Forest Tree Associated Bacterial Diffusible and Volatile Organic Compounds against Various Phytopathogenic Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial and Fungal Strains
2.2. In Vitro Antifungal Activity
2.3. Analysis of the Antagonistic Substances In Vitro
2.4. Detection of Genes Encoding Antibiotics and HCN in ST–TJ4
2.5. Antifungal Activity of VOCs of Strain ST–TJ4
2.6. Analysis of Volatile Organic Compounds (VOCs)
2.7. Identification of Strain ST-TJ4
2.8. Statistical Analysis
3. Results
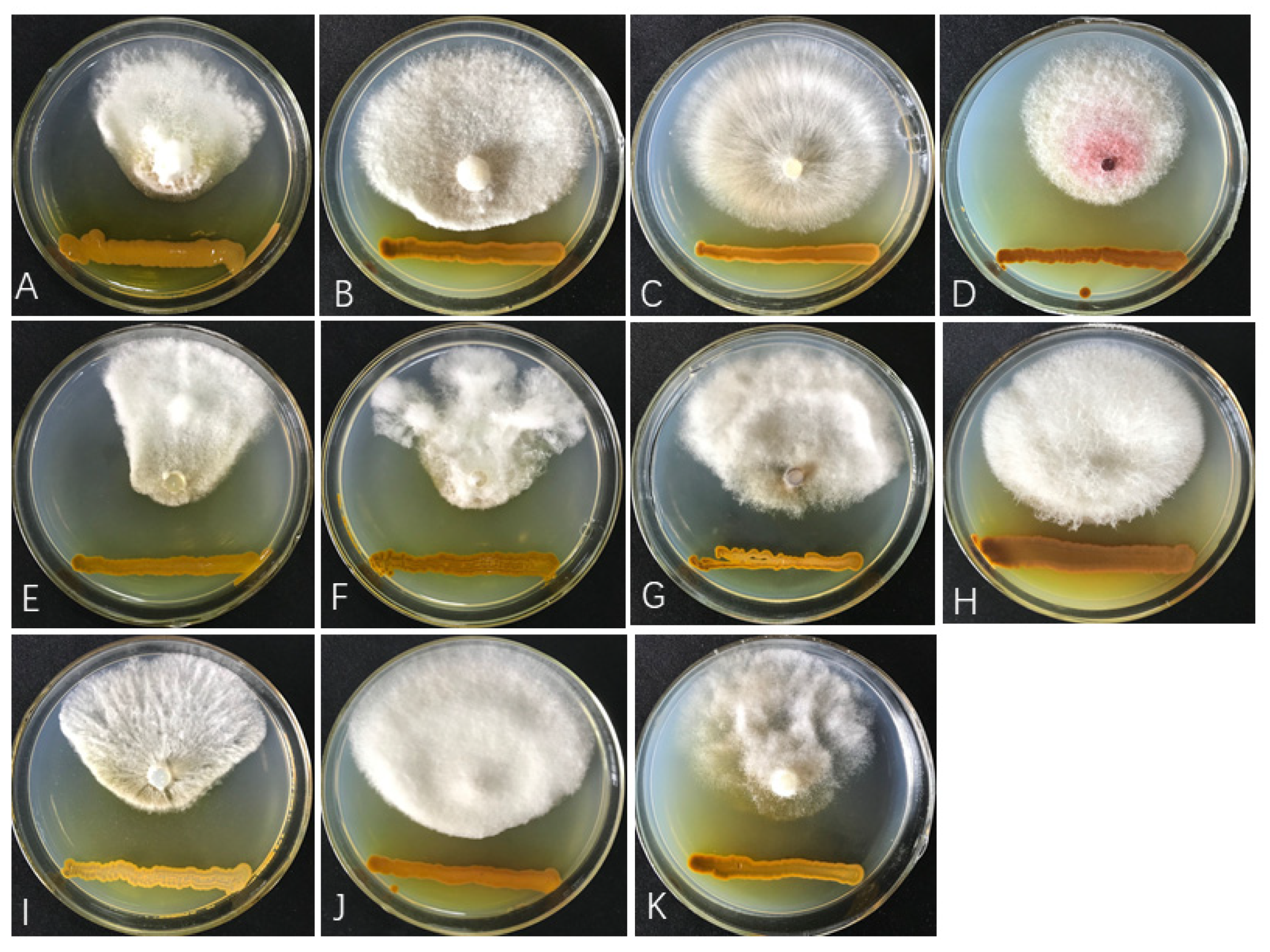
3.1. Antagonistic Effects of Diffusible Substances Produced by ST–TJ4
3.2. Siderophore and cell wall Degradation Enzyme Activities of ST–TJ4
3.3. Detection of Antibiotic and HCN Encoding Genes in ST-TJ4
3.4. The Antifungal Spectrum of ST-TJ4 VOCs
3.5. GC-MS/MS Analysis of VOCs Produced by ST–TJ4
3.6. Identification of Strain ST–TJ4
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, S.; Li, K. Antagonistic activity and mechanism of an isolated Streptomyces corchorusii stain AUH-1 against phytopathogenic fungi. World J. Microbiol. Biotechnol. 2019, 35, 145. [Google Scholar] [CrossRef]
- Fletcher, J.; Bender, C.; Budowle, B. Plant pathogen forensics: Capabilities, needs, and recommendations. Microbiol. Mol. Biol. Rev. 2006, 70, 450. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.E.; Peterson, J.C. Surveillance of occupational, accidental, and incidental exposure to organophosphate pesticides using urine alkyl phosphate and phenolic metabolite measurements. Ann. N. Y. Acad. Sci. 1997, 837, 257–268. [Google Scholar]
- Wang, S.-L.; Yieh, T.-C.; Shih, I.-L. Production of antifungal compounds by Pseudomonas aeruginosa K-187 using shrimp and crab shell powder as a carbon source. Enzym. Microb. Technol. 1999, 25, 142–148. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; de Jonge, R.; Berendsen, R.L. The Soil-Borne Supremacy. Trends Plant Sci. 2016, 21, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Sarma, B.K.; Singh, K.P. Management of Sclerotium rolfsii with integration of non-conventional chemicals, vermicompost and Pseudomonas syringae. World J. Microbiol. Biotechnol. 2008, 24, 517–522. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.F.; Xing, Z.; Gao, B.L.; Zhang, L.Q. Engineering the bacterial endophyte Burkholderia pyrrocinia JK-SH007 for the control of lepidoptera larvae by introducing the cry218 genes of Bacillus thuringiensis. Biotechnol. Biotechnol. Equip. 2017, 31, 1167–1172. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.; Wang, C.; Song, X.F.; Ding, X.L.; Wu, L.M.; Wu, H.J.; Gao, X.W.; Borriss, R. Bacillus velezensis FZB42 in 2018: The gram-positive model strain for plant growth promotion and biocontrol. Front. Microbiol. 2018, 9, 2491. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, T.; Liu, Y. Volatile organic compounds produced by Pseudomonas chlororaphis subsp. aureofaciens SPS-41 as biological fumigants to control Ceratocystis fimbriata in postharvest sweet potatoes. J. Agric. Food Chem. 2019, 67, 3702–3710. