, T.; Vagnoli, P.; Ortiz-Julien, A.; Esti, M. Pre-fermentative cold maceration in presence of non-Saccharomyces strains: Evolution of chromatic characteristics of Sangiovese red wine elaborated by sequential inoculation. Food Res. Int. 2018, 107, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; Gil, J.V.; Manzanares, P. Challenges of the Non-Conventional Yeast Wickerhamomyces anomalus in Winemaking. Fermentation 2018, 4, 68. [Google Scholar] [CrossRef]
- Mannazzu, I.; Domizio, P.; Carboni, G.; Zara, S.; Zara, G.; Comitini, F.; Budroni, M.; Ciani, M. Yeast killer toxins: From ecological significance to application. Crit. Rev. Biotechnol. 2019, 39, 603–617. [Google Scholar] [CrossRef]
- Velázquez, R.; Zamora, E.; Álvarez, M.L.; Hernández, L.M.; Ramírez, M. Effects of new Torulaspora delbrueckii killer yeasts on the must fermentation kinetics and aroma compounds of white table wine. Front. Microbiol. 2015, 6, 1222. [Google Scholar] [CrossRef]
- Liu, G.-L.; Chi, Z.; Wang, G.-Y.; Wang, Z.-P.; Li, Y.; Chi, Z.-M. Yeast killer toxins, molecular mechanisms of their action and their applications. Crit. Rev. Biotechnol. 2015, 35, 222–234. [Google Scholar] [CrossRef]
- Mehlomakulu, N.N.; Setati, M.E.; Divol, B. Non-Saccharomyces Killer Toxins: Possible Biocontrol Agents Against Brettanomyces in Wine? S. Afr. J. Enol. Viticult. 2015, 36, 94–104. [Google Scholar] [CrossRef]
- Oro, L.; Ciani, M.; Bizzaro, D.; Comitini, F. Evaluation of damage induced by Kwkt and Pikt zymocins against Brettanomyces/Dekkera spoilage yeast, as compared to sulphur dioxide. J. Appl. Microbiol. 2016, 121, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.L.; Sáez, J.S.; del Monaco, S.; Lopes, C.A.; Sangorrín, M.P. TdKT, a new killer toxin produced by Torulaspora delbrueckii effective against wine spoilage yeasts. Int. J. Food Microbiol. 2016, 217, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Kuchen, B.; Maturano, Y.P.; Mestre, M.V.; Combina, M.; Toro, M.E.; Vazquez, F. Selection of Native Non-Saccharomyces Yeasts with Biocontrol Activity against Spoilage Yeasts in Order to Produce Healthy Regional Wines. Fermentation 2019, 5, 60. [Google Scholar] [CrossRef]
- Infowine Non Saccharomyces contro Brett: Nuove Prospettive nell’Impiego di Lieviti ad Attività Antimicrobica—Video—Piacenza. Available online: https://www.infowine.com/it/video/non_saccharomyces_contro_brett_nuove_prospettive_nellimpiego_di_lieviti_ad_attivita_antimicrobica_sc_13312.htm (accessed on 17 February 2020).
