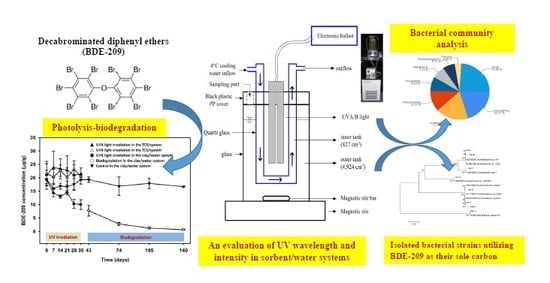
Characterization of a Sequential UV Photolysis-Biodegradation Process for Treatment of Decabrominated Diphenyl Ethers in Sorbent/Water Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Bacterial Mixed Cultures
2.2. BDE-209-Contaminated Sorbent/Water Systems
2.3. A Sequential Treatment Process Involving UV Photolysis Followed by Biodegradation
2.3.1. The SSR Design
2.3.2. Degradation of BDE-209 in the SSR by Sequential Treatment
2.4. BDE-209 and PBDEs Analysis
2.5. Bacterial Community Analysis
2.6. Bacterial Strains Capable of Utilizing BDE-209 as a Sole Carbon Source in the Sorbent/Water System
3. Results
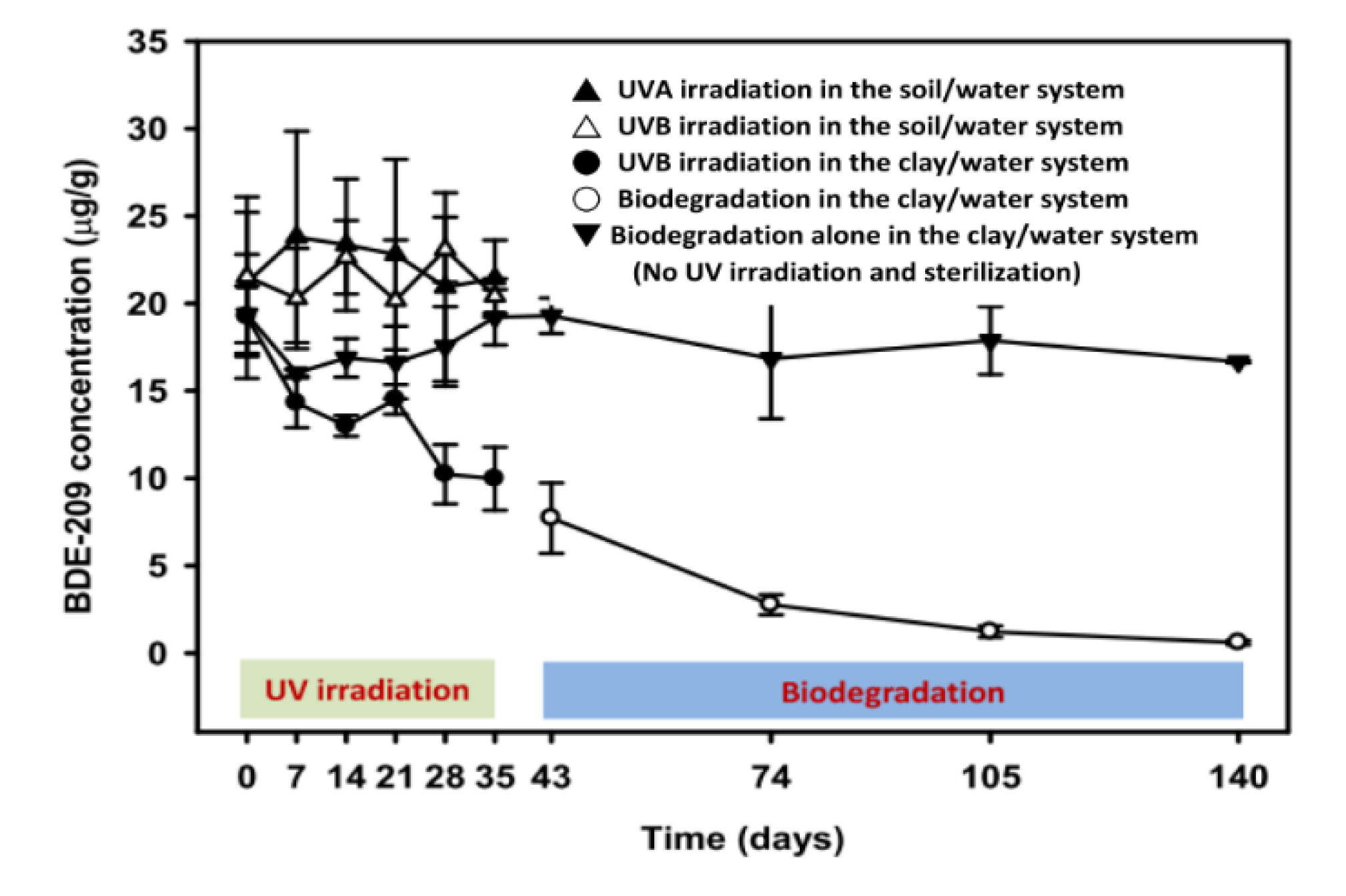
3.1. BDE-209 Removal
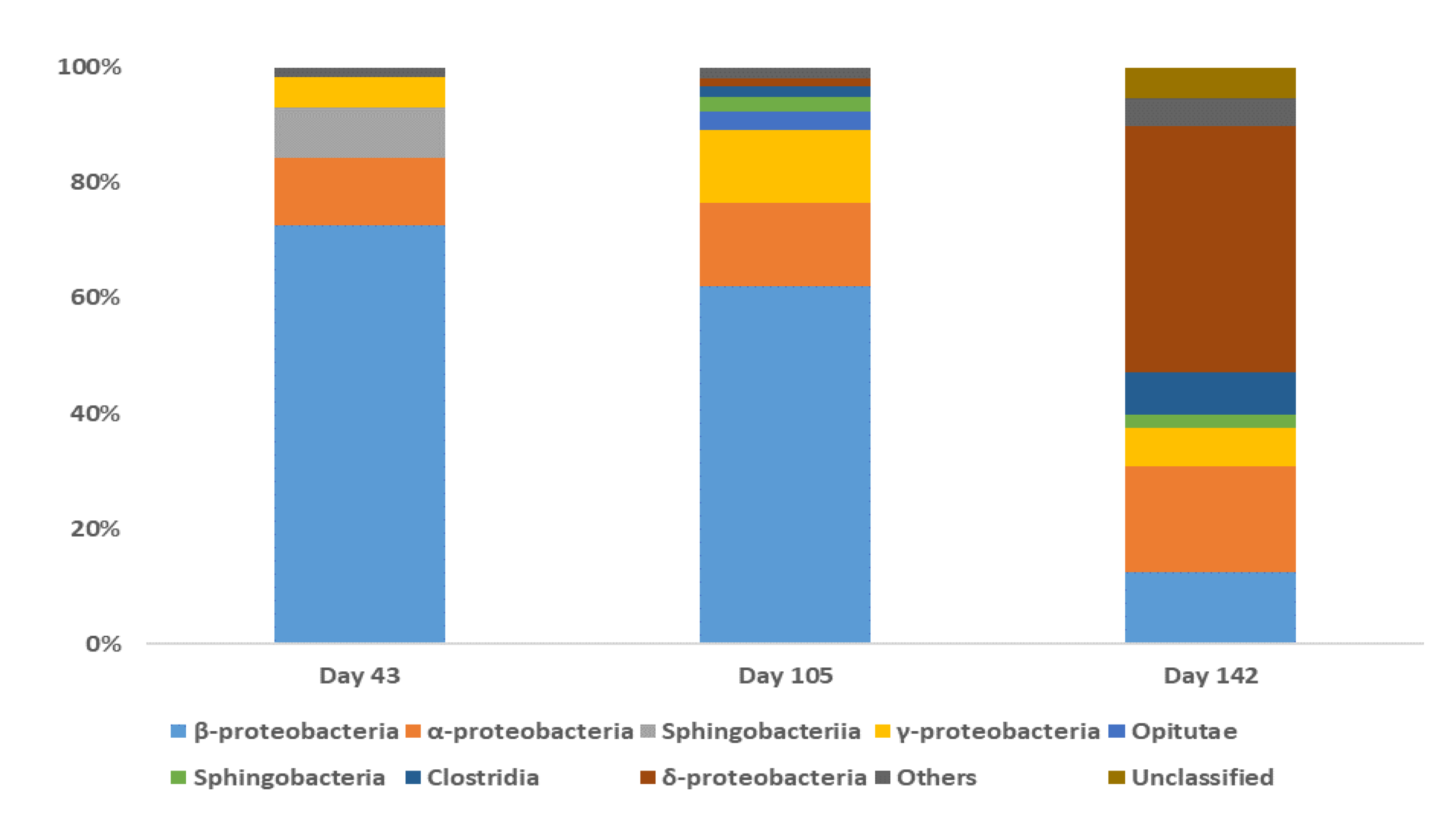
3.2. Bacterial Community Analysis
3.3. Isolated Bacterial Strains That Have the Potential to Utilize BDE-209 as a Sole Carbon Source
4. Discussion
4.1. A Comparison of BDE-209 Degradation in Sorbent/Water System
4.2. Influence of UV Irradiation on BDE-209 Degradation in a Clay/Water System
4.3. Bacterial Strains Isolated Involved in the BDE-209 Biodegradation in a Clay/Water System
4.4. The Bacterial Communities Involved in the BDE-209 Biodegradation in a Clay/Water System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McGrath, T.J.; Ball, A.S.; Clarke, B.O. Critical review of soil contamination by polybrominated diphenyl ethers (PBDEs) and novel brominated flame retardants (NBFRs); concentrations, sources and congener profiles. Environ. Pollut. 2017, 230, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Pagano, J.; McGoldrick, D.J.; Chen, D.; Crimmins, B.S.; Hopke, P.K.; Milligan, M.S.; Murphy, E.W.; Holsen, T.M. Legacy polybrominated diphenyl ethers (PBDEs) trends in top predator fish of the Laurentian Great Lakes (GL) from 1979 to 2016: Will concentrations continue to decrease? Environ. Sci. Technol. 2019, 53, 6650–6659. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Sun, Y.; Wang, Y.; Liang, B.; Chen, T.; Zheng, D.; Zhao, X.; Zhou, X.; Sun, Z.; Shi, Z. Cardiovascular toxicity of decabrominated diphenyl ethers (BDE-209) and decabromodiphenyl ethane (DBDPE) in rats. Chemosphere 2019, 223, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Z.; Gu, L.; Yin, R.; Li, H.; Zhang, X.; Cao, T.; Jiang, C. Toxic effects of decabromodiphenyl ether (BDE-209) on human embryonic kidney cells. Front. Genet. 