Oxidative Stress and Antioxidant Responses of Phormidium ambiguum and Microcystis aeruginosa Under Diurnally Varying Light Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cyanobacteria Cultures and Incubation
2.2. Experimental Setup and Procedure
2.3. H2O2 Concentration
2.4. GPX-Activity Assay
2.5. CAT-Activity Assay
2.6. APX-Activity Assay
2.7. SOD-Activity Assay
2.8. Data Analysis
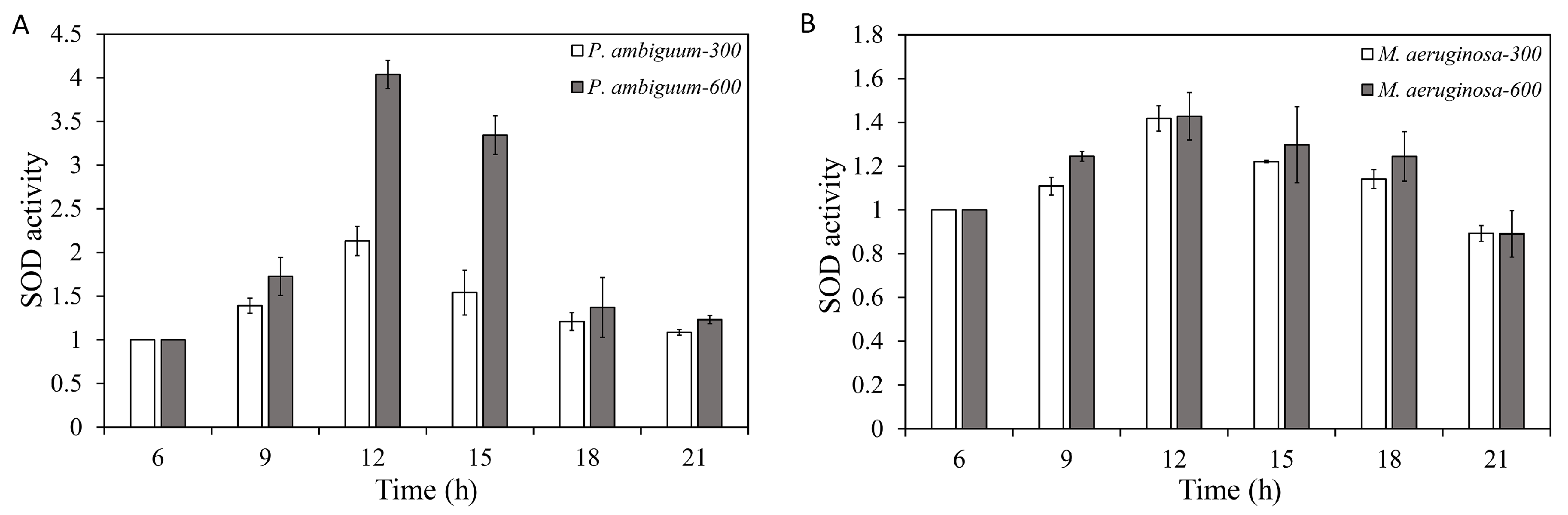
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pham, T.-L.; Utsumi, M. An overview of the accumulation of microcystins in aquatic ecosystems. J. Environ. Manag. 2018, 213, 520–529. [Google Scholar] [CrossRef]
- Trolle, D.; Nielsen, A.; Andersen, H.E.; Thodsen, H.; Olesen, J.E.; Børgesen, C.D.; Refsgaard, J.C.; Sonnenborg, T.O.; Karlsson, I.B.; Christensen, J.P.; et al. Effects of changes in land use and climate on aquatic ecosystems: Coupling of models and decomposition of uncertainties. Sci. Total Environ. 2019, 657, 627–633. [Google Scholar] [CrossRef]
- Izaguirre, G.; Hwang, C.J.; Krasner, S.W.; McGuire, M.J. Geosmin and 2-Methylisoborneol from cyanobacteria in three water supply systems. Appl. Environ. Microbiol. 1982, 43, 708–714. [Google Scholar] [CrossRef] [Green Version]
- Butakova, E.A. Specific features of odor-causing compounds (geosmin and 2-methylisoborneol) as secondary metabolites of cyanobacteria. Russ. J. Plant Physiol. 2013, 60, 507–510. [Google Scholar] [CrossRef]
- Kakimoto, M.; Ishikawa, T.; Miyagi, A.; Saito, K.; Miyazaki, M.; Asaeda, T.; Yamaguchi, M.; Uchimiya, H.; Kawai-Yamada, M. Culture temperature affects gene expression and metabolic pathways in the 2-methylisoborneol-producing cyanobacterium Pseudanabaena galeata. J. Plant Physiol. 2014, 171, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Monserrat, J.M.; Yunes, J.S.; Bianchini, A. Effects of Anabaena spiroides (cyanobacteria) aqueous extracts on the acetylcholinesterase activity of aquatic species. Environ. Toxicol. Chem. 2001, 20, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Pflugmacher, S. Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat. Toxicol. 2004, 70, 169–178. [Google Scholar] [CrossRef]
- Ghadouani, A.; Pinel-Alloul, B.; Prepas, E.E. Effects of experimentally induced cyanobacterial blooms on crustacean zooplankton communities. Freshw. Biol. 2003, 48, 363–381. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; McCarthy, M.J.; Zhu, G.; Qin, B.; Li, Y.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar] [CrossRef]
- Rodríguez-Molares, A.; Dickson, S.; Hobson, P.; Howard, C.; Zander, A.; Burch, M. Quantification of the ultrasound induced sedimentation of Microcystis aeruginosa. Ultrason. Sonochem. 2014, 21, 1299–1304. [Google Scholar] [CrossRef]
- Rajasekhar, P.; Fan, L.; Nguyen, T.; Roddick, F.A. A review of the use of sonication to control cyanobacterial blooms. Water Res. 2012, 46, 4319–4329. [Google Scholar] [CrossRef]