- Bozoudi, D.; Tsaltas, D. The Multiple and Versatile Roles of Aureobasidium pullulans in the Vitivinicultural Sector. Fermentation 2018, 4, 85. [Google Scholar] [CrossRef]
- Guzzon, R.; Franciosi, E.; Larcher, R. A new resource from traditional wines: Characterisation of the microbiota of “Vino Santo” grapes as a biocontrol agent against Botrytis cinerea. Eur. Food Res. Technol. 2014, 239, 117–126. [Google Scholar] [CrossRef]
- Lorenzini, M.; Zapparoli, G. Epiphytic bacteria from withered grapes and their antagonistic effects on grape-rotting fungi. Int. J. Food Microbiol. 2020, 319, 108505. [Google Scholar] [CrossRef]
- Nadai, C.; Junior, W.J.F.L.; Favaron, F.; Giacomini, A.; Corich, V. Biocontrol activity of Starmerella bacillaris yeast against blue mold disease on apple fruit and its effect on cider fermentation. PLoS ONE 2018, 13, e0204350. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Droby, S. Metschnikowia fructicola, a new ascosporic yeast with potential for biocontrol of postharvest fruit rots. Syst. Appl. Microbiol. 2001, 24, 395–399. [Google Scholar] [CrossRef]
- Karabulut, O.A.; Smilanick, J.L.; Gabler, F.M.; Mansour, M.; Droby, S. Near-harvest applications of Metschnikowia fructicola, ethanol, and sodium bicarbonate to control postharvest diseases of grape in central California. Plant Disease 2003, 87, 1384–1389. [Google Scholar] [CrossRef]
- Torriani, S.; Tornielli, G. Metschnikowia fructicola, un lievito contro la botrite. Vite&Vino 2019, 2, 38. [Google Scholar]
- Bagheri, B.; Zambelli, P.; Vigentini, I.; Bauer, F.F.; Setati, M.E. Investigating the Effect of Selected Non-Saccharomyces Species on Wine Ecosystem Function and Major Volatiles. Front. Bioeng. Biotechnol. 2018, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Esteve-Zarzoso, B.; Arroyo, T. Non-Saccharomyces yeasts: Biotechnological role for wine production. In Grape and Wine Biotechnology; Morata, A., Ed.; IntechOpen: London, UK, 2016; pp. 249–271. [Google Scholar]
- Nardi, T.; Panero, L.; Petrozziello, M.; Guaita, M.; Tsolakis, C.; Cassino, C.; Vagnoli, P.; Bosso, A. Managing wine quality using Torulaspora delbrueckii and Oenococcus oeni starters in mixed fermentations of a red Barbera wine. Eur. Food Res. Technol. 2019, 245, 293–307. [Google Scholar] [CrossRef]
- Nardi, T.; Bordiga, M. Fermentation Process. In Post-Fermentation and-Distillation Technology: Stabilization, Aging, and Spoilage; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- OIV Code of Good Vitivinicultural Practices in Order to Avoid or Limit Contamination by Brettanomyces. Available online: http://www.oiv.int/en/technical-standards-and-documents/good-practices-guidelines/code-of-good-vitivinicultural-practices-in-order-to-avoid-or-limit-contamination-by-brettanomyces (accessed on 18 February 2020).
- Alexandre, H.; Charpentier, C. Biochemical aspects of stuck and sluggish fermentation in grape must. J. Ind. Microbiol. Biotechnol. 1998, 20, 20–27. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Duc, C.; Pradal, M.; Sanchez, I.; Noble, J.; Tesniere, C.; Blondin, B. A set of nutrient limitations trigger yeast cell death in a nitrogen-dependent manner during wine alcoholic fermentation. PLoS ONE 2017, 12, e0184838. [Google Scholar] [CrossRef]
- Childs, B.C.; Bohlscheid, J.C.; Edwards, C.G. Impact of available nitrogen and sugar concentration in musts on alcoholic fermentation and subsequent wine spoilage by Brettanomyces bruxellensis. Food Microbiol. 2015, 46, 604–609. [Google Scholar] [CrossRef]
- Noble, J.; Sanchez, I.; Blondin, B. Identification of new Saccharomyces cerevisiae variants of the MET2 and SKP2 genes controlling the sulfur assimilation pathway and the production of undesirable sulfur compounds during alcoholic fermentation. Microb. Cell Fact. 2015, 14, 68. [Google Scholar] [CrossRef]
- Morgan, S.C.; Haggerty, J.J.; Johnston, B.; Jiranek, V.; Durall, D.M. Response to Sulfur Dioxide Addition by Two Commercial Saccharomyces cerevisiae Strains. Fermentation 2019, 5, 69. [Google Scholar] [CrossRef]
- Mira de Orduña, R.; Lamon, J. Acetaldehyde Management during Winemaking. Available online: https://www.winemak-in.com/en/publications/acetaldehyde-management-during-winemaking (accessed on 18 February 2020).
