Nitrogen Preferences during Alcoholic Fermentation of Different Non-Saccharomyces Yeasts of Oenological Interest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Fermentation Conditions and Sampling
2.3. Nitrogen Analysis
2.4. Metabolite Analysis
2.5. Statistical Analysis
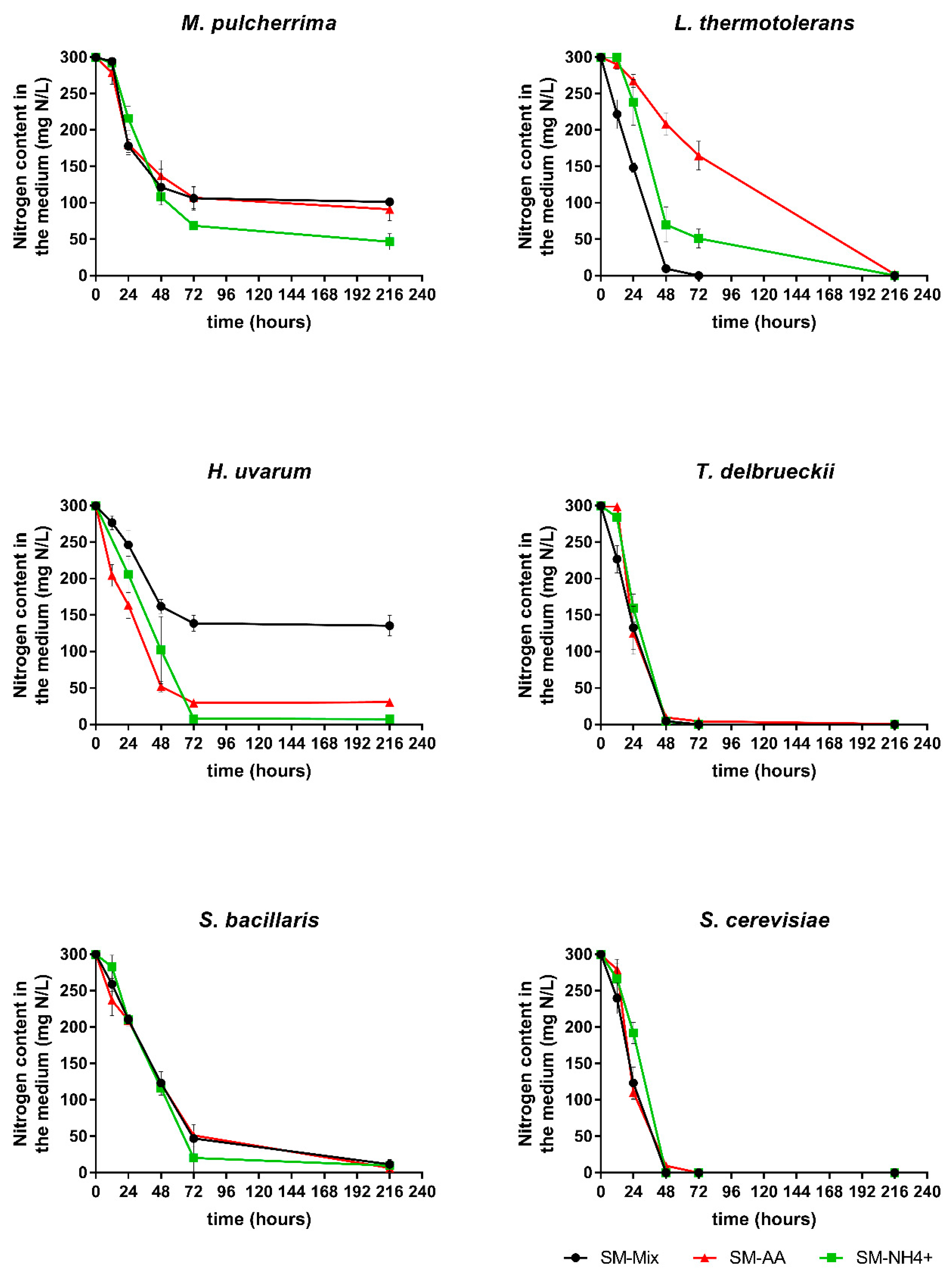
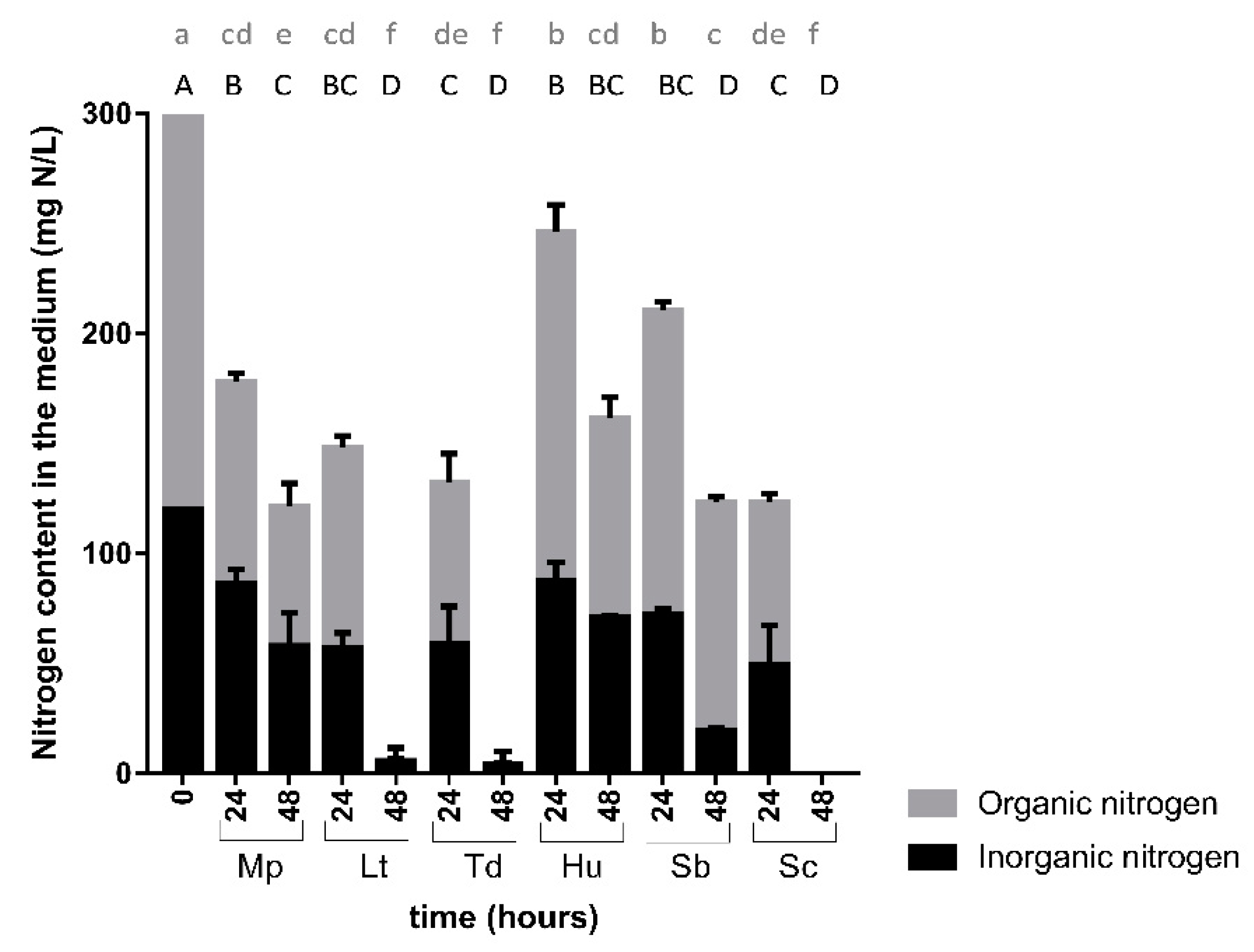
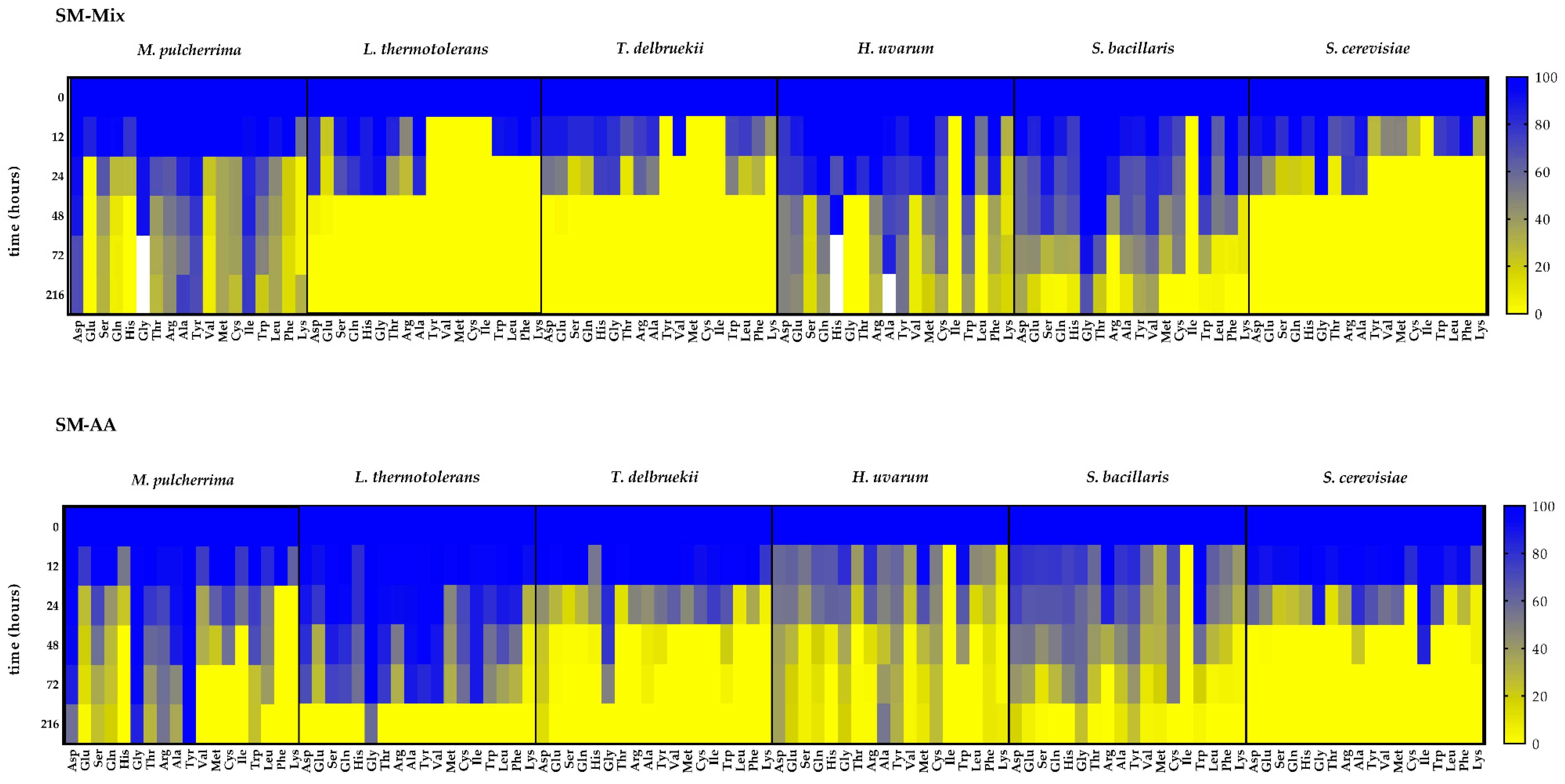
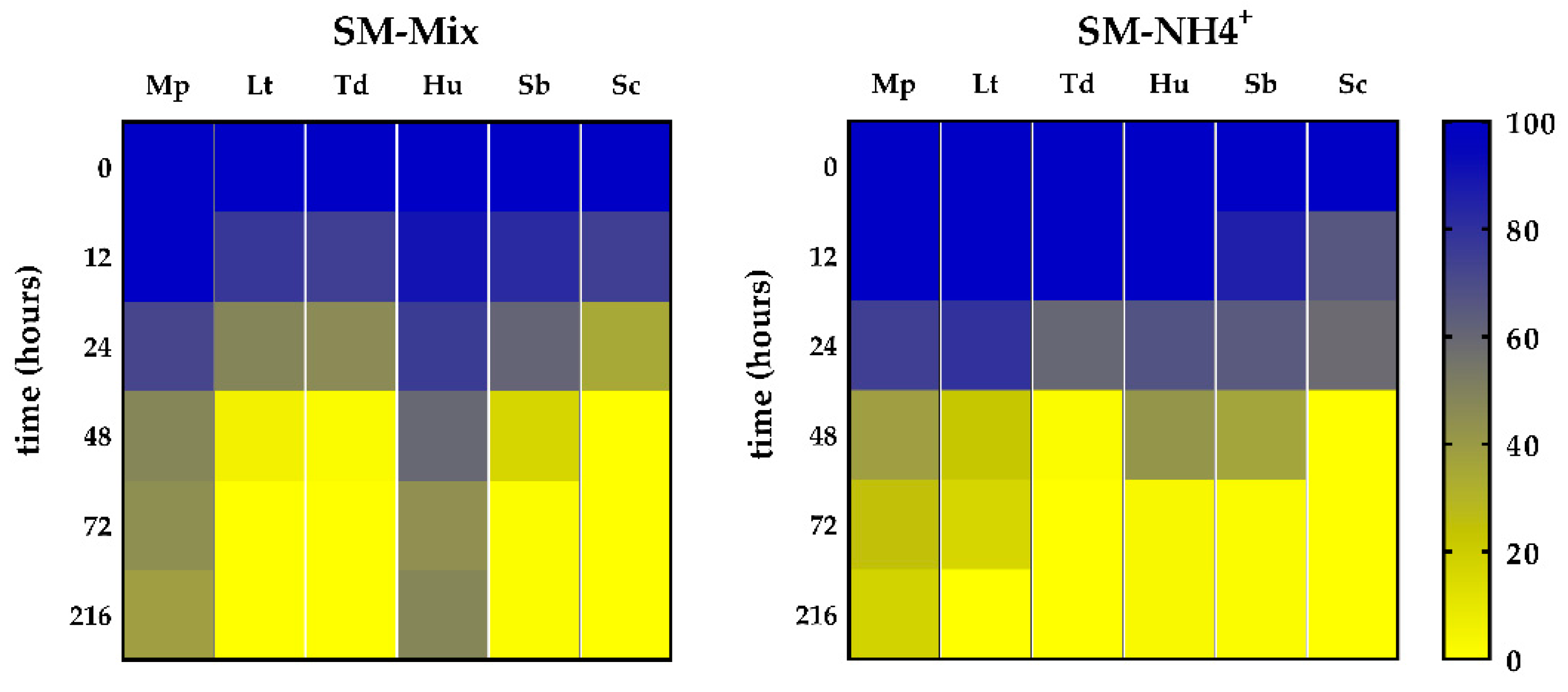
3. Results
3.1. Fermentation Kinetics
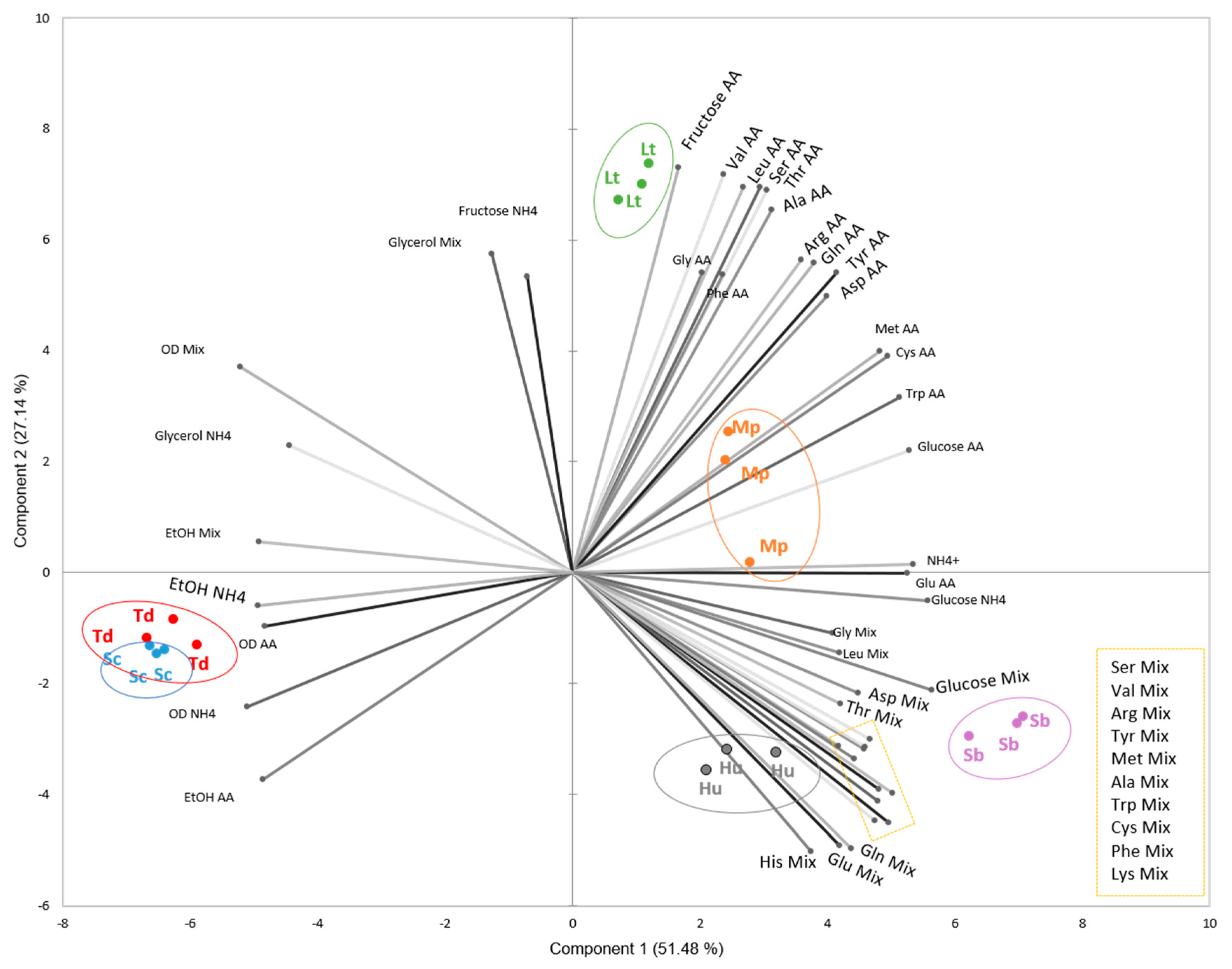
3.2. Nitrogen Consumption and Preferences
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology: The Microbiology of Wine and Vinifications, 2nd ed.; Jhon Wiley & Sons, Ltd.: Chichester, UK, 2006; pp. 22–53. [Google Scholar] [CrossRef]
- Lleixà, J.; Manzano, M.; Mas, A.; del Portillo, M.C. Saccharomyces and non-Saccharomyces competition during microvinification under different sugar and nitrogen conditions. Front. Microbiol. 2016, 7, 1959. [Google Scholar] [CrossRef]
- Renault, P.; Coulon, J.; Moine, V.; Thibon, C.; Bely, M. Enhanced 3-sulfanylhexan-1-ol production in sequential mixed fermentation with Torulaspora delbrueckii/Saccharomyces cerevisiae reveals a situation of synergistic interaction between two industrial strains. Front. Microbiol. 2016, 7, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albertin, W.; Zimmer, A.; Miot-Sertier, C.; Bernard, M.; Coulon, J.; Moine, V.; Colonna-Ceccaldi, B.; Bely, M.; Marullo, P.; Masneuf-Pomarede, I. Combined effect of the Saccharomyces cerevisiae lag phase and the non-Saccharomyces consortium to enhance wine fruitiness and complexity. Appl. Microbiol. Biotechnol. 2017, 101, 7603–7620. [Google Scholar] [CrossRef] [PubMed]
- Andorrà, I.; Berradre, M.; Mas, A.; Esteve-Zarzoso, B.; Guillamón, J.M. Effect of pure and mixed cultures of the main wine yeast species on grape must fermentations. Eur. Food Res. Technol. 2010, 231, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Lleixà, J.; Martín, V.; del Portillo, M.C.; Carrau, F.; Beltran, G.; Mas, A. Comparison of Fermentation and Wines Produced by Inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 338. [Google Scholar] [CrossRef] [Green Version]
- Renault, P.; Coulon, J.; Revel, G.; De Barbe, J.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef] [Green Version]
- Quirós, M.; Rojas, V.; Gonzalez, R.; Morales, P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food Microbiol. 2014, 181, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Medina, K.; Boido, E.; Dellacassa, E.; Carrau, F. Growth of non-Saccharomyces yeasts affects nutrient availability for Saccharomyces cerevisiae during wine fermentation. Int. J. Food Microbiol. 2012, 157, 245–250. [Google Scholar] [CrossRef]