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Sui, J. Isolation and characterization of antagonistic bacteria Paenibacillus jamilae HS-26 and their effects on plant growth. Biomed. Res. Int. 2019, 3638926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frikha-Gargouri, O.; Ben Abdallah, D.; Ghorbel, I.; Charfeddine, I.; Jlaiel, L.; Triki, M.A.; Tounsi, S. Lipopeptides from a novel Bacillus methylotrophicus 39b strain suppress Agrobacterium crown gall tumours on tomato plants. Pest Manag. Sci. 2017, 73, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Maindad, D.V.; Kasture, V.M.; Chaudhari, H.; Dhavale, D.D.; Chopade, B.A.; Sachdev, D.P. Characterization and fungal inhibition activity of siderophore from wheat rhizosphere associated Acinetobacter calcoaceticus strain HIRFA32. Indian J. Microbiol. 2014, 54, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordero, P.; Principe, A.; Jofre, E.; Mori, G.; Fischer, S. Inhibition of the phytopathogenic fungus Fusarium proliferatum by volatile compounds produced by Pseudomonas. Arch. Microbiol. 2014, 196, 803–809. [Google Scholar] [CrossRef]
- Velivelli, S.L.S.; Kromann, P.; Lojan, P. Identification of mVOCs from andean rhizobacteria and field evaluation of bacterial and mycorrhizal inoculants on growth of potato in its center of origin. Microb. Ecol. 2015, 69, 652–667. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, B.; Liu, H.; Han, J.; Zhang, Y. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol. Control. 2017, 105, 27–39. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K.; Zhang, X. A two-component histidine kinase, MoSLN1, is required for cell wall integrity and pathogenicity of the rice blast fungus, Magnaporthe oryzae. Curr. Genet. 2010, 56, 517–528. [Google Scholar] [CrossRef]
- Lim, S.M.; Yoon, M.; Choi, G.J. Diffusible and volatile antifungal compounds produced by an antagonistic Bacillus velezensis G341 against various phytopathogenic fungi. Plant Pathol. J. 2017, 33, 488–498. [Google Scholar] [CrossRef] [Green Version]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Han, J.; Shim, H.; Shin, J.; Kim, K.S. Antagonistic activities of Bacillus spp. strains isolated from Tidal Flat Sediment towards anthracnose pathogens Colletotrichum acutatum and C. gloeosporioides in South Korea. Plant Pathol. J. 2015, 31, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Suryadi, Y.; Susilowati, D.; Lestari, P.; Priyatno, T.; Samudra, I.; Hikmawati, N.; Mubarik, N. Characterization of bacterial isolates producing chitinase and glucanase for biocontrol of plant fungal pathogens. J. Agric. Technol. 2014, 10, 983–999. [Google Scholar]
- Huang, L.; Li, Q.; Hou, Y. Bacillus velezensis strain HYEB5-6 as a potential biocontrol agent against anthracnose on Euonymus japonicus. Biocontrol Sci Technol. 2017, 27, 636–653. [Google Scholar] [CrossRef]
- Ausubel, F.M.; Brent, R.; Kingston, R.E.; Moore, D.D.; Seidman, J.G.; Smith, J.A.; Struhl, K. Current Protocols in Molecular Biology; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Raaijmakers, J.M.; Weller, D.M.; Thomashow, L.S. Frequency of antibiotic-producing Pseudomonas spp. in natural environments. Appl. Environ. Microbiol. 1997, 63, 881–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Gao, Q.; Hamada, M.S. Potential of Pseudomonas chlororaphis subsp aurantiaca Strain Pcho10 as a biocontrol agent against Fusarium graminearum. Phytopathology 2014, 104, 1289–1297. [Google Scholar] [CrossRef] [Green Version]