- Grandgirard, J.; Poinsot, D.; Krespi, L.; Nenon, J.-P.; Cortesero, A.M. Costs of secondary parasitism in the facultative hyperparasitoid Pachycrepoideus dubius: Does host size matter? Èntomol. Exp. Appl. 2002, 103, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Lake, M.; Madsen, M.; Brokaw, T.; Moon, R.; Beardon, C.; Cassell, C.; Collins, D. Mason Lake; Lake Stewardship Consulting: Belfair, WA, USA, 2003. [Google Scholar]
- Jančula, D.; Maršálek, B. Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere 2011, 85, 1415–1422. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidler, W. Phycobilisome and Phycobiliprotein Structures. In The Molecular Biology of Cyanobacteria; Bryant, D.A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; Volume 2, ISBN 978-0-7923-3273-2. [Google Scholar]
- Liu, L.; Chen, H.; Liu, M.; Yang, J.R.; Xiao, P.; Wilkinson, D.M. Response of the eukaryotic plankton community to the cyanobacterial biomass cycle over 6 years in two subtropical reservoirs. ISME J. 2019, 13, 2196–2208. [Google Scholar] [CrossRef] [Green Version]
- Joset, F.; Jeanjean, R.; Hagemann, M. Dynamics of the response of cyanobacteria to salt stress: Deciphering the molecular events. Physiol. Plant. 1996, 96, 738–744. [Google Scholar] [CrossRef]
- Sinetova, M.; Los, D.A. New insights in cyanobacterial cold stress responses: Genes, sensors, and molecular triggers. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 2391–2403. [Google Scholar] [CrossRef] [PubMed]
- Babele, P.K.; Kumar, J.; Chaturvedi, V. Proteomic de-regulation in cyanobacteria in response to abiotic stresses. Front. Microbiol. 2019, 10, 1315. [Google Scholar] [CrossRef] [Green Version]
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.; Hamilton, D.P.; Brookes, J. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Dillon, J.G.; Tatsumi, C.M.; Tandingan, P.G.; Castenholz, R.W. Effect of environmental factors on the synthesis of scytonemin, a UV-screening pigment, in a cyanobacterium (Chroococcidiopsis sp.). Arch. Microbiol. 2002, 177, 322–331. [Google Scholar] [CrossRef]
- Dobretsov, S.; Abed, R.M.M.; Al Maskari, S.M.S.; Al Sabahi, J.N.; Victor, R. Cyanobacterial mats from hot springs produce antimicrobial compounds and quorum-sensing inhibitors under natural conditions. Environ. Biol. Fishes 2010, 23, 983–993. [Google Scholar] [CrossRef]
- Celeste, A.J.; Beaulieu, K.M.; Bradley, P.M. Environmental factors that influence cyanobacteria and Geosmin occurrence in reservoirs. Curr. Perspect. Contam. Hydrol. Water Resour. Sustain. 2013. [Google Scholar] [CrossRef] [Green Version]
- Watson, S. Cyanobacterial and eukaryotic algal odour compounds: Signals or by-products? A review of their biological activity. Phycologia 2003, 42, 332–350. [Google Scholar] [CrossRef]
- Paerl, H.W.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef]
- Paerl, H.W. Mitigating harmful cyanobacterial blooms in a human- and climatically-impacted world. Life 2014, 4, 988–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, R.P.; Madamwar, D.; Incharoensakdi, A. Bloom dynamics of cyanobacteria and their toxins: Environmental health impacts and mitigation strategies. Front. Microbiol. 2015, 6, 223. [Google Scholar] [CrossRef] [Green Version]
