The Role of Cyanobacterial External Layers in Mass Transfer: Evidence from Temperature Shock Experiments by Noninvasive Microtest Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culturing
2.2. Experimental Design
2.3. Light Microscopy
2.4. Photosynthetic Activity Determination
2.5. Measurement of NH4+ and O2 Fluxes by NMT
2.6. Data Processing and Statistical Analysis
3. Results and Discussion
3.1. Evaluation of External Layer Extraction
3.2. Comparison of Net NH4+ and O2 Fluxes of External-Layer-Retaining and Stripped Samples
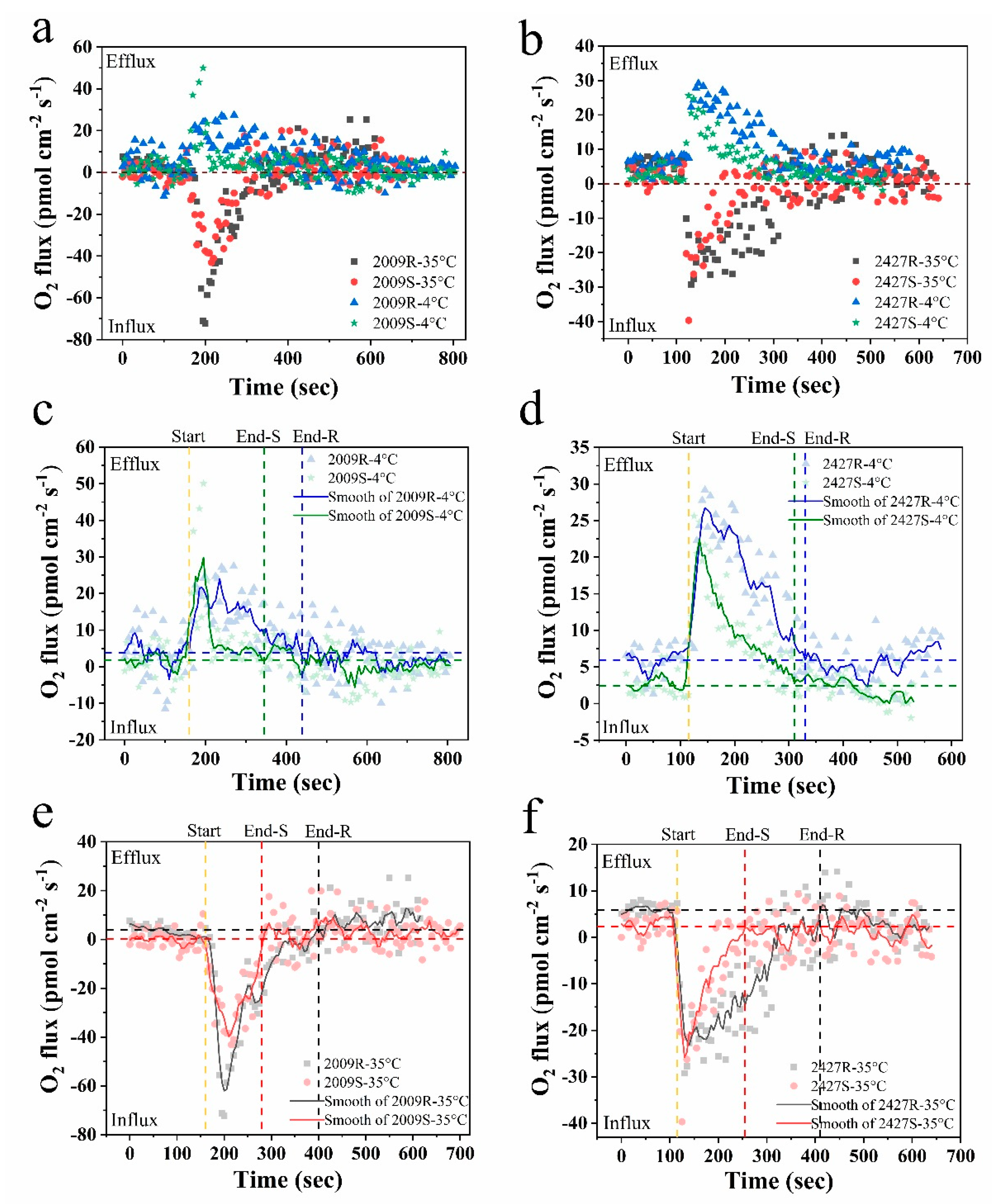
3.3. The Responses of External-Layer-Retaining and Stripped Samples to Instantaneous Temperature Shock
3.4. Contribution of External Layers to the Dominance of Cyanobacteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bennett, H.S. Morphological aspects of extracellular polysaccharides. J. Histochem. Cytochem. 1963, 11, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Leak, L.V. Fine structure of the mucilaginous sheath of Anabaena sp. J. Ultrastruct. Res. 1967, 21, 61–74. [Google Scholar] [CrossRef]
- Bayer, M.E.; Thurrow, H. Polysaccharide capsule of Escherichia coli: Microscope study of its size, structure, and sites of synthesis. J. Bacteriol. 1977, 130, 911–936. [Google Scholar] [CrossRef] [Green Version]
- Walsby, A.E. Mucilage secretion and the movements of blue-green algae. Protoplasma 1968, 65, 223–238. [Google Scholar] [CrossRef]
- Plude, J.L.; Parker, D.L.; Schommer, O.J.; Timmerman, R.J.; Hagstrom, S.A.; Joers, J.M.; Hnasko, R. Chemical characterization of polysaccharide from the slime layer of the cyanobacterium Microcystis flos-aquae C3-40. Appl. Environ. Microb. 1991, 57, 1696–1700. [Google Scholar] [CrossRef] [Green Version]
- Otero, A.; Vincenzini, M. Extracellular polysaccharide synthesis by Nostoc strains as affected by N source and light intensity. J. Biotechnol. 2003, 102, 143–152. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, H.; Yu, G.; Yang, L. Towards understanding the role of extracellular polymeric substances in cyanobacterial Microcystis aggregation and mucilaginous bloom formation. Chemosphere 2014, 117, 815–822. [Google Scholar] [CrossRef]
- Tan, X.; Shu, X.; Duan, Z.; Parajuli, K. Two types of bound extracellular polysaccharides and their roles in shaping the size and tightness of Microcystis colonies. J. Appl. Phycol. 2020, 32, 255–262. [Google Scholar] [CrossRef]
- Kumar, D.; Kaštánek, P.; Adhikary, S.P. Exopolysaccharides from cyanobacteria and microalgae and their commercial application. Curr. Sci. 2018, 115, 234–241. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Exocellular polysaccharides in microalgae and cyanobacteria: Chemical features, role and enzymes and genes involved in their biosynthesis. In The Physiology of Microalgae. Developments in Applied Phycology; Borowitzka, M., Beardall, J., Raven, J., Eds.; Springer: Cham, Switzerland, 2016; Volume 6. [Google Scholar]
- De Philippis, R.; Vincenzini, M. Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol. Rev. 1998, 22, 151–175. [Google Scholar] [CrossRef]