- Svercel, M.; Duffy, B.; Defago, G. PCR amplification of hydrogen cyanide biosynthetic locus hcnAB in Pseudomonas spp. J. Microbiol. Methods 2007, 70, 209–213. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Roquigny, R.; Novinscak, A.; Arseneault, T.; Joly, D.L.; Filion, M. Transcriptome alteration in Phytophthora infestans in response to phenazine-1-carboxylic acid production by Pseudomonas fluorescens strain LBUM223. BMC Genomics 2018, 19, 474. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Kong, W.L.; Wu, X.Q.; Zhao, Y.J. Effects of Rahnella aquatilis JZ-GX1 on treat chlorosis induced by iron deficiency in Cinnamomum camphora. J. Plant Growth Regul. 2019. [Google Scholar] [CrossRef]
- Tortora, M.L.; Díaz-Ricci, J.C.; Pedraza, R.O. Azospirillum brasilense siderophores with antifungal activity against Colletotrichum acutatum. Arch. Microbiol. 2011, 193, 275–286. [Google Scholar] [CrossRef]
- Yu, S.; Teng, C.; Liang, J.; Song, T.; Dong, L.; Bai, X.; Jin, Y.; Qu, J. Characterization of siderophore produced by Pseudomonas syringae BAF.1 and its inhibitory effects on spore germination and mycelium morphology of Fusarium oxysporum. J. Microbiol. 2017, 55, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J. Fungal cell wall chitinases and glucanases. Microbiol. SGM 2004, 150, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Cabib, E.; Roh, D.H.; Schmidt, M.; Crotti, L.B.; Varma, A. The yeast cell wall and septum as paradigms of cell growth and morphogenesis. J. Biol. Chem. 2001, 276, 19679–19682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, J.; Goh, J.; Yoo, S. A putative MAP kinase, MCK1, is required for cell wall integrity and pathogenicity of the rice blast fungus, Magnaporthe oryzae. Mol. Plant Microbe Interact. 2008, 21, 525–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, J.; Weber, R. Introduction to Fungi, 3rd ed.; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Lenardon, M.D.; Munro, C.A.; Gow, N.A.R. Chitin synthesis and fungal pathogenesis. Curr. Opin. Microbiol. 2010, 13, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pareek, S.S.; Ravi, I.; Sharma, V. Induction of beta-1,3-glucanase and chitinase in Vigna aconitifolia inoculated with Macrophomina phaseolina. J. Plant Interact. 2014, 9, 434–439. [Google Scholar] [CrossRef] [Green Version]
- Rovera, M.; Pastor, N.; Niederhauser, M.; Rosas, S.B. Evaluation of Pseudomonas chlororaphis subsp aurantiaca SR1 for growth promotion of soybean and for control of Macrophomina phaseolina. Biocontrol Sci. Technol. 2014, 24, 1012–1025. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yang, N. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat. Commun. 2018, 9, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Raio, A.; Puopolo, G.; Cimmino, A.; Danti, R.; Della Rocca, G.; Evidente, A. Biocontrol of cypress canker by the phenazine producer Pseudomonas chlororaphis subsp aureofaciens strain M71. Biol. Control. 2011, 58, 133–138. [Google Scholar] [CrossRef]