- Li, E.; De Orduña, R.M. Acetaldehyde kinetics of enological yeast during alcoholic fermentation in grape must. J. Ind. Microbiol. Biotechnol. 2017, 44, 229–236. [Google Scholar] [CrossRef]
- Coetzee, C.; Buica, A.; Du Toit, W.J. The Use of SO2 to Bind Acetaldehyde in Wine: Sensory Implications. S. Afr. J. Enol. Viticult. 2018, 39, 1–6. [Google Scholar]
- Osborne, J.P.; de Orduña, R.M.; Pilone, G.J.; Liu, S.-Q. Acetaldehyde metabolism by wine lactic acid bacteria. FEMS Microbiol. Lett. 2000, 191, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Blondin, B.; Noble, J.; Sanchez, I. Method for Controlling the Production of Sulphites, of Hydrogen Sulphide and of Acetaldehyde by Yeasts—Global Patent Index—EP 2807247 B1; European Patent Office: Munich, Germany, 2017. [Google Scholar]
- Binati, R.L.; Lemos Junior, W.J.F.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Inte. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef] [PubMed]
- Andorrà, I.; Martín, L.; Nart, E.; Puxeu, M.; Hidalgo, C.; Ferrer-Gallego, R. Effect of grape juice composition and nutrient supplementation on the production of sulfur dioxide and carboxylic compounds by Saccharomyces cerevisiae. Aust. J. Grape Wine Res. 2018, 24, 260–266. [Google Scholar] [CrossRef]
- Masneuf-Pomarède, I.; Mansour, C.; Murat, M.-L.; Tominaga, T.; Dubourdieu, D. Influence of fermentation temperature on volatile thiols concentrations in Sauvignon blanc wines. Int. J. Food Microbiol. 2006, 108, 385–390. [Google Scholar]
- Torija, M.J.; Beltran, G.; Novo, M.; Poblet, M.; Guillamón, J.M.; Mas, A.; Rozes, N. Effects of fermentation temperature and Saccharomyces species on the cell fatty acid composition and presence of volatile compounds in wine. Int. J. Food Microbiol. 2003, 85, 127–136. [Google Scholar] [CrossRef]
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.S.; Agosin, E. Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Appl. Microbiol. Biotechnol. 2007, 77, 675–687. [Google Scholar] [CrossRef]
- Galitsky, C.; Worrell, E.; Radspieler, A.; Healy, P.; Zechiel, S. BEST Winery Guidebook: Benchmarking and Energy and Water Savings Tool for the Wine Industry; Alameda Lab: Alameda, CA, USA, 2005. [Google Scholar]
- Malvoni, M.; Congedo, P.M.; Laforgia, D. Analysis of energy consumption: A case study of an Italian winery. Energy Procedia 2017, 126, 227–233. [Google Scholar] [CrossRef]
- Flint, D.J.; Golicic, S.L. Searching for competitive advantage through sustainability. Int. J. Phys. Distrib. Logist. Manag. 2009, 39, 841–860. [Google Scholar] [CrossRef]
- Szolnoki, G. A cross-national comparison of sustainability in the wine industry. J. Clean. Prod. 2013, 53, 243–251. [Google Scholar] [CrossRef]
- Galletto, L.; Barisan, L. Carbon footprint as a lever for sustained competitive strategy in developing a smart oenology: Evidence from an exploratory study in Italy. Sustainability 2019, 11, 1483. [Google Scholar] [CrossRef]
- Giovenzana, V.; Beghi, R.; Vagnoli, P.; Iacono, F.; Guidetti, R.; Nardi, T. Evaluation of Energy Saving Using a New Yeast Combined with Temperature Management in Sparkling Base Wine Fermentation. Am. J. Enol. Viticult. 2016, 67, 308–314. [Google Scholar] [CrossRef]
- Schwinn, M.; Durner, D.; Wacker, M.; Delgado, A.; Fischer, U. Impact of fermentation temperature on required heat dissipation, growth and viability of yeast, on sensory characteristics and on the formation of volatiles in Riesling. Aust. J. Grape Wine Res. 2019, 25, 173–184. [Google Scholar] [CrossRef]
