Importance of Initial Interfacial Steps during Chalcopyrite Bioleaching by a Thermoacidophilic Archaeon
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Culture Conditions
2.2. Mineral
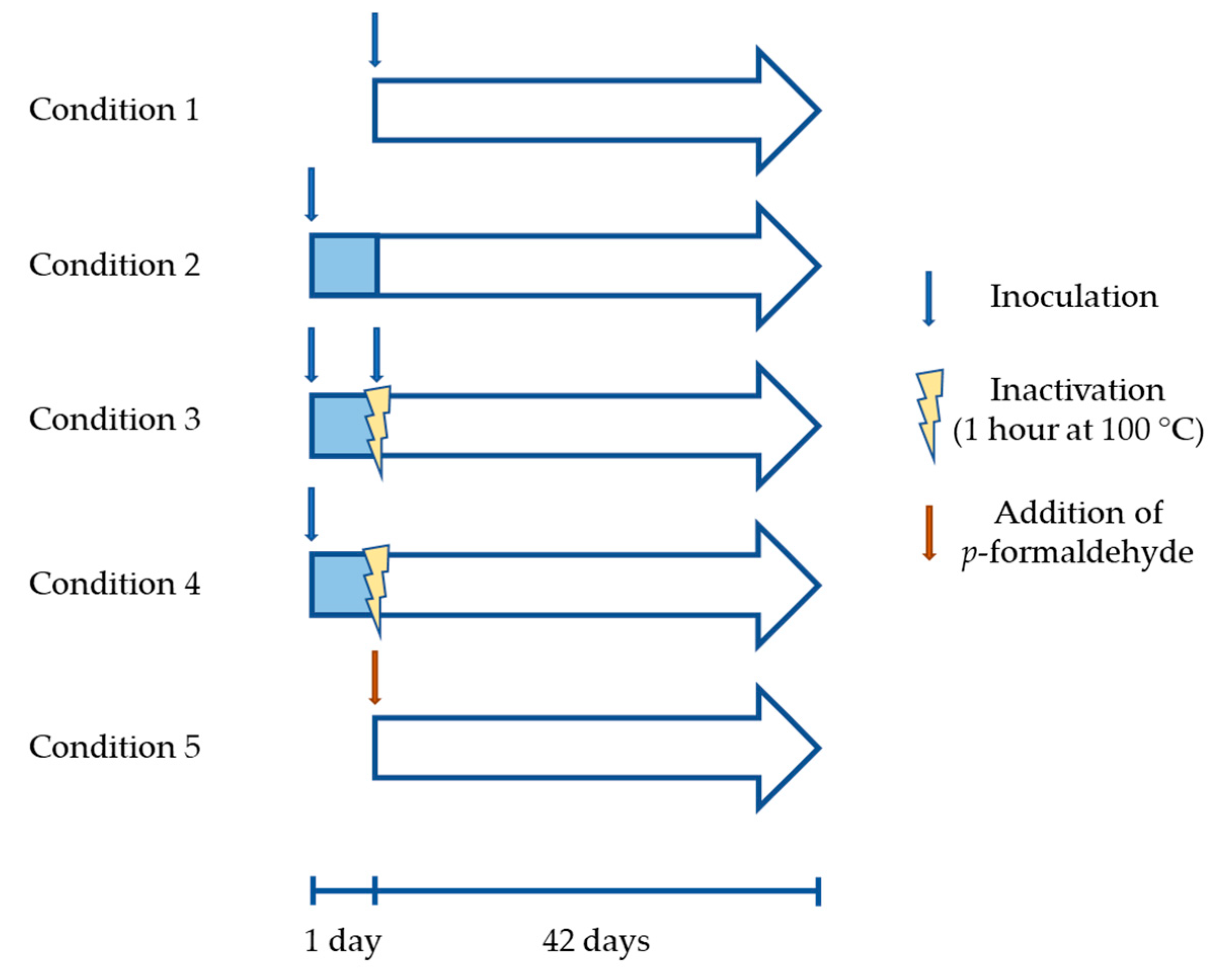
2.3. Bioleaching Experiments
2.4. Fluorescent In Situ Hybridization
2.5. EPS Staining
3. Results and Discussion
3.1. Adhesion to Chalcopyrite Surface
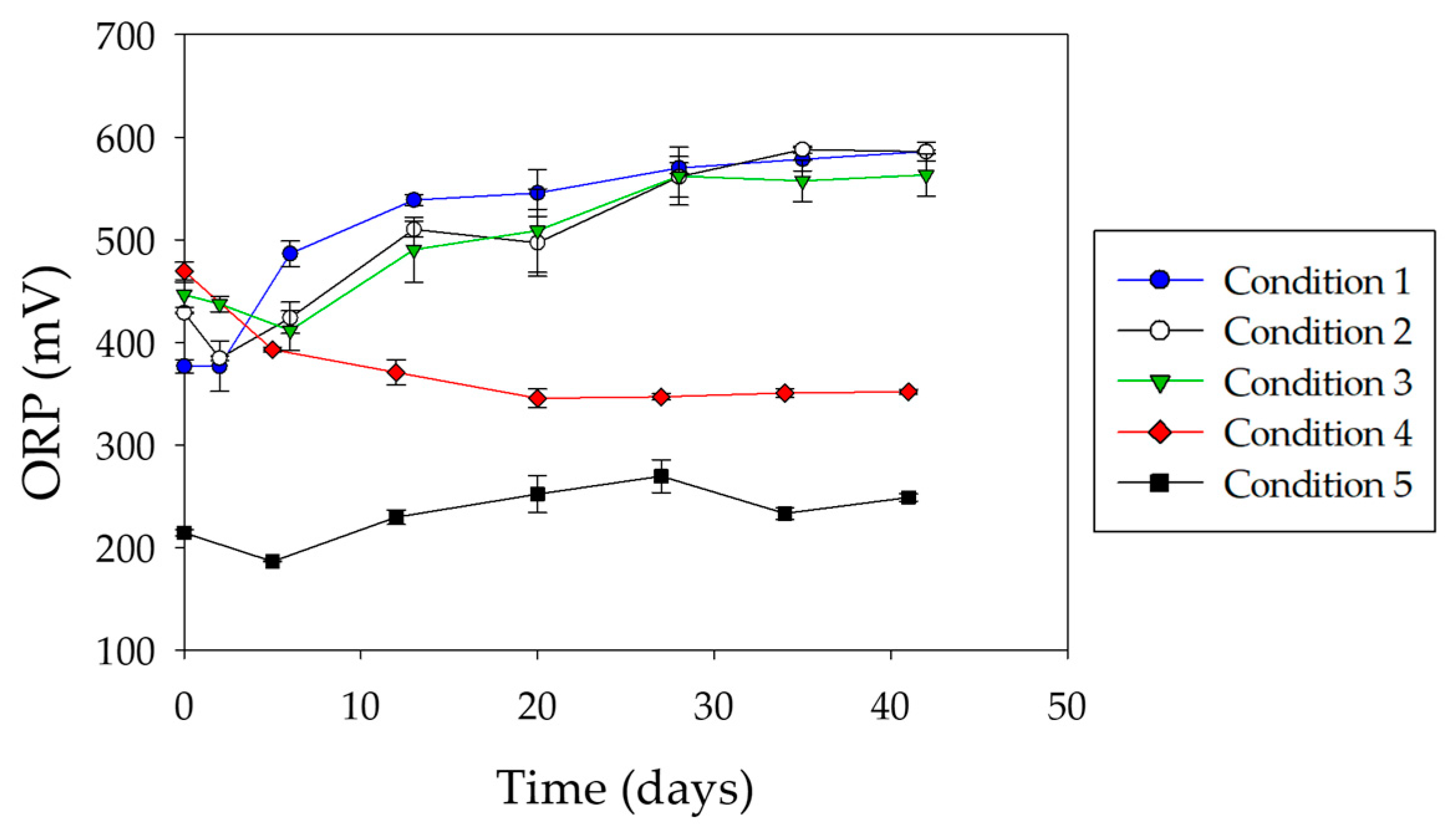
3.2. Chalcopyrite Bioleaching
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Urbieta, M.S.; Donati, E.R.; Chan, K.-G.; Shahar, S.; Sin, L.L.; Goh, K.M. Thermophiles in the genomic era: Biodiversity, science, and applications. Biotechnol. Adv. 2015, 33, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Eswari, J.S.; Dhagat, S.; Sen, R.; Eswari, J.S.; Dhagat, S.; Sen, R. Biosurfactants, Bioemulsifiers, and Biopolymers from Thermophilic Microorganisms. In Thermophiles for Biotech Industry; Springer: Singapore, 2019; pp. 87–97. [Google Scholar]
- Mehta, R.; Singhal, P.; Singh, H.; Damle, D.; Sharma, A.K. Insight into thermophiles and their wide-spectrum applications. 3 Biotech 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Straub, C.T.; Counts, J.A.; Nguyen, D.M.N.; Wu, C.-H.; Zeldes, B.M.; Crosby, J.R.; Conway, J.M.; Otten, J.K.; Lipscomb, G.L.; Schut, G.J.; et al. Biotechnology of extremely thermophilic archaea. FEMS Microbiol. Rev. 2018, 012, 543–578. [Google Scholar] [CrossRef]
- Sato, T.; Atomi, H. Novel metabolic pathways in Archaea. Curr. Opin. Microbiol. 2011, 14, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, A.H.; Boxall, N.J.; Gumulya, Y.; Khaleque, H.N.; Morris, C.; Bohu, T.; Cheng, K.Y.; Usher, K.M.; Lakaniemi, A.M. Recent progress in biohydrometallurgy and microbial characterisation. Hydrometallurgy 2018, 180, 7–25. [Google Scholar] [CrossRef]
- Castro, C.; Donati, E. Effects of different energy sources on cell adhesion and bioleaching of a chalcopyrite concentrate by extremophilic archaeon Acidianus copahuensis. Hydrometallurgy 2016, 162, 49–56. [Google Scholar] [CrossRef]