- Filion, M.; Roquigny, R.; Novinscak, A.; Joly, D.L. Transcriptome alteration in Phytophthora infestans in response to phenazine-1-carboxylic acid production by Pseudomonas fluorescens LBUM223. Phytopathology 2018, 108S, 5–6. [Google Scholar]
- Fang, R.; Lin, J.; Yao, S. Promotion of plant growth, biological control and induced systemic resistance in maize by Pseudomonas aurantiaca JD37. Ann. Microbiol. 2013, 63, 1177–1185. [Google Scholar] [CrossRef]
- Sharma, P.K.; Munir, R.I.; Plouffe, J.; Shah, N.; de Kievit, T.; Levin, D.B. Polyhydroxyalkanoate (PHA) polymer accumulation and pha gene expression in phenazine (phz(-)) and pyrrolnitrin (prn(-)) defective mutants of Pseudomonas chlororaphis PA23. Polymers 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.Y.; Oh, S.A.; Anderson, A.J.; Neiswender, J.; Kim, J.C.; Kim, Y.C. Production of the antifungal compounds phenazine and pyrrolnitrin from Pseudomonas chlororaphis O6 is differentially regulated by glucose. Lett. Appl. Microbiol. 2011, 52, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Wu, N.; Hale, L.; Wu, W.; Wu, D.; Guo, Y. Characterisation of Pseudomonas chlororaphis subsp aurantiaca strain Pa40 with the ability to control wheat sharp eyespot disease. Ann. Appl. Biol. 2013, 163, 444–453. [Google Scholar]
- Gong, A.; Dong, F.; Hu, M. Antifungal activity of volatile emitted from Enterobacter asburiae Vt-7 against Aspergillus flavus and aflatoxins in peanuts during storage. Food Control 2019, 106, 106718. [Google Scholar] [CrossRef]
- Hunziker, L.; Boenisch, D.; Groenhagen, U.; Bailly, A.; Schulz, S.; Weisskopf, L. Pseudomonas Strains naturally associated with potato plants produce volatiles with high potential for inhibition of Phytophthora infestans. Appl. Environ. Microb. 2015, 81, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Guevara-Avendano, E.; Adriana Bejarano-Bolivar, A.; Kiel-Martinez, A. Avocado rhizobacteria emit volatile organic compounds with antifungal activity against Fusarium solani, Fusarium sp. associated with Kuroshio shot hole borer, and Colletotrichum gloeosporioides. Microbiol. Res. 2019, 219, 74–83. [Google Scholar] [CrossRef]
- Tagele, S.B.; Lee, H.G.; Kim, S.W.; Lee, Y.S. Phenazine and 1-Undecene producing Pseudomonas chlororaphis subsp. aurantiaca Strain KNU17Pc1 for growth promotion and disease suppression in Korean maize cultivars. J. Microbiol. Biotechnol. 2019, 29, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Liu, Q.; Cheng, L.; Lei, Y.; Zhang, A. Synthesis and antimicrobial activities of polysiloxane-containing quaternary ammonium salts on bacteria and phytopathogenic fungi. React. Funct. Polym. 2014, 85, 36–44. [Google Scholar] [CrossRef]
- Rajer, F.U.; Wu, H.; Xie, Y.; Xie, S.; Raza, W.; Tahir HA, S.; Gao, X. Volatile organic compounds produced by a soil-isolate, Bacillus subtilis FA26 induce adverse ultra-structural changes to the cells of Clavibacter michiganensis ssp. sepedonicus the causal agent of bacterial ring rot of potato. Microbiology 2017, 163, 523–530. [Google Scholar] [CrossRef]