- Rollero, S.; Bloem, A.; Ortiz-Julien, A.; Camarasa, C.; Divol, B. Altered fermentation performances, growth, and metabolic footprints reveal competition for nutrients between yeast species inoculated in synthetic grape juice-like medium. Front. Microbiol. 2018, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Bisson, L.F. Stuck and sluggish fermentations. Am. J. Enol. Vitic. 1999, 50, 107–119. [Google Scholar]
- Carrau, F.M.; Medina, K.; Farina, L.; Boido, E.; Henschke, P.A.; Dellacassa, E. Production of fermentation aroma compounds by Saccharomyces cerevisiae wine yeasts: Effects of yeast assimilable nitrogen on two model strains. FEMS Yeast Res. 2008, 8, 1196–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairbairn, S.; McKinnon, A.; Musarurwa, H.T.; Ferreira, A.C.; Bauer, F.F. The Impact of Single Amino Acids on Growth and Volatile Aroma Production by Saccharomyces cerevisiae Strains. Front. Microbiol. 2017, 8, 2554. [Google Scholar] [CrossRef] [Green Version]
- González, B.; Vázquez, J.; Morcillo-Parra, M.A.; Mas, A.; Torija, M.J.; Beltran, G. The production of aromatic alcohols in non-Saccharomyces wine yeast is modulated by nutrient availability. Food Microbiol. 2018, 74, 64–74. [Google Scholar] [CrossRef]
- Jiranek, V.; Langridge, P.; Henschke, P.A. Amino acid and ammonium utilization by Saccharomyces cerevisiae wine yeasts from a chemically defined medium. Am. J. Enol. Vitic. 1995, 46, 75–83. [Google Scholar]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Gobert, A.; Tourdot-Maréchal, R.; Morge, C.; Sparrow, C.; Liu, Y.; Quintanilla-Casas, B.; Vichi, S.; Alexandre, H. Non-Saccharomyces Yeasts nitrogen source preferences: Impact on sequential fermentation and wine volatile compounds profile. Front. Microbiol. 2017, 8, 2175. [Google Scholar] [CrossRef] [Green Version]
- Ingledew, W.M.; Kunkee, R. Factors Influencing Sluggish Fermentations of Grape Juice. Am. J. Enol. Vitic. 1985, 36, 65–76. [Google Scholar]
- Butzke, C.E.; Bisson, L.F. Diagnosis and rectification of stuck and sluggish fermentations. Am. J. Enol. Vitic. 2000, 51, 168–177. [Google Scholar]
- Martínez-Moreno, R.; Quirós, M.; Morales, P.; Gonzalez, R. New insights into the advantages of ammonium as a winemaking nutrient. Int. J. Food Microbiol. 2014, 177, 128–135. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Chiva, R.; Sancho, M.; Beltran, G.; Arroyo-López, F.N.; Guillamón, J.M. Nitrogen requirements of commercial wine yeast strains during fermentation of a synthetic grape must. Food Microbiol. 2012, 31, 25–32. [Google Scholar] [CrossRef]