2014, 5, 118. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tsang, D.C.W.; Wang, Y.; Li, Y.; Yang, X. The photodegradation of polybrominated diphenyl ethers (PBDEs) in various environmental matrices: Kinetics and mechanisms. Chem. Eng. Sci. 2016, 297, 74–96. [Google Scholar] [CrossRef]
- Söderström, G.; Sellström, U.; De Wit, C.A.; Tysklind, M. Photolytic debromination of decabromodiphenyl ether (BDE 209). Environ. Sci. Technol. 2004, 38, 127–132. [Google Scholar] [CrossRef]
- Hua, I.; Kang, N.; Jafvert, C.T.; Fábrega-Duque, J.R. Heterogeneous photochemical reactions of decabromodiphenyl ether. Environ. Toxicol. Chem. 2003, 22, 798–804. [Google Scholar] [CrossRef]
- Robles-González, I.V.; Fava, F.; Poggi-Varaldo, H.M. A review on slurry bioreactors for bioremediation of soils and sediments. Microb. Cell Fact. 2008, 7, 5. [Google Scholar] [CrossRef]
- Chou, H.-L.; Chang, Y.-T.; Liao, Y.-F.; Lin, C.-H. Biodegradation of decabromodiphenyl ether (BDE-209) by bacterial mixed cultures in the sorbent/water system. Int. Biodeterior. Biodegrad. 2013, 85, 671–682. [Google Scholar] [CrossRef]
- Chou, H.-L.; Hwa, M.-Y.; Lee, Y.-C.; Chang, Y.-J.; Chang, Y.-T. Microbial degradation of decabromodiphenyl ether (DBDE) in soil slurry microcosms. Environ. Sci. Pollut. R. Int. 2016, 23, 5255–5267. [Google Scholar] [CrossRef]
- Winsley, T.; Dorst, J.M.; Van Brown, M.V.; Ferrari, B.C. Capturing greater 16S rRNA gene sequence diversity within the domain bacteria. Appl. Environ. Microbiol. 2012, 78, 5938–5941. [Google Scholar] [CrossRef] [PubMed]
- Kadali, K.K.; Simons, K.L.; Skuza, P.P.; Moore, R.B.; Ball, A.S. A complementary approach to identifying and assessing the remediation potential of hydrocarbonoclastic bacteria. J. Microbiol. Methods 2012, 88, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Filley, T.R.; Jafvert, C.T.; Nies, L.; Hua, I.; Bezares-Cruz, J. Photodegradation of decabromodiphenyl ether adsorbed onto clay minerals, metal oxide, and sediment. Environ. Sci. Technol. 2006, 40, 215–220. [Google Scholar] [CrossRef]
- Mamane, H.; Ducoste, J.J.; Linden, K.G. Effect of particles on ultraviolet light penetration in natural and engineered systems. Appl. Opt. 2006, 45, 1844–1856. [Google Scholar] [CrossRef] [PubMed]
- Dimou, A.D.; Sakkas, V.A.; Albanis, T.A. Trifluralin photolysis in natural waters and under the presence of isolated organic matter and nitrate ions: Kinetics and photoproduct analysis. J. Photochem. Photobiol. A 2004, 163, 473–480. [Google Scholar] [CrossRef]
- Chiou, C.T. Partition and Adsorption of Organic Contaminants in Environmental Systems; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; pp. 112–178. ISBN 978-0-471-23325-1. [Google Scholar]
- Zhao, C.; Yan, M.; Zhong, H.; Liu, Z.; Shi, L.; Chen, M.; Zeng, G.; Song, B.; Shao, B.; Feng, H. Biodegradation of polybrominated diphenyl ethers and strategies for acceleration: A review. Int. Biodeterior. Biodegrad. 2018, 129, 23–32. [Google Scholar] [CrossRef]
- Boyd, S.A.; Sheng, G.; Teppen, B.J.; Johnston, C.T. Mechanisms for the adsorption of substituted nitrobenzenes by smectite clays. Environ. Sci. Technol. 2001, 35, 4227–4234. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, E.; Hu, Y. Impact of mineral micropores on transport and fate of organic contaminants: A review. J. Contam. Hydrol. 2012, 129–130, 80–90. [Google Scholar] [CrossRef]
- Khaled, A.; Richard, C.; Redin, L.; Niinipuu, M.; Jansson, S.; Jaber, F.; Sleiman, M. Characterization and photodegradation of polybrominated diphenyl ethers in car seat fabrics from end-of-life vehicles. Environ. Sci. Technol. 2018, 52, 1216–1224. [Google Scholar] [CrossRef]
- Suh, Y.W.; Buettner, G.R.; Venkataraman, S.; Treimer, S.E.; Robertson, L.W.; Ludewig, G. UVA/B-induced formation of free radicals from decabromodiphenyl ether. Environ. Sci. Technol. 2009, 43, 2581–2588. [Google Scholar] [CrossRef]
- Son, H.S.; Choi, S.B.; Zoh, K.D.; Khan, E. Effects of ultraviolet intensity and wavelength on the photolysis of triclosan. Water Sci. Technol. 2007, 55, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Lehto, K.M.; Vuorimaa, E.; Lemmetyinen, H. Photolysis of polycyclic aromatic hydrocarbons (PAHs) in dilute aqueous solutions detected by fluorescence. J. Photochem. Photobiol. 2000, A136, 53–60. [Google Scholar] [CrossRef]
- Ronen, Z.; Vasiluk, L.; Abeliovich, A.; Nejidat, A. Activity and survival of tribromophenol-degrading bacteria in a contaminated desert soil. Soil Biol. Biochem. 2000, 32, 1643–1650. [Google Scholar] [CrossRef]
- Ahmed, M.; Focht, D.D. Degradation of polychlorinated biphenyls by two species of Achromobacter. Can. J. Microbiol. 1973, 19, 47–52. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Zhang, W.; Niu, L.; Du, J.; Cai, W.; Wang, J. Isolation and characterization of two novel psychrotrophic decabromodiphenyl ether-degrading bacteria from river sediments. Environ. Sci. Pollut. Res. Int. 2016, 23, 10371–10381. [Google Scholar] [CrossRef]
- Yang, C.-W.; Huang, H.-W.; Chao, W.-L.; Chang, B.-V. Bacterial communities associated with aerobic degradation of polybrominated diphenyl ethers from river sediments. Environ. Sci. Pollut. Res. Int. 2015, 22, 3810–3819. [Google Scholar] [CrossRef]
- Xu, M.; Chen, X.; Qiu, M.; Zeng, X.; Xu, J.; Deng, D.; Sun, G.; Li, X.; Guo, J. Bar-Coded pyrosequencing reveals the responses of PBDE-degrading microbial communities to electron donor amendments. PLoS ONE 2012, 7, e30439. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Z.-Z.; Wu, X.-J.; Xu, Y.-X.; Su, X.-L.; Zhang, M.; Wang, J.-X. Biodegradation of decabromodiphenyl ether (BDE-209) by a metal resistant strain, Bacillus cereus JP12. Bioresour. Technol. 2013, 149, 8–15. [Google Scholar] [CrossRef]
- Fahy, A.; McGenity, T.J.; Timmis, K.N.; Ball, A.S. Heterogeneous aerobic benzene-degrading communities in oxygen-depleted groundwaters. FEMS Microbiol. Ecol. 2006, 58, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-T.; Chou, H.-L.; Li, H.; Boyd, S.A. Variation of microbial communities in aquatic sediments under long-term exposure to decabromodiphenyl ether and UVA irradiation. Sustainability 2019, 11, 3773. [Google Scholar] [CrossRef]
- Singleton, D.R.; Ramirez, L.G.; Aitken, M.D. Characterization of a polycyclic aromatic hydrocarbon degradation gene cluster in a phenanthrene-degrading Acidovorax strain. Appl. Environ. Microbiol. 2009, 75, 2613–2620. [Google Scholar] [CrossRef]
- Jiang, L.; Luo, C.; Zhang, D.; Song, M.; Sun, Y.; Zhang, G. Biphenyl-metabolizing microbial community and a functional operon revealed in e waste-contaminated soil. Environ. Sci. Technol. 2018, 52, 8558–8567. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Gan, L.; Wang, N.; Quan, X.; Logan, B.E.; Chen, G. Mineralization of pentachlorophenol with enhanced degradation and power generation from air cathode microbial fuel cells. Biotechnol. Bioeng. 2012, 109, 2211–2221. [Google Scholar] [CrossRef]
- Shih, Y.H.; Chou, H.L.; Peng, Y.H.; Chang, C.Y. Synergistic effect of microscale zerovalent iron particles combined with anaerobic sludges on the degradation of decabromodiphenyl ether. Bioresour. Technol. 2012, 108, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Z.; Gong, A.; Qiu, L.; Zhang, W.; Li, J.; Li, F.; Bai, Y.; Li, J.; Gao, G. Cell changes and differential proteomic analysis during biodegradation of decabromodiphenyl ether (BDE-209) by Pseudomonas aeruginosa. RSC Adv. 2019, 9, 25048–25055. [Google Scholar] [CrossRef]
- Shi, G.; Yin, H.; Ye, J.; Peng, H.; Li, J.; Luo, C. Aerobic biotransformation of decabromodiphenyl ether (PBDE-209) by Pseudomonas aeruginosa. Chemosphere 2013, 93, 1487–1493. [Google Scholar] [CrossRef]
- Robrock, K.R.; Coelhan, M.; Sedlak, D.L.; Alvarez-Cohent, L. Aerobic biotransformation of polybrominated diphenyl ethers (PBDEs) by bacterial isolates. Environ. Sci. Technol. 2009, 43, 5705–5711. [Google Scholar] [CrossRef]
- Tiirola, M.A.; Mannisto, M.K.; Puhakka, J.A.; Kulomaa, M.S. Isolation and characterization of Novosphingobium sp. strain MT1, a dominant polychlorophenol-degrading strain in a groundwater bioremediation system. Appl. Environ. Microbiol. 2002, 68, 173–180. [Google Scholar] [CrossRef]
- Luo, Y.R.; Kang, S.G.; Kim, S.J.; Kim, M.R.; Li, N.; Lee, J.H.; Kwon, K.K. Genome sequence of benzo(a)pyrene-degrading bacterium Novosphingobium pentaromativorans US6-1. J. Bacteriol. 2012, 194, 907. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Wang, C.H.; Jiang, J.; Kwon, S.W.; Sun, L.N.; Shen, W.B.; He, J. Novosphingobium chloroacetimidivorans sp. nov., a chloroacetamide herbicide-degrading bacterium isolated from activated sludge. Int. J. Syst. Evol. Microbiol. 2014, 64, 2573–2578. [Google Scholar] [CrossRef]