- Reynolds, C.S. Variability in the provision and function of mucilage in phytoplankton: Facultative responses to the environment. Hydrobiology 2007, 578, 37–45. [Google Scholar] [CrossRef]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Z.; Huang, Q.; Qin, B.Q. Characteristics and roles of Microcystis extracellular polymeric substances (EPS) in cyanobacterial blooms: A short review. J. Freshw. Ecol. 2018, 33, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Burford, M.A.; Carey, C.C.; Hamilton, D.P.; Huisman, J.; Paerl, H.W.; Wood, S.A.; Wulff, A. Perspective: Advancing the research agenda for improving understanding of cyanobacteria in a future of global change. Harmful Algae 2020, 91, 101601. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Xiao, Y.; Zhu, L.; Wu, Z.; Liu, J.; Hu, C.; Song, L. The role of microcystins in maintaining colonies of bloom-forming Microcystis spp. Environ. Microbiol. 2012, 14, 730–742. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Xiao, M.; Li, M.; Reynolds, C.S. Colony formation in the cyanobacterium Microcystis. Biol. Rev. 2018, 93, 1399–1420. [Google Scholar] [CrossRef] [Green Version]
- Fang, F.; Yang, L.; Gan, L.; Guo, L.; Hu, Z.; Yuan, S.; Chen, Q.; Jiang, L. DO, pH, and Eh microprofiles in cyanobacterial granules from Lake Taihu under different environmental conditions. J. Appl. Phycol. 2014, 26, 1689–1699. [Google Scholar] [CrossRef]
- Lange, W. Speculations on a possible essential function of the gelatinous sheath of blue-green algae. Can. J. Micobiol. 1976, 22, 1181–1185. [Google Scholar] [CrossRef]
- Hou, J.; Yang, Y.; Wang, P.; Wang, C.; Miao, L.; Wang, X.; Lv, B.; You, G.; Liu, Z. Effects of CeO2, CuO, and ZnO nanoparticles on physiological features of Microcystis aeruginosa and the production and composition of extracellular polymeric substances. Environ. Sci. Pollut. Res. 2017, 24, 226–235. [Google Scholar] [CrossRef]
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.P.; Hamilton, D.P.; Brookes, J.D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Tan, X.; Parajuli, K.; Upadhyay, S.; Zhang, D.; Shu, X.; Liu, Q. Colony formation in two Microcystis morphotypes: Effects of temperature and nutrient availability. Harmful Algae 2018, 72, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Griffith, A.W.; Gobler, C.J. Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 2020, 91, 101590. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Bose, J. Application of non-invasive microelectrode flux measurements in plant stress physiology. In Plant Electrophysiology; Volkov, A.G., Ed.; Springer: New York, NY, USA, 2012; pp. 91–126. [Google Scholar]
- Chen, H.; Zhang, Y.M.; He, C.L.; Wang, Q. Ca2+ signal transduction related to neutral lipid synthesis in an oil-producing green alga Chlorella sp. C2. Plant Cell Physiol. 2014, 55, 634–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strieth, D.; Stiefelmaier, J.; Wrabl, B.; Schwing, J.; Schmeckebier, A.; Nonno, S.D.; Muffler, K.; Ulber, R. A new strategy for a combined isolation of EPS and pigments from cyanobacteria. J. Appl. Phycol. 2020. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Zhang, C.; Meng, S.; Li, Y.; Zhao, Z. Net NH4+ and NO3− fluxes, and expression of NH4+ and NO3− transporter genes in roots of Populus simonii after acclimation to moderate salinity. Trees 2014, 28, 1813–1821. [Google Scholar] [CrossRef]