- Monteiro, F.; Bisson, L. Biological assay of nitrogen content of grape juice and prediction of sluggish fermentations. Am. J. Enol. Vitic. 1991, 42, 47–57. [Google Scholar]
- Bell, A.A.; Ough, C.S.; Kliewer, W.M. Effects on Must and Wine Composition, Rates of Fermentation, and Wine Quality of Nitrogen Fertilization of Vitis Vinifera Var. Thompson Seedless Grapevines. Am. J. Enol. Vitic. 1979, 30, 124–129. [Google Scholar]
- Torija, M.J.; Beltran, G.; Novo, M.; Poblet, M.; Rozès, N.; Mas, A.; Guillamón, J.M. Effect of organic acids and nitrogen source on alcoholic fermentation: Study of their buffering capacity. J. Agric. Food Chem. 2003, 51, 916–922. [Google Scholar] [CrossRef]
- Beltran, G.; Esteve-Zarzoso, B.; Rozès, N.; Mas, A.; Guillamón, J.M. Influence of the timing of nitrogen additions during synthetic grape must fermentations on fermentation kinetics and nitrogen consumption. J. Agric. Food Chem. 2005, 53, 996–1002. [Google Scholar] [CrossRef]
- Mendes-Ferreira, A.; Mendes-Faia, A.; Leao, C. Growth and fermentation patterns of Saccharomyces cerevisiae under different ammonium concentrations and its implications in winemaking industry. J. Appl. Microbiol. 2004, 97, 540–545. [Google Scholar] [CrossRef]
- Vilanova, M.; Ugliano, M.; Varela, C.; Siebert, T.; Pretorius, I.S.; Henschke, P.A. Assimilable nitrogen utilisation and production of volatile and non-volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine yeasts. Appl. Microbiol. Biotechnol. 2007, 77, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Martí, E.; Aranda, A.; Mendes-Ferreira, A.; Mendes-Faia, A.; del Olmo, M.L. The nature of the nitrogen source added to nitrogen depleted vinifications conducted by a Saccharomyces cerevisiae strain in synthetic must affects gene expression and the levels of several volatile compounds. Antonie Van Leeuwenhoek 2007, 92, 61–75. [Google Scholar] [CrossRef]
- Gobert, A.; Tourdot-Maréchal, R.; Sparrow, C.; Morge, C.; Alexandre, H. Influence of nitrogen status in wine alcoholic fermentation. Food Microbiol. 2019, 83, 71–85. [Google Scholar] [CrossRef]
- Kemsawasd, V.; Viana, T.; Ardö, Y.; Arneborg, N. Influence of nitrogen sources on growth and fermentation performance of different wine yeast species during alcoholic fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 10191–10207. [Google Scholar] [CrossRef]