- Takatsugi, K.; Sasaki, K.; Hirajima, T. Mechanism of the enhancement of bioleaching of copper from enargite by thermophilic iron-oxidizing archaea with the concomitant precipitation of arsenic. Hydrometallurgy 2011, 109, 90–96. [Google Scholar] [CrossRef]
- Watling, H.R.; Collinson, D.M.; Corbett, M.K.; Shiers, D.W.; Kaksonen, A.H.; Watkin, E.L.J. Saline-water bioleaching of chalcopyrite with thermophilic, iron(II)- and sulfur-oxidizing microorganisms. Res. Microbiol. 2016, 167, 546–554. [Google Scholar] [CrossRef]
- Abbas, S. Bioleaching of Molybdenum by Two New Thermophilic Strains Isolated and Characterized. Iran. J. Chem. Chem. Eng. 2017, 36, 183–194. [Google Scholar]
- Astudillo, C.; Acevedo, F. Effect of CO2 air enrichment in the biooxidation of a refractory gold concentrate by Sulfolobus metallicus adapted to high pulp densities. Hydrometallurgy 2009, 97, 94–97. [Google Scholar] [CrossRef]
- Castro, C.; Donati, E.R. Improving zinc recovery by thermoacidophilic archaeon Acidianus copahuensis using tetrathionate. Trans. Nonferrous Met. Soc. China 2016, 26, 3004–3014. [Google Scholar] [CrossRef]
- Li, S.; Zhong, H.; Hu, Y.; Zhao, J.; He, Z.; Gu, G. Bioleaching of a low-grade nickel–copper sulfide by mixture of four thermophiles. Bioresour. Technol. 2014, 153, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Batty, J.D.; Rorke, G.V. Development and commercial demonstration of the BioCOPTM thermophile process. Hydrometallurgy 2006, 83, 83–89. [Google Scholar] [CrossRef]
- Neale, J.; Robertson, S.; Muller, H.; Gericke, M. Integrated piloting of a thermophilic bioleaching process for the treatment of a low-grade nickel-copper sulphide concentrate. J. S. Afr. I. Min. Metall. 2009, 109, 173–193. [Google Scholar]
- Castro, C.; Urbieta, M.S.; Plaza Cazón, J.; Donati, E.R. Metal biorecovery and bioremediation: Whether or not thermophilic are better than mesophilic microorganisms. Bioresour. Technol. 2019, 279, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation—part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Afzal Ghauri, M.; Okibe, N.; Barrie Johnson, D. Attachment of acidophilic bacteria to solid surfaces: The significance of species and strain variations. Hydrometallurgy 2007, 85, 72–80. [Google Scholar] [CrossRef]