- Li, H.B.; Zheng, X.W.; Tao, L.X.; Yang, Y.J.; Gao, L.; Xiong, J. Aeration increases cadmium (Cd) retention by enhancing iron plaque formation and regulating pectin synthesis in the roots of rice (Oryza sativa) seedlings. Rice 2019, 12, 28. [Google Scholar] [CrossRef] [Green Version]
- Fellows, A.P.; Casford, M.T.L.; Davies, P.B. Spectral Analysis and Deconvolution of the Amide I Band of Proteins Presenting with High-Frequency Noise and Baseline Shifts. Appl. Spectrosc. 2020. [Google Scholar] [CrossRef]
- Tan, X.; Shu, X.; Guo, J.; Parajuli, K.; Zhang, X.; Duan, Z. Effects of low-frequency ultrasound on Microcystis aeruginosa from cell Inactivation to disruption. Bull. Environ. Contam. Toxicol. 2018, 101, 117–123. [Google Scholar] [CrossRef]
- Beecraft, L.; Watson, S.B.; Smith, R.E.H. Innate resistance of PSII efficiency to sunlight stress is not an advantage for cyanobacteria compared to eukaryotic phytoplankton. Aquat. Ecol. 2019, 53, 347–364. [Google Scholar] [CrossRef]
- Francko, D.; Taylor, S.R.; Thomas, B.J.; McIntosh, D. Effect of low-dose ultrasonic treatment on physiological variables in Anabaena flos-aquae and Selenastrum capricornutum. Biotechnol. Lett. 1990, 12, 219–224. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, P.; Liu, H.; Wang, B. Ultrasonic damages on cyanobacterial photosynthesis. Ultrason. Sonochem. 2006, 13, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Purcell, D.; Parsons, S.A.; Jefferson, B. The influence of ultrasound frequency and power, on the algal species Microcystis aeruginosa, Aphanizomenon flos-aquae, Scenedesmus subspicatus and Melosira sp. Environ. Technol. 2013, 34, 2477–2490. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Song, L. Physiological comparison between colonial and unicellular forms of Microcystis aeruginosa Kütz. (Cyanobacteria). Phycologia 2008, 47, 98–104. [Google Scholar] [CrossRef]
- Marañón, E. Cell size as a key determinant of phytoplankton metabolism and community structure. Annu. Rev. Mar. Sci. 2015, 7, 241–264. [Google Scholar] [CrossRef] [Green Version]
- Sand-Jensen, K. Ecophysiology of gelatinous Nostoc colonies: Unprecedented slow growth and survival in resource-poor and harsh environments. Ann. Bot. 2014, 114, 17–33. [Google Scholar] [CrossRef]
- Beardall, J.; Allen, D.; Bragg, J.; Finkel, Z.V.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Richardson, A.; Raven, J.A. Allometry and stoichiometry of unicellular, colonial and multicellular phytoplankton. New Phytol. 2009, 181, 295–309. [Google Scholar] [CrossRef]
- Padfield, D.; Yvon-Durocher, G.; Buckling, A.; Jennings, S.; Yvon-Durocher, G. Rapid evolution of metabolic traits explains thermal adaptation in phytoplankton. Ecol. Lett. 2016, 19, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Schaum, C.-E.; Barton, S.; Bestion, E.; Buckling, A.; Garcia-Carreras, B.; Lopez, P.; Lowe, C.; Pawar, S.; Smirnoff, N.; Trimmer, M.; et al. Adaptation of phytoplankton to a decade of experimental warming linked to increased photosynthesis. Nat. Ecol. Evol. 2017, 1, 0094. [Google Scholar] [CrossRef]