- Prior, K.J.; Bauer, F.F.; Divol, B. The utilisation of nitrogenous compounds by commercial non-Saccharomyces yeasts associated with wine. Food Microbiol. 2019, 79, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Rollero, S.; Bloem, A.; Ortiz-Julien, A.; Camarasa, C.; Divol, B. Fermentation performances and aroma production of non-conventional wine yeasts are influenced by nitrogen preferences. FEMS Yeast Res. 2018, 18, foy055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Seguinot, P.; Sanchez, I.; Ortiz-Julien, A.; Heras, J.M.; Querol, A.; Camarasa, C.; Guillamón, J.M. Nitrogen sources preferences of non-Saccharomyces yeasts to sustain growth and fermentation under winemaking conditions. Food Microbiol. 2020, 85, 103287. [Google Scholar] [CrossRef] [PubMed]
- Englezos, V.; Cocolin, L.; Rantsiou, K.; Ortiz-Julien, A.; Bloem, A.; Dequin, S.; Camarasa, C. Specific Phenotypic Traits of Starmerella bacillaris Related to Nitrogen Source Consumption and Central Carbon Metabolite Production during Wine Fermentation. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollero, S.; Bloem, A.; Ortiz-Julien, A.; Bauer, F.F.; Camarasa, C.; Divol, B. A comparison of the nitrogen metabolic networks of Kluyveromyces marxianus and Saccharomyces cerevisiae. Environ Microbiol. 2019, 21, 4076–4409. [Google Scholar] [CrossRef]
- Seguinot, P.; Bloem, A.; Brial, P.; Meudec, E.; Ortiz-Julien, A.; Camarasa, C. Analysing the impact of the nature of the nitrogen source on the formation of volatile compounds to unravel the aroma metabolism of two non-Saccharomyces strains. Int. J. Food Microbiol. 2020, 316, 108441. [Google Scholar] [CrossRef]
- Andorrà, I.; Berradre, M.; Mas, A.; Esteve-Zarzoso, B.; Guillamón, J.M. Effect of mixed culture fermentations on yeast populations and aroma profile. LWT 2012, 49, 8–13. [Google Scholar] [CrossRef]
- Taillandier, P.; Lai, Q.P.; Julien-Ortiz, A.; Brandam, C. Interactions between Torulaspora delbrueckii and Saccharomyces cerevisiae in wine fermentation: Influence of inoculation and nitrogen content. World J. Microbiol. Biotechnol. 2014, 30, 1959–1967. [Google Scholar] [CrossRef] [Green Version]
- Alonso-del-Real, J.; Pérez-Torrado, R.; Querol, A.; Barrio, E. Dominance of wine Saccharomyces cerevisiae strains over S. kudriavzevii in industrial fermentation competitions is related to an acceleration of nutrient uptake and utilization. Environ. Microbiol. 2019, 21, 1627–1644. [Google Scholar] [CrossRef] [Green Version]
- Curiel, J.A.; Morales, P.; Gonzalez, R.; Tronchoni, J. Different non-Saccharomyces yeast species stimulate nutrient consumption in S. cerevisiae mixed cultures. Front. Microbiol. 2017, 8, 2121. [Google Scholar] [CrossRef] [PubMed]