- Gerbaux, V.; Briffox, C.; Dumont, A.; Krieger, S. Influence of inoculation with malolactic bacteria on volatile phenols in wines. Am. J. Enol. Viticult. 2009, 60, 233–235. [Google Scholar]
- Nardi, T.; Vagnoli, P.; Minacci, A.; Gautier, S.; Sieczkowski, N. Evaluating the impact of a fungal-origin chitosan preparation on Brettanomyces bruxellensis in the context of wine aging. Wine Stud. 2014, 3. [Google Scholar] [CrossRef]
- Coulon, J.; Perello, M.C.; Lonvaud-Funel, A.; De Revel, G.; Renouf, V. Brettanomyces bruxellensis evolution and volatile phenols production in red wines during storage in bottles. J. Appl. Microbiol. 2010, 108, 1450–1458. [Google Scholar] [CrossRef]
- Bauer, R.; Dicks, L.M.T. Control of malolactic fermentation in wine. A review. S. Afr. J. Enol. Vitic. 2004, 25, 74–88. [Google Scholar] [CrossRef]
- Bartowsky, E.J. Bacterial spoilage of wine and approaches to minimize it. Lett. Appl. Microbiol. 2009, 48, 149–156. [Google Scholar] [CrossRef]
- Alexandre, H.; Costello, P.J.; Remize, F.; Guzzo, J.; Guilloux-Benatier, M. Saccharomyces cerevisiae–Oenococcus oeni interactions in wine: Current knowledge and perspectives. Int. J. Food Microbiol. 2004, 93, 141–154. [Google Scholar] [CrossRef]
- Lerm, E.; Engelbrecht, L.; Du Toit, M. Malolactic fermentation: The ABC’s of MLF. S. Afr. J. Enol. Vitic. 2010, 31, 186–212. [Google Scholar] [CrossRef]
- Liu, S.-Q. Malolactic fermentation in wine–beyond deacidification. J. Appl. Microbiol. 2002, 92, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Terrade, N.; de Orduña, R.M. Determination of the essential nutrient requirements of wine-related bacteria from the genera Oenococcus and Lactobacillus. Int. J. Food Microbiol. 2009, 133, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rousseaux, S.; Tourdot-Maréchal, R.; Sadoudi, M.; Gougeon, R.; Schmitt-Kopplin, P.; Alexandre, H. Wine microbiome, a dynamic world of microbial interactions. Crit. Rev. Food Sci. Nutr. 2017, 57, 856–873. [Google Scholar] [CrossRef] [PubMed]
- Bartle, L.; Sumby, K.; Sundstrom, J.; Jiranek, V. The microbial challenge of winemaking: Yeast-bacteria compatibility. FEMS Yeast Res. 2019, 19, foz040. [Google Scholar] [CrossRef] [PubMed]
- Sumby, K.M.; Bartle, L.; Grbin, P.R.; Jiranek, V. Measures to improve wine malolactic fermentation. Appl. Microbiol. Biotechnol. 2019, 103, 2033–2051. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Forcisi, S.; Harir, M.; Deleris-Bou, M.; Krieger-Weber, S.; Lucio, M.; Longin, C.; Degueurce, C.; Gougeon, R.D.; Schmitt-Kopplin, P.; et al. New molecular evidence of wine yeast-bacteria interaction unraveled by non-targeted exometabolomic profiling. Metabolomics 2016, 12, 1–16. [Google Scholar] [CrossRef]
- Berbegal, C.; Borruso, L.; Fragasso, M.; Tufariello, M.; Russo, P.; Brusetti, L.; Spano, G.; Capozzi, V. A Metagenomic-Based Approach for the Characterization of Bacterial Diversity Associated with Spontaneous Malolactic Fermentations in Wine. Int. J. Mol. Sci. 2019, 20, 3980. [Google Scholar] [CrossRef]
- Krieger-Weber, S. Application of yeast and bacteria as starter cultures. In Biology of Microorganisms on Grapes, in Must and in Wine; Springer: Berlin/Heidelberg, Germany, 2017; pp. 605–634. [Google Scholar]