- Briand, J.-F.; Leboulanger, C.; Humbert, J.-F.; Bernard, C.; Dufour, P. Cylindrospermopsis raciborskii (cyanobacteria) invasion at mid-latitudes: Selection, wide physiological tolerance, orglobalwarming? J. Phycol. 2004, 40, 231–238. [Google Scholar] [CrossRef]
- Rücker, J.; Tingwey, E.I.; Wiedner, C.; Anu, C.M.; Nixdorf, B. Impact of the inoculum size on the population of Nostocales cyanobacteria in a temperate lake. J. Plankton Res. 2009, 31, 1151–1159. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Hu, C.; Visser, P.M.; Ma, R. Diurnal changes of cyanobacteria blooms in Taihu Lake as derived from GOCI observations. Limnol. Oceanogr. 2018, 63, 1711–1726. [Google Scholar] [CrossRef] [Green Version]
- Saha, R.; Liu, D.; Hoynes-O’Connor, A.; Liberton, M.; Yu, J.; Bhattacharyya-Pakrasi, M.; Balássy, A.; Zhang, F.; Moon, T.S.; Maranas, C.D.; et al. Diurnal regulation of cellular processes in the Cyanobacterium. mBio 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Anthony, J.R.; Warczak, K.L.; Donohue, T.J. A transcriptional response to singlet oxygen, a toxic byproduct of photosynthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 6502–6507. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymanska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Latifi, A.; Ruiz, M.; Zhang, C.-C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, P.; Ibelings, B.W.; Bormans, M.; Huisman, J. Artificial mixing to control cyanobacterial blooms: A review. Aquat. Ecol. 2015, 50, 423–441. [Google Scholar] [CrossRef] [Green Version]
- Berrendero, E.; Valiente, E.F.; Perona, E.; Gómez, C.L.; Loza, V.; Martin, M.D.L.; Ángeles, M.; Mateo, P. Nitrogen fixation in a non-heterocystous cyanobacterial mat from a mountain river. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Xiao, M.; Li, M.; Reynolds, C.S. Colony formation in the cyanobacteriumMicrocystis. Biol. Rev. 2018, 93, 1399–1420. [Google Scholar] [CrossRef] [Green Version]
- Princiotta, S.D.; Hendricks, S.P.; White, D.S. Production of Cyanotoxins by Microcystis aeruginosa mediates interactions with the Mixotrophic flagellate Cryptomonas. Toxins 2019, 11, 223. [Google Scholar] [CrossRef] [Green Version]
- Teneva, I.; Dzhambazov, B.; Koleva-Valkova, L.; Mladenov, R.; Schirmer, K. Toxic potential of five freshwater Phormidium species (Cyanoprokaryota). Toxicon 2005, 45, 711–725. [Google Scholar] [CrossRef]
- Rippka, R.; Stanier, R.Y.; Deruelles, J.; Herdman, M.; Waterbury, J.B. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111. [Google Scholar] [CrossRef] [Green Version]
- Jana, S.; Choudhuri, M.A. Effects of plant growth regulators on Hill activity of submerged aquatic plants during induced senescence. Aquat. Bot. 1984, 18, 371–376. [Google Scholar] [CrossRef]
- Senousy, H.H.; Ellatif, S.A.; Ali, S. Assessment of the antioxidant and anticancer potential of different isolated strains of cyanobacteria and microalgae from soil and agriculture drain water. Environ. Sci. Pollut. Res. 2020, 27, 18463–18474. [Google Scholar] [CrossRef] [PubMed]