- Beltran, G.; Novo, M.; Rozes, N.; Mas, A.; Guillamon, J. Nitrogen catabolite repression in during wine fermentations. FEMS Yeast Res. 2004, 4, 625–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Alonso, S.; Hermosín-Gutiérrez, I.; Esteban, G.-R. Simultaneous HPLC Analysis of Biogenic Amines, Amino Acids, and Ammonium Ion as Aminoenone Derivatives in Wine and Beer Samples. J. Agric. Food Chem. 2007, 55, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Quirós, M.; Gonzalez-Ramos, D.; Tabera, L.; Gonzalez, R. A new methodology to obtain wine yeast strains overproducing mannoproteins. Int. J. Food Microbiol. 2010, 139, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [Green Version]
- Bauer, F.F.; Pretorius, I.S. Yeast Stress Response and Fermentation Efficiency: How to Survive the Making of Wine—A Review. S. Afr. J. Enol. Vitic. 2000, 21, 27–51. [Google Scholar] [CrossRef]
- Beltran, G.; Torija, M.J.; Novo, M.; Ferrer, N.; Poblet, M.; Guillamón, J.M.; Rozès, N.; Mas, A. Analysis of yeast populations during alcoholic fermentation: A six year follow-up study. Syst. Appl. Microbiol. 2002, 25, 287–293. [Google Scholar] [CrossRef]
- Torija, M.J.; Rozès, N.; Poblet, M.; Guillamón, J.M.; Mas, A. Yeast population dynamics in spontaneous fermentations: Comparison between two different wine-producing areas over a period of three years. Antonie Van Leeuwenhoek 2001, 79, 345–352. [Google Scholar] [CrossRef]
- De Koker, S. Nitrogen Utilisation of Selected Non-Saccharomyces Yeasts and the Impact on Volatile Compound Production. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, December 2015. [Google Scholar]
- Lleixà, J.; Martín, V.; Giorello, F.; del Portillo, M.C.; Carrau, F.; Beltran, G.; Mas, A. Analysis of the NCR Mechanisms in Hanseniaspora vineae and Saccharomyces cerevisiae During Winemaking. Front. Genet. 2019, 9, 747. [Google Scholar] [CrossRef] [Green Version]
- Ljungdahl, P.O. Amino-acid-induced signalling via the SPS-sensing pathway in yeast. Biochem. Soc. Trans. 2009, 37, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Marini, A.-M.; Soussi-Boudekou, S.; Vissers, S.; Andre, B. A Family of Ammonium Transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 1997, 17, 4282–4293. [Google Scholar] [CrossRef] [Green Version]
- Seixas, I.; Barbosa, C.; Mendes-Faia, A.; Güldener, U.; Tenreiro, R.; Mendes-Ferreira, A.; Mira, N.P. Genome sequence of the non-conventional wine yeast Hanseniaspora guilliermondii UTAD222 unveils relevant traits of this species and of the Hanseniaspora genus in the context of wine fermentation. DNA Res. 2019, 26, 67–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, T.G. Nitrogen metabolism in Saccharomyces cerevisiae. In The Molecular Biology of the Yeast Saccharomyces, Metabolism and Gene Expression; Strathern, J.N., Jones, E.W., Broach, J.B., Eds.; Cold Spring Harbor Laboratory: New York, NY, USA, 1982; pp. 399–462. [Google Scholar]