- Lonvaud-Funel, A. Biogenic amines in wines: Role of lactic acid bacteria. FEMS Microbiol. Lett. 2001, 199, 9–13. [Google Scholar] [CrossRef]
- Marques, A.P.; Leitão, M.C.; San Romão, M.V. Biogenic amines in wines: Influence of oenological factors. Food Chem. 2008, 107, 853–860. [Google Scholar] [CrossRef]
- . Capozzi, V.; Russo, P.; Ladero, V.; Fernández, M.; Fiocco, D.; Alvarez, M.A.; Grieco, F.; Spano, G. Biogenic amines degradation by Lactobacillus plantarum: toward a potential application in wine. Front. Microbiol. 2012, 3, 122. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Costello, P.J.; Chambers, P.J. Emerging trends in the application of malolactic fermentation. Aust. J. Grape Wine Res. 2015, 21, 663–669. [Google Scholar] [CrossRef]
- Guzzon, R.; Villega, T.R.; Pedron, M.; Malacarne, M.; Nicolini, G.; Larcher, R. Simultaneous yeast–bacteria inoculum. A feasible solution for the management of oenological fermentation in red must with low nitrogen content. Ann. Microbiol. 2013, 63, 805–808. [Google Scholar] [CrossRef]
- Knoll, C.; Fritsch, S.; Schnell, S.; Grossmann, M.; Krieger-Weber, S.; Du Toit, M.; Rauhut, D. Impact of different malolactic fermentation inoculation scenarios on Riesling wine aroma. World J. Microbiol. Biotechnol. 2012, 28, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Guzzon, R.; Davide, S.; Villegas, T.R.; Malacarne, M.; Larcher, R.; Nardi, T.; Vagnoli, P.; Krieger-Weber, S. Exploitation of Simultaneous Alcoholic and Malolactic Fermentation of Incrocio Manzoni, a Traditional Italian White Wine. S. Afr. J. Enol. Viticult. 2016, 37, 124–131. [Google Scholar] [CrossRef][Green Version]
- Mo, S.; Mo, F. Differenti protocolli di gestione della fermentazione malolattica in vini Barbera d’Asti. In L’ENOLOGO; Assoenologi: Milano, Italy, 2009; pp. 77–81. [Google Scholar]
- CDTI VINYSOST—EU Project. Available online: http://vinysost.com/ (accessed on 26 February 2020).
- Vagnoli, P. “I Giorni della Sostenibilità 2020” Seminari Formazione Avanzata—Personal Communication; CIRVE, Università di Padova: Padova, Italy, 2020. [Google Scholar]
- Jackowetz, J.N.; Mira de Orduña, R. Metabolism of SO2 binding compounds by Oenococcus oeni during and after malolactic fermentation in white wine. Int. J. Food Microbiol. 2012, 155, 153–157. [Google Scholar] [CrossRef]
- Osborne, J.P.; Dubé Morneau, A.; Mira de Orduna, R. Degradation of free and sulfur-dioxide-bound acetaldehyde by malolactic lactic acid bacteria in white wine. J. Appl. Microbiol. 2006, 101, 474–479. [Google Scholar] [CrossRef]
- Taillandier, P.; Joannis-Cassan, C.; Jentzer, J.-B.; Gautier, S.; Sieczkowski, N.; Granes, D.; Brandam, C. Effect of a fungal chitosan preparation on Brettanomyces bruxellensis, a wine contaminant. J. Appl. Microbiol. 2015, 118, 123–131. [Google Scholar] [CrossRef]
- Valera, M.J.; Sainz, F.; Mas, A.; Torija, M.J. Effect of chitosan and SO2 on viability of Acetobacter strains in wine. Int. J. Food Microbiol. 2017, 246, 1–4. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- EU Commission Regulation (EU) No 53/2011 of 21 January 2011 Amending Regulation (EC) No 606/2009 Laying down Certain Detailed Rules for Implementing Council Regulation (EC) No 479/2008 as Regards the Categories of Grapevine Products, Oenological Practices and the Applicable Restrictions; Official Journal L019/2011. Available online: http://data.europa.eu/eli/reg/2011/53/oj/eng (accessed on 25 February 2020).