- Lazazzara, V.; Perazzolli, M.; Pertot, I.; Biasioli, F.; Puopolo, G.; Cappellin, L. Growth media affect the volatilome and antimicrobial activity against Phytophthora infestans in four Lysobacter type strains. Microbiol. Res. 2017, 201, 52–62. [Google Scholar] [CrossRef] [PubMed]






| Primer | Sequence | Target | Antibiotic or Related Pathways | Size (bp) |
|---|---|---|---|---|
| pca2a | TTGCCAAGCCTCGCTCCAAC | phzCD | 1-Phenazinecarboxylic acid | 1150 |
| pca3b | CCGCGTTGTTCCTCGTTCAT | |||
| PHZ1 | GGCGACATGGTCAACGG | phz | Phenazine biosynthesis | 1408 |
| PHZ2 | CGGCTGGCGGCGTATTC | |||
| PRNCF | CCACAAGCCCGGCCAGGAGC | prnC | Pyrrolnitrin | 786 |
| PRNCR | GAGAAGAGCGGGTCGATGAAGCC | |||
| PM1 | TGCGGCATGGGCGTGTGCCATTGCTGCCTGG | hcnAB | Hydrogen cyanide | 570 |
| PM2 | CCGCTCTTGATCTGCAATTGCAGGCC |
| Target Pathogens | Percent Inhibition (%) | Mean ± SE |
|---|---|---|
| Diffusible | Volatile | |
| Botryosphaeria berengeriana | 53.49 ± 4.8 cd | 73.76 ± 1.7 bcd |
| Colletotrichum tropicale | 21.46 ± 2.0 a | 86.25 ± 5.1 cd |
| Cytospora chrysosperma | 23.18 ± 6.9 ab | 82.79 ± 0.5 cd |
| Fusarium graminearum | 28.04 ± 8.4 ab | 55.62 ± 7.1 ab |
| Fusarium oxysporum | 41.11 ± 5.3 bcd | 40.4 ± 5.5 a |
| Fusicoccus aesculi | 35.98 ± 9.9 abc | 72.95 ± 13.38 bcd |
| Pestalotiopsis versicolor | 49.00 ± 4.9 cd | 62.31 ± 15.6 abc |
| Phomopsis ricinella | 59.91 ± 3.9 d | 73.03 ± 7.4 bcd |
| Phytophthora cinnamomi | 50.58 ± 5.2 cd | 91.35 ± 1.1 d |
| Rhizoctonia solani | 19.87 ± 4.4 a | 90.63 ± 0.4 d |
| Sphaeropsis sapinea | 56.73 ± 2.6 d | 71.97 ± 3.6 bcd |
| Retention Time (min) | Relative Peak Area (%) | CAS# | Compound |
|---|---|---|---|
| 2.04 | 1.42 | 5874-90-8 | l-Ala-l-Ala-l-Ala |
| 6.85 | 1.14 | 541-05-9 | octamethylcyclotetrasiloxane |
| 8.65 | 75.97 | 821-95-4 | 1-undecene |
| 8.95 | 1.06 | 2078-13-9 | 4-hydroxybenzoic acid |
| 12.04 | 0.54 | 53044-27-2 | phosphonoacetic acid |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, W.-L.; Li, P.-S.; Wu, X.-Q.; Wu, T.-Y.; Sun, X.-R. Forest Tree Associated Bacterial Diffusible and Volatile Organic Compounds against Various Phytopathogenic Fungi. Microorganisms 2020, 8, 590. https://doi.org/10.3390/microorganisms8040590
Kong W-L, Li P-S, Wu X-Q, Wu T-Y, Sun X-R. Forest Tree Associated Bacterial Diffusible and Volatile Organic Compounds against Various Phytopathogenic Fungi. Microorganisms. 2020; 8(4):590. https://doi.org/10.3390/microorganisms8040590
Chicago/Turabian StyleKong, Wei-Liang, Pu-Sheng Li, Xiao-Qin Wu, Tian-Yu Wu, and Xiao-Rui Sun. 2020. "Forest Tree Associated Bacterial Diffusible and Volatile Organic Compounds against Various Phytopathogenic Fungi" Microorganisms 8, no. 4: 590. https://doi.org/10.3390/microorganisms8040590
APA StyleKong, W. -L., Li, P. -S., Wu, X. -Q., Wu, T. -Y., & Sun, X. -R. (2020). Forest Tree Associated Bacterial Diffusible and Volatile Organic Compounds against Various Phytopathogenic Fungi. Microorganisms, 8(4), 590. https://doi.org/10.3390/microorganisms8040590