- Ljungdahl, P.O.; Daignan-Fornier, B. Regulation of amino acid, nucleotide, and phosphate metabolism in Saccharomyces cerevisiae. Genetics 2012, 190, 885–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, A.; Sancho, M.; Beltran, G.; Guillamon, J.M.; Warringer, J. Replenishment and mobilization of intracellular nitrogen pools decouples wine yeast nitrogen uptake from growth. Appl. Microbiol. Biotechnol. 2016, 100, 3255–3265. [Google Scholar] [CrossRef]
- Cubillos, F.A.; Louis, E.J.; Liti, G. Generation of a large set of genetically tractable haploid and diploid Saccharomyces strains. FEMS Yeast Res. 2009, 9, 1217–1225. [Google Scholar] [CrossRef] [Green Version]
- Salinas, F.; Cubillos, A.; Soto, D.; Garcia, V.; Bergström, A.; Warringer, J.; Ganga, M.A.; Louis, E.J.; Liti, G.; Martinez, C. The Genetic Basis of Natural Variation in Oenological Traits in Saccharomyces cerevisiae. PLoS ONE 2012, 7, e49640. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, A.; Beltran, G.; Warringer, J.; Guillamón, J.M. Genetic Basis of Variations in Nitrogen Source Utilization in Four Wine Commercial Yeast Strains. PLoS ONE 2013, 8, e67166. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, A.; Durand, C.; Loira, N.; Durrens, P.; Sherman, D.J.; Marullo, P. QTL dissection of lag phase in wine fermentation reveals a new translocation responsible for Saccharomyces cerevisiae adaptation to sulfite. PLoS ONE 2014, 9, e86298. [Google Scholar] [CrossRef] [Green Version]
- Marullo, P.; Aigle, M.; Bely, M.; Masneuf-Pomarède, I.; Durrens, P.; Dubourdieu, D.; Yvert, G. Single QTL mapping and nucleotide-level resolution of a physiologic trait in wine Saccharomyces cerevisiae strains. FEMS Yeast Res. 2007, 7, 941–952. [Google Scholar] [CrossRef] [Green Version]
- Hagman, A.; Säll, T.; Piškur, J. Analysis of the yeast short-term Crabtree effect and its origin. FEBS J. 2014, 281, 4805–4814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masneuf-Pomarede, I.; Bely, M.; Marullo, P.; Albertin, W. The Genetics of Non-conventional Wine Yeasts: Current Knowledge and Future Challenges. Front. Microbiol. 2016, 6, 1563. [Google Scholar] [CrossRef]
- Bely, M.; Stoeckle, P.; Masneuf-Pomarède, I.; Dubourdieu, D. Impact of mixed Torulaspora delbrueckii-Saccharomyces cerevisiae culture on high-sugar fermentation. Int. J. Food Microbiol. 2008, 122, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Crépin, L.; Nidelet, T.; Sanchez, I.; Dequin, S.; Camarasa, C. Sequential use of nitrogen compounds by Saccharomyces cerevisiae during wine fermentation: A model based on kinetic and regulation characteristics of nitrogen permeases. Appl. Environ. Microbiol. 