- Petrova, B.; Cartwright, Z.M.; Edwards, C.G. Effectiveness of chitosan preparations against Brettanomyces bruxellensis grown in culture media and red wines. Oeno One 2016, 50, 49–56. [Google Scholar] [CrossRef]
- Zuehlke, J.M.; Petrova, B.; Edwards, C.G. Advances in the control of wine spoilage by Zygosaccharomyces and Dekkera/Brettanomyces. Annu. Rev. Food Sci. Technol. 2013, 4, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Malfeito-Ferreira, M. Two Decades of “Horse Sweat” Taint and Brettanomyces Yeasts in Wine: Where do We Stand Now? Beverages 2018, 4, 32. [Google Scholar] [CrossRef]
- Bastard, A.; Coelho, C.; Briandet, R.; Canette, A.; Gougeon, R.D.; Alexandre, H.; Guzzo, J.; Weidmann, S. Effect of biofilm formation by Oenococcus oeni on malolactic fermentation and the release of aromatic compounds in wine. Front. Microbiol. 2016, 7, 613. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed]
- Bovo, B.; Nardi, T.; Fontana, F.; Carlot, M.; Giacomini, A.; Corich, V. Acidification of grape marc for alcoholic beverage production: Effects on indigenous microflora and aroma profile after distillation. Int. J. Food Microbiol. 2012, 152, 100–106. [Google Scholar] [CrossRef]
- Campanaro, S.; Treu, L.; Vendramin, V.; Bovo, B.; Giacomini, A.; Corich, V. Metagenomic analysis of the microbial community in fermented grape marc reveals that Lactobacillus fabifermentans is one of the dominant species: Insights into its genome structure. Appl. Microbiol. Biotechnol. 2014, 98, 6015–6037. [Google Scholar] [CrossRef]
- Maragkoudakis, P.A.; Nardi, T.; Bovo, B.; D’Andrea, M.; Howell, K.S.; Giacomini, A.; Corich, V. Biodiversity, dynamics and ecology of bacterial community during grape marc storage for the production of grappa. Int. J. Food Microbiol. 2013, 162, 143–151. [Google Scholar] [CrossRef]
- Iacumin, L.; Manzano, M.; Cecchini, F.; Orlic, S.; Zironi, R.; Comi, G. Influence of specific fermentation conditions on natural microflora of pomace in “Grappa” production. World J. Microbiol. Biotechnol. 2012, 28, 1747–1759. [Google Scholar] [CrossRef]
- Bovo, B.; Fontana, F.; Giacomini, A.; Corich, V. Effects of yeast inoculation on volatile compound production by grape marcs. Ann. Microbiol. 2011, 61, 117–124. [Google Scholar] [CrossRef]
- Bordiga, M. Valorization of Wine Making by-Products; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity—A review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Karpe, A.V.; Beale, D.J.; Harding, I.H.; Palombo, E.A. Optimization of degradation of winery-derived biomass waste by Ascomycetes. J. Chem. Technol. Biotechnol. 2015, 90, 1793–1801. [Google Scholar] [CrossRef]
- Zepf, F.; Jin, B. Bioconversion of grape marc into protein rich animal feed by microbial fungi. Chem. Eng. Process Tech. 2013, 1, 1011–1018. [Google Scholar]
- Sotiropoulou, E.I.; Liouni, M.; Calokerinos, A.C.; Nerantzis, E. Utilization of grape pomace for the production of microbial protein-A review. In Proceedings of the 5th International Conference on Sustainable Solid Waste Management, Athens, Greece, 21–24 June 2017; pp. 21–24. [Google Scholar]
- Avantaggiato, G.; Greco, D.; Damascelli, A.; Solfrizzo, M.; Visconti, A. Assessment of multi-mycotoxin adsorption efficacy of grape pomace. J. Agricult. Food Chem. 2014, 62, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Gude, V.G. Wastewater treatment in microbial fuel cells–an overview. J. Clean. Prod. 2016, 122, 287–307. [Google Scholar] [CrossRef]