- Bellenberg, S.; Díaz, M.; Noël, N.; Sand, W.; Poetsch, A.; Guiliani, N.; Vera, M. Biofilm formation, communication and interactions of leaching bacteria during colonization of pyrite and sulfur surfaces. Res. Microbiol. 2014, 165, 773–781. [Google Scholar] [CrossRef]
- Castro, C.; Zhang, R.; Liu, J.; Bellenberg, S.; Neu, T.R.; Donati, E.; Sand, W.; Vera, M. Biofilm formation and interspecies interactions in mixed cultures of thermo-acidophilic archaea Acidianus spp. and Sulfolobus metallicus. Res. Microbiol. 2016, 167. [Google Scholar] [CrossRef]
- Zhang, R.; Neu, T.R.; Blanchard, V.; Vera, M.; Sand, W. Biofilm dynamics and EPS production of a thermoacidophilic bioleaching archaeon. N. Biotechnol. 2019, 51, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Z.; Nie, Z.Y.; Yang, Y.; Pan, X.; Xia, X.; Zhou, Y.H.; Xia, J.L.; Zhang, L.J.; Zhen, X.J.; Yang, H.Y. In situ characterization of change in superficial organic components of thermoacidophilic archaeon Acidianus manzaensis YN-25. Res. Microbiol. 2018, 169, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Valdebenito-Rolack, E.; Ruiz-Tagle, N.; Abarzúa, L.; Aroca, G.; Urrutia, H. Characterization of a hyperthermophilic sulphur-oxidizing biofilm produced by archaea isolated from a hot spring. Electron. J. Biotechnol. 2017, 25, 58–63. [Google Scholar] [CrossRef]
- Giaveno, M.A.; Urbieta, M.S.; Ulloa, J.R.; González Toril, E.; Donati, E.R. Physiologic Versatility and Growth Flexibility as the Main Characteristics of a Novel Thermoacidophilic Acidianus Strain Isolated from Copahue Geothermal Area in Argentina. Microb. Ecol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, M.E. Nitrogen Fixation by Thiobacillus ferrooxidans. J. Gen. Microbiol. 1978, 105, 215–218. [Google Scholar] [CrossRef]
- Kolthoff, I.M.; Sandell, E.B.; Meehan, E.J.; Bruckenstein, S. Análisis Químico Cuantitativo, 6th ed.; Niger: Buenos Aires, Argentina, 1998. [Google Scholar]