2012, 78, 8102–8111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Ríos, E.; Gutiérrez, A.; Salvadó, Z.Z.; Arroyo-López, F.N.; Guillamon, J.M. The Fitness Advantage of Commercial Wine Yeasts in Relation to the Nitrogen Concentration, Temperature, and Ethanol Content under Microvinification Conditions. Appl. Environ. Microbiol. 2014, 80, 704–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras, A.; Hidalgo, C.; Schmidt, S.; Henschke, P.A.; Curtin, C.; Varela, C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 2015, 205, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Venturin, C.; Boze, H.; Moulin, G.; Galzy, P. Influence of oxygen limitation on glucose metabolism in Hanseniaspora uvarum K5 grown in chemostat. Biotechnol. Lett. 1995, 17, 537–542. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Starmerella bacillaris and Saccharomyces cerevisiae mixed fermentations to reduce ethanol content in wine. Appl. Microbiol. Biotechnol. 2016, 100, 5515–5526. [Google Scholar] [CrossRef]
- Mestre Furlani, M.V.; Maturano, Y.P.; Combina, M.; Mercado, L.A.; Toro, M.E.; Vazquez, F. Selection of non-Saccharomyces yeasts to be used in grape musts with high alcoholic potential: A strategy to obtain wines with reduced ethanol content. FEMS Yeast Res. 2017, 17, fox010. [Google Scholar] [CrossRef] [Green Version]


| Mp | Lt | Td | Hu | Sb | Sc | |
|---|---|---|---|---|---|---|
| SM-Mix | 12.00 ± 2.27 A | 18.17 ± 1.96 A | 20.67 ± 0.56 A | 5.16 ± 0.51 B | 7.06 ± 0.67 A | 16.76 ± 0.70 A |
| SM-NH4+ | 13.91 ± 1.03 A | 8.29 ± 3.27 B | 14.13 ± 0.60 B | 8.71 ± 0.71 A | 5.91 ± 1.45 A | 16.95 ± 1.05 A |
| SM-AA | 10.33 ± 2.54 A | 10.45 ± 2.15 B | 14.40 ± 2.87 B | 5.47 ± 1.12 B | 7.73 ± 0.59 A | 18.65 ± 2.03 A |
| Glucose | Fructose | Glycerol | Glycerol Yield | Acetic Acid | Acetic Acid Yield | Ethanol | Ethanol Yield | ||
|---|---|---|---|---|---|---|---|---|---|
| g/L | g/L | g/L | g/g | g/L | mg/g | % (v/v) | g/g | ||
| Mp | SM-Mix | 31.81 ± 2.68 | 49.85 ± 2.88 | 10.30 ± 0.50 | 0.06 ± 0.04 | 1.90 ± 0.10 | 15.91 ± 0.99 | 5.36 ± 0.11 | 0.36 ± 0.01 |
| SM-AA | 30.77 ± 3.24 | 51.76 ± 2.80 | 6.87 ± 0.76 | 0.05 ± 0.01 | 0.98 ± 0.16 | 7.61 ± 0.20 | 5.59 ± 0.70 | 0.32 ± 0.05 | |
| SM-NH4+ | 25.35 ± 2.00 | 43.46 ± 2.69 | 8.32 ± 0.25 | 0.06 ± 0.00 | 0.86 ± 0.44 | 8.04 ± 2.66 | 6.03 ± 0.32 | 0.36 ± 0.01 | |
| Lt | SM-Mix | 3.15 ± 0.12 | 2.12 ± 2.99 | 10.10 ± 0.40 | 0.03 ± 0.02 | 0.23 ± 0.20 | 1.20 ± 0.84 | 9.55 ± 1.34 | 0.39 ± 0.05 |
| SM-AA | 22.14 ± 2.15 | 54.78 ± 5.22 | 4.25 ± 1.38 | 0.03 ± 0.01 | 1.01 ± 0.29 | 8.74 ± 0.21 | 6.3 ± 0.94 | 0.34 ± 0.06 | |
| SM-NH4+ | 22.65 ± 0.16 | 50.08 ± 0.34 | 6.034 ± 1.12 | 0.04 ± 0 | 0.27 ± 0.08 | 1.58 ± 0.13 | 5.84 ± 0.48 | 0.32 ± 0.08 | |