- Cercado-Quezada, B.; Delia, M.-L.; Bergel, A. Testing various food-industry wastes for electricity production in microbial fuel cell. Bioresour. Technol. 2010, 101, 2748–2754. [Google Scholar] [CrossRef]
- Logan, B.E.; Regan, J.M. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006, 14, 512–518. [Google Scholar] [CrossRef]
- Penteado, E.D.; Fernandez-Marchante, C.M.; Zaiat, M.; Cañizares, P.; Gonzalez, E.R.; Rodrigo, M.A. Energy recovery from winery wastewater using a dual chamber microbial fuel cell. J. Chem. Technol. Biotechnol. 2016, 91, 1802–1808. [Google Scholar] [CrossRef]
- Penteado, E.D.; Fernandez-Marchante, C.M.; Zaiat, M.; Gonzalez, E.R.; Rodrigo, M.A. Influence of carbon electrode material on energy recovery from winery wastewater using a dual-chamber microbial fuel cell. Environ. Technol. 2017, 38, 1333–1341. [Google Scholar] [CrossRef]
- Sciarria, T.P.; Merlino, G.; Scaglia, B.; D’Epifanio, A.; Mecheri, B.; Borin, S.; Licoccia, S.; Adani, F. Electricity generation using white and red wine lees in air cathode microbial fuel cells. J. Power Sources 2015, 274, 393–399. [Google Scholar] [CrossRef]
- Biovale. Available online: https://www.progettoager.it/index.php/settori/trasferimento-tecnologico-i-progetti/trasferimento-tecnologico-i-progetti-biovale (accessed on 28 February 2020).
- Insam, H.; Riddech, N.; Klammer, S. Microbiology of Composting; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Santos, M.; Diánez, F.; del Valle, M.G.; Tello, J.C. Grape marc compost: Microbial studies and suppression of soil-borne mycosis in vegetable seedlings. World J. Microbiol. Biotechnol. 2008, 24, 1493–1505. [Google Scholar] [CrossRef]
- Patti, A.F.; Issa, G.; Smernik, R.; Wilkinson, K. Chemical composition of composted grape marc. Water Sci. Technol. 2009, 60, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Viel, A.; Stellin, F.; Carlot, M.; Nadai, C.; Concheri, G.; Stevanato, P.; Squartini, A.; Corich, V.; Giacomini, A. Characteristics of Compost Obtained from Winemaking Byproducts. Waste Biomass Valoriz. 2018, 9, 2021–2029. [Google Scholar] [CrossRef]
- Majbar, Z.; Lahlou, K.; Ben Abbou, M.; Ammar, E.; Triki, A.; Abid, W.; Nawdali, M.; Bouka, H.; Taleb, M.; El Haji, M. Co-composting of Olive Mill Waste and Wine-Processing Waste: An Application of Compost as Soil Amendment. J. Chem. 2018, 2018, 7918583. [Google Scholar] [CrossRef]
- Excessive, K. Using composted grape marc in the vineyard. Water Sci. Technol. 2018, 60, 1265–1271. [Google Scholar]
- Diánez, F.; Santos, D.M.; Tello, J.C. Suppressive effects of grape marc compost on phytopathogenic oomycetes. Arch. Phytopathol. Plant Prot. 2007, 40, 1–18. [Google Scholar] [CrossRef]

© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardi, T. Microbial Resources as a Tool for Enhancing Sustainability in Winemaking. Microorganisms 2020, 8, 507. https://doi.org/10.3390/microorganisms8040507
Nardi T. Microbial Resources as a Tool for Enhancing Sustainability in Winemaking. Microorganisms. 2020; 8(4):507. https://doi.org/10.3390/microorganisms8040507
Chicago/Turabian StyleNardi, Tiziana. 2020. "Microbial Resources as a Tool for Enhancing Sustainability in Winemaking" Microorganisms 8, no. 4: 507. https://doi.org/10.3390/microorganisms8040507
APA StyleNardi, T. (2020). Microbial Resources as a Tool for Enhancing Sustainability in Winemaking. Microorganisms, 8(4), 507. https://doi.org/10.3390/microorganisms8040507