- Macadam, J.W.; Nelson, C.J.; Sharp, R.E. Peroxidase activity in the leaf elongation zone of tall fescue: I. spatial distribution of Ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiol. 1992, 99, 872–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by Ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Ewing, J.F.; Janero, D.R. Microplate superoxide dismutase assay employing a Nonenzymatic superoxide generator. Anal. Biochem. 1995, 232, 243–248. [Google Scholar] [CrossRef]
- Pruchniak, M.P.; Arazna, M.; Demkow, U. Biochemistry of oxidative stress. Adv. Exp. Med. Biol. 2015, 878, 9–19. [Google Scholar] [CrossRef]
- Lin, G.G.; Scott, J.G. ROS function in redox signaling. Curr. Biol. 2012, 100, 130–134. [Google Scholar]
- Exposito-Rodriguez, M.; Laissue, P.P.; Yvon-Durocher, G.; Smirnoff, N.; Mullineaux, P.M. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat. Commun. 2017, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Page†, M.T.; Sultana, N.; Paszkiewicz, K.; Florance, H.; Smirnoff, N. The influence of ascorbate on anthocyanin accumulation during high light acclimation in Arabidopsis thaliana: Further evidence for redox control of anthocyanin synthesis. Plant Cell Environ. 2011, 35, 388–404. [Google Scholar] [CrossRef]
- Virtanen, O.; Valev, D.; Kruse, O.; Wobbe, L.; Tyystjarvi, E. Photoinhibition and continuous growth of the wild-type and a high-light tolerant strain of Chlamydomonas reinhardtii. Photosynthetica 2019, 57, 617–626. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim. Biophys. Acta (BBA)-Bioenerg. 2006, 1757, 742–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collén, J.; Pedersén, M. Production, scavenging and toxicity of hydrogen peroxide in the green seaweedUlva rigida. Eur. J. Phycol. 1996, 31, 265–271. [Google Scholar] [CrossRef]
- Estervig, D.; Wang, R.J. Sister chromatid exchanges and chromosome aberrations in human cells induced by H2O2 and other photoproducts generated in fluorescent light-exposed medium. Photochem. Photobiol. 1984, 40, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Vanderauwera, S.; Zimmermann, P.; Rombauts, S.; Vandenabeele, S.; Langebartels, C.; Gruissem, W.; Inzé, D.; Van Breusegem, F. Genome-wide analysis of hydrogen peroxide-regulated gene expression in arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 2005, 139, 806–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brutemark, A.; Engstrom-Ost, J.; Vehmaa, A.; Gorokhova, E. Growth, toxicity and oxidative stress of a cultured cyanobacterium (Dolichospermum sp.) under different CO2/pH and temperature conditions. Phycol. Res. 2015, 63, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2010, 155, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhetaer, G.; Asaeda, T.; Jayasanka, S.M.; Baniya, M.B.; Abeynayaka, H.D.; Rashid, M.H.; Yan, H. Effects of light intensity and exposure period on the growth and stress responses of two cyanobacteria species: Pseudanabaena galeata and Microcystis aeruginosa. Water 2020, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Zevenboom, W.; Mur, L.R. Growth and photosynthetic response of the cyanobacterium Microcystis aeruginosa in relation to photoperiodicity and irradiance. Arch. Microbiol. 1984, 139, 232–239. [Google Scholar] [CrossRef]