| Td | SM-Mix | 3.03 ± 0.11 | 0.52 ± 0.17 | 7.72 ± 0.59 | 0.04 ± 0 | 1.16 ± 0.14 | 5.88 ± 0.59 | 9.92 ± 0.76 | 0.40 ± 0.02 |
| SM-AA | 3.10 ± 0.40 | 1.60 ± 2.20 | 4.81 ± 0.02 | 0.02 ± 0 | 1.32 ± 0.32 | 7.65 ± 0.31 | 10.44 ± 0.75 | 0.42 ± 0.03 | |
| SM-NH4+ | 2.60 ± 0.28 | 3.10 ± 1.60 | 7.80 ± 1.11 | 0.04 ± 0 | 0.52 ± 0.21 | 3.31 ± 0.20 | 10.54 ± 0.29 | 0.43 ± 0.01 | |
| Hu | SM-Mix | 38.78 ± 3.98 | 24.38 ± 2.29 | 5.70 ± 0.63 | 0.04 ± 0.01 | 0.18 ± 0.06 | 1.33 ± 0.40 | 6.79 ± 0.18 | 0.39 ± 0.02 |
| SM-AA | 36.80 ± 1.00 | 28.92 ± 0.95 | 3.95 ± 0.60 | 0.03 ± 0 | 0.18 ± 0.03 | 1.29 ± 0.17 | 6.80 ± 0.46 | 0.37 ± 0.06 | |
| SM-NH4+ | 32.98 ± 3.91 | 22.26 ± 1.99 | 6.31 ± 0.29 | 0.04 ± 0 | 0.30 ± 0.14 | 2.00 ± 0.99 | 7.47 ± 0.54 | 0.38 ± 0.03 | |
| Sb | SM-Mix | 46.77 ± 6.72 | 1.24 ± 0.35 | 6.32 ± 1.21 | 0.04 ± 0.01 | 0.51 ± 0.26 | 3.60 ± 1.43 | 8.61 ± 1.32 | 0.41 ± 0.01 |
| SM-AA | 17.60 ± 18.86 | 0.00 ± 0.00 | 6.08 ± 1.83 | 0.03 ± 0.01 | 0.39 ± 0.06 | 2.12 ± 0.16 | 9.09 ± 0.76 | 0.39 ± 0.02 | |
| SM-NH4+ | 61.72 ± 12.05 | 0.00 ± 0.00 | 8.60 ± 0.97 | 0.06 ± 0.02 | 0.24 ± 0.20 | 2.65 ± 0.56 | 7.51 ± 0.83 | 0.43 ± 0.03 | |
| Sc | SM-Mix | 3.17 ± 0.50 | 1.97 ± 1.90 | 7.00 ± 0.37 | 0.04 ± 0 | 0.66 ± 0.22 | 3.37 ± 0.95 | 9.73 ± 0.19 | 0.39 ± 0.01 |
| SM-AA | 3.05 ± 0.09 | 2.10 ± 1.40 | 5.38 ± 0.45 | 0.03 ± 0 | 0.71 ± 0.28 | 3.64 ± 1.18 | 10.28 ± 0.19 | 0.42 ± 0.01 | |
| SM-NH4+ | 3.61 ± 0.41 | 2.36 ± 1.74 | 7.98 ± 0.97 | 0.04 ± 0 | 0.82 ± 0.11 | 4.19 ± 0.44 | 9.85 ± 0.43 | 0.40 ± 0.01 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roca-Mesa, H.; Sendra, S.; Mas, A.; Beltran, G.; Torija, M.-J. Nitrogen Preferences during Alcoholic Fermentation of Different Non-Saccharomyces Yeasts of Oenological Interest. Microorganisms 2020, 8, 157. https://doi.org/10.3390/microorganisms8020157
Roca-Mesa H, Sendra S, Mas A, Beltran G, Torija M-J. Nitrogen Preferences during Alcoholic Fermentation of Different Non-Saccharomyces Yeasts of Oenological Interest. Microorganisms. 2020; 8(2):157. https://doi.org/10.3390/microorganisms8020157
Chicago/Turabian StyleRoca-Mesa, Helena, Sonia Sendra, Albert Mas, Gemma Beltran, and María-Jesús Torija. 2020. "Nitrogen Preferences during Alcoholic Fermentation of Different Non-Saccharomyces Yeasts of Oenological Interest" Microorganisms 8, no. 2: 157. https://doi.org/10.3390/microorganisms8020157
APA StyleRoca-Mesa, H., Sendra, S., Mas, A., Beltran, G., & Torija, M.-J. (2020). Nitrogen Preferences during Alcoholic Fermentation of Different Non-Saccharomyces Yeasts of Oenological Interest. Microorganisms, 8(2), 157. https://doi.org/10.3390/microorganisms8020157