- Coram-Uliana, N.J.; van Hille, R.P.; Kohr, W.J.; Harrison, S.T.L. Development of a method to assay the microbial population in heap bioleaching operations. Hydrometallurgy 2006, 83, 237–244. [Google Scholar] [CrossRef]
- Amann, R.I.; Ludwig, W.; Schleifer, K. Phylogenetic Identification and In Situ Detection of Individual Microbial Cells without Cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [CrossRef]
- Amann, R.I.; Krumholz, L.; Stahl, D.A. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J. Bacteriol. 1990, 172, 762–770. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 1–26. [Google Scholar] [CrossRef]
- Castro, C.; Vera, M.; Donati, E.; Sand, W. Visualization of attachment and colonization of pyrite surfaces by a novel species of Acidianus. Adv. Mat. Res. 2013, 825, 70–73. [Google Scholar]
- Zhao, H.; Zhang, Y.; Zhang, X.; Qian, L.; Sun, M.; Yang, Y.; Zhang, Y.; Wang, J.; Kim, H.; Qiu, G. The dissolution and passivation mechanism of chalcopyrite in bioleaching: An overview. Miner. Eng. 2019, 136, 140–154. [Google Scholar] [CrossRef]
- Méndez-Tovar, M.; García-Meza, J.V.; González, I. Electrochemical monitoring of Acidithiobacillus thiooxidans biofilm formation on graphite surface with elemental sulfur. Bioelectrochemistry 2019, 128, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Bellenberg, S.; Castro, L.; Neu, T.R.; Sand, W.; Vera, M. Colonization and biofilm formation of the extremely acidophilic archaeon Ferroplasma acidiphilum. Hydrometallurgy 2014, 150, 245–252. [Google Scholar] [CrossRef]
- Zhang, R.; Neu, T.R.; Zhang, Y.; Bellenberg, S.; Kuhlicke, U.; Li, Q.; Sand, W.; Vera, M. Visualization and analysis of EPS glycoconjugates of the thermoacidophilic archaeon Sulfolobus metallicus. Appl. Microbiol. Biotechnol. 2015, 99, 7343–7356. [Google Scholar] [CrossRef]
- Van Wolferen, M.; Orell, A.; Albers, S.V. Archaeal biofilm formation. Nat. Rev. Microbiol. 2018, 16, 699–713. [Google Scholar] [CrossRef]
- Schopf, S.; Wanner, G.; Rachel, R.; Wirth, R. An archaeal bi-species biofilm formed by Pyrococcus furiosus and Methanopyrus kandleri. Arch. Microbiol. 2008, 190, 371–377. [Google Scholar] [CrossRef]