- Barton, S.; Jenkins, J.; Buckling, A.; Schaum, C.-E.; Smirnoff, N.; Raven, J.A.; Yvon-Durocher, G. Evolutionary temperature compensation of carbon fixation in marine phytoplankton. Ecol. Lett. 2020, 23, 722–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wang, Y.; Shu, X.; Zhang, Q. Physiological responses of soil crust-forming cyanobacteria to diurnal temperature variation. J. Basic Microbiol. 2013, 53, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.K.; Vimercati, L. Growth of cyanobacterial soil crusts during diurnal freeze-thaw cycles. J. Microbiol. 2019, 57, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiao, M.; Zhang, P.; Hamilton, D.P. Morphospecies-dependent disaggregation of colonies of the cyanobacterium Microcystis under high turbulent mixing. Water Res. 2018, 141, 340–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Tian, L.; Ren, C.; Xu, C.; Wang, Y.; Li, L. Extracellular polysaccharide synthesis in a bloom-forming strain of Microcystis aeruginosa: Implications for colonization and buoyancy. Sci. Rep. 2019, 9, 1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan Can, H.; Gurbuz, F.; Odabaşı, M. Partial characterization of cyanobacterial extracellular polymeric substances for aquatic ecosystems. Aquat. Ecol. 2019, 53, 431–440. [Google Scholar] [CrossRef]
- Borah, D.; Nainamalai, S.; Gopalakrishnan, S.; Rout, J.; Alharbi, N.S.; Alharbi, S.A.; Nooruddin, T. Biolubricant potential of exopolysaccharides from the cyanobacterium Cyanothece epiphytica. Appl. Microbiol. Biot. 2018, 102, 3635–3647. [Google Scholar] [CrossRef]
- Pannard, A.; Pedrono, J.; Bormans, M.; Briand, E.; Claquin, P.; Lagadeuc, Y. Production of exopolymers (EPS) by cyanobacteria: Impact on the carbon-to-nutrient ratio of the particulate organic matter. Aquat. Ecol. 2016, 50, 29–44. [Google Scholar] [CrossRef]
- Margalef, R. Our Biosphere; Ecology Institute: Oldendorf, Germany, 1997; p. 176. [Google Scholar]



| Strains | Code | Size | Form | External Layer Types |
|---|---|---|---|---|
| Nostoc sp. | FACHB-599 | 100–600 μm | Colony | Sheath |
| Nostoc sp. | FACHB-2009 | 100–600 μm | Colony | Sheath |
| Microcystis aeruginosa | FACHB-1338 | 200–500 μm | Colony | Slime |
| Microcystis sp. | FACHB-2427 | 200–500 μm | Colony | Slime |
| Strains | Code | Tr | Ma | Flw | |||
|---|---|---|---|---|---|---|---|
| 4 °C | 35 °C | 4 °C | 35 °C | 4 °C | 35 °C | ||
| Nostoc sp. | 2009R | 280 | 240 | 3650.25 | −4317.74 | 13.04 | −17.99 |
| 2009S | 185 | 120 | 1758.13 | −2951.77 | 9.50 | −24.60 | |
| Microcystis sp. | 2427R | 215 | 295 | 3650.11 | −2898.03 | 16.98 | −9.82 |
| 2427S | 195 | 140 | 2052.27 | −1386.07 | 10.52 | −9.90 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Liu, L.; Li, Z.; Cheng, Y. The Role of Cyanobacterial External Layers in Mass Transfer: Evidence from Temperature Shock Experiments by Noninvasive Microtest Technology. Microorganisms 2020, 8, 861. https://doi.org/10.3390/microorganisms8060861
Xiao Y, Liu L, Li Z, Cheng Y. The Role of Cyanobacterial External Layers in Mass Transfer: Evidence from Temperature Shock Experiments by Noninvasive Microtest Technology. Microorganisms. 2020; 8(6):861. https://doi.org/10.3390/microorganisms8060861
Chicago/Turabian StyleXiao, Yan, Lingxin Liu, Zhe Li, and Yuran Cheng. 2020. "The Role of Cyanobacterial External Layers in Mass Transfer: Evidence from Temperature Shock Experiments by Noninvasive Microtest Technology" Microorganisms 8, no. 6: 861. https://doi.org/10.3390/microorganisms8060861