- Edge, R.; McGarvey, D.; Truscott, T. The carotenoids as anti-oxidants—A review. J. Photochem. Photobiol. B Biol. 1997, 41, 189–200. [Google Scholar] [CrossRef]
- Paerl, H.W. Cyanobacterial carotenoids: Their roles in maintaining optimal photosynthetic production among aquatic bloom forming genera. Oecologia 1984, 61, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Niu, X.; Song, Q.; Li, Y.; Zhang, R.; Zou, D.; Lai, S.; Yang, Z.; Tang, Z.; Zhou, S.; et al. Physiological and biochemical responses of Microcystis aeruginosa to phosphine. Environ. Pollut. 2019, 247, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Borase, D.; Dhar, D.W.; Singh, N. Diversity indices and growth parameters of cyanobacteria from three lakes of Rajasthan. Vegetos-Int. J. Plant Res. 2013, 26, 377. [Google Scholar] [CrossRef]
- Golden, S.S.; Ishiura, M.; Johnson, C.H.; Kondo, T. Cyanobacterial circadian rhythms. Annu. Rev. Plant Biol. 1997, 48, 327–354. [Google Scholar] [CrossRef] [PubMed]






| Condition | Parameter | R2 | p Value |
|---|---|---|---|
| M. aeruginosa–300 1 | SOD | 0.780 | p < 0.01 |
| APX | 0.652 | p < 0.01 | |
| CAT | 0.893 | p < 0.01 | |
| GPX | 0.539 | p < 0.05 | |
| AOX | 0.856 | p < 0.01 | |
| M. aeruginosa-600 | SOD | 0.526 | p < 0.05 |
| APX | 0.683 | p < 0.01 | |
| CAT | 0.924 | p < 0.01 | |
| GPX | 0.692 | p < 0.01 | |
| AOX | 0.720 | p < 0.01 | |
| P. ambiguum-300 | SOD | 0.748 | p < 0.01 |
| APX | 0.962 | p < 0.01 | |
| CAT | 0.824 | p < 0.01 | |
| GPX | 0.383 | p > 0.05 | |
| AOX | 0.803 | p < 0.01 | |
| P. ambiguum-600 | SOD | 0.784 | p < 0.01 |
| APX | 0.738 | p < 0.01 | |
| CAT | 0.830 | p < 0.01 | |
| GPX | 0.624 | p < 0.01 | |
| AOX | 0.796 | p < 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhetaer, G.; Jayasanka, S.M.D.H.; Fujino, T. Oxidative Stress and Antioxidant Responses of Phormidium ambiguum and Microcystis aeruginosa Under Diurnally Varying Light Conditions. Microorganisms 2020, 8, 890. https://doi.org/10.3390/microorganisms8060890
Muhetaer G, Jayasanka SMDH, Fujino T. Oxidative Stress and Antioxidant Responses of Phormidium ambiguum and Microcystis aeruginosa Under Diurnally Varying Light Conditions. Microorganisms. 2020; 8(6):890. https://doi.org/10.3390/microorganisms8060890
Chicago/Turabian StyleMuhetaer, Guligena, Senavirathna M.D.H. Jayasanka, and Takeshi Fujino. 2020. "Oxidative Stress and Antioxidant Responses of Phormidium ambiguum and Microcystis aeruginosa Under Diurnally Varying Light Conditions" Microorganisms 8, no. 6: 890. https://doi.org/10.3390/microorganisms8060890
APA StyleMuhetaer, G., Jayasanka, S. M. D. H., & Fujino, T. (2020). Oxidative Stress