- Vilcáez, J. On the Mechanisms of Chalcopyrite Bioleaching with Thermophiles. Austin J. Earth Sci. 2015, 2, 1–4. [Google Scholar]
- Wang, J.; Gan, X.; Zhao, H.; Hu, M.; Li, K.; Qin, W.; Qiu, G. Dissolution and passivation mechanisms of chalcopyrite during bioleaching: DFT calculation, XPS and electrochemistry analysis. Miner. Eng. 2016, 98, 264–278. [Google Scholar] [CrossRef]
- Liddell, K.C. Shrinking core models in hydrometallurgy: What students are not being told about the pseudo-steady approximation. Hydrometallurgy 2005, 79, 62–68. [Google Scholar] [CrossRef]
- Safari, V.; Arzpeyma, G.; Rashchi, F.; Mostoufi, N. A shrinking particle-shrinking core model for leaching of a zinc ore containing silica. Int. J. Miner. Process. 2009, 93, 79–83. [Google Scholar] [CrossRef]
- MacCarthy, J.; Nosrati, A.; Skinner, W.; Addai-Mensah, J. Atmospheric acid leaching mechanisms and kinetics and rheological studies of a low grade saprolitic nickel laterite ore. Hydrometallurgy 2016, 160, 26–37. [Google Scholar] [CrossRef]
- Akcil, A.; Ciftci, H.; Deveci, H. Role and contribution of pure and mixed cultures of mesophiles in bioleaching of a pyritic chalcopyrite concentrate. Miner. Eng. 2007, 20, 310–318. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safar, C.; Castro, C.; Donati, E. Importance of Initial Interfacial Steps during Chalcopyrite Bioleaching by a Thermoacidophilic Archaeon. Microorganisms 2020, 8, 1009. https://doi.org/10.3390/microorganisms8071009
Safar C, Castro C, Donati E. Importance of Initial Interfacial Steps during Chalcopyrite Bioleaching by a Thermoacidophilic Archaeon. Microorganisms. 2020; 8(7):1009. https://doi.org/10.3390/microorganisms8071009
Chicago/Turabian StyleSafar, Camila, Camila Castro, and Edgardo Donati. 2020. "Importance of Initial Interfacial Steps during Chalcopyrite Bioleaching by a Thermoacidophilic Archaeon" Microorganisms 8, no. 7: 1009. https://doi.org/10.3390/microorganisms8071009
APA StyleSafar, C., Castro, C., & Donati, E. (2020). Importance of Initial Interfacial Steps during Chalcopyrite Bioleaching by a Thermoacidophilic Archaeon. Microorganisms, 8(7), 1009. https://doi.org/10.3390/microorganisms8071009