- Mohn, W.W.; Kennedy, K.J. Reductive dehalogenation of chlorophenols by Desulfomonile tiedjei DCB-1. Appl. Environ. Microbiol. 1992, 58, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Nam, I.H.; Murugesan, K.; Schnidt, S.; Crowley, D.E.; Chang, Y.S. Biodegradation of diphenyl ether and transformation of selected brominated congeners by Sphingomonas sp. PH-07. Appl. Microbiol. Biotechnl. 2007, 77, 187–194. [Google Scholar] [CrossRef] [PubMed]


| No. | Biodegradation Sampling Day | Bacterial Species (Similarity) | Response to BDE-209 Utilization |
|---|---|---|---|
| NH-1 | 43 | Acinetobacter sp. (99%) | 2 |
| NH-2 | Achromobacter sp. (99%) | 3 | |
| NH-3 | Bacillus sp. (99%) | 1 | |
| NH-4 | Achromobacter sp. (99%) | 2 | |
| NH-5 | 105 | Pseudomonas sp. (99%) | 1 |
| NH-6 | Achromobacter sp. (99%) | 2 | |
| NH-7 | 140 | Acinetobacter sp. (99%) | 2 |
| NH-8 | Acinetobacter sp. (99%) | 1 | |
| Level | 1:Weak | 2:Medium | 3:Strong |
| OD590 | <0.1 | 0.1–0.3 | >0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-T.; Chao, W.-L.; Chen, H.-Y.; Li, H.; Boyd, S.A. Characterization of a Sequential UV Photolysis-Biodegradation Process for Treatment of Decabrominated Diphenyl Ethers in Sorbent/Water Systems. Microorganisms 2020, 8, 633. https://doi.org/10.3390/microorganisms8050633
Chang Y-T, Chao W-L, Chen H-Y, Li H, Boyd SA. Characterization of a Sequential UV Photolysis-Biodegradation Process for Treatment of Decabrominated Diphenyl Ethers in Sorbent/Water Systems. Microorganisms. 2020; 8(5):633. https://doi.org/10.3390/microorganisms8050633
Chicago/Turabian StyleChang, Yi-Tang, Wei-Liang Chao, Hsin-Yu Chen, Hui Li, and Stephen A. Boyd. 2020. "Characterization of a Sequential UV Photolysis-Biodegradation Process for Treatment of Decabrominated Diphenyl Ethers in Sorbent/Water Systems" Microorganisms 8, no. 5: 633. https://doi.org/10.3390/microorganisms8050633
APA StyleChang, Y.-T., Chao, W.-L., Chen, H.-Y., Li, H., & Boyd, S. A. (2020). Characterization of a Sequential UV Photolysis-Biodegradation Process for Treatment of Decabrominated Diphenyl Ethers in Sorbent/Water Systems. Microorganisms, 8(5), 633. https://doi.org/10.3390/microorganisms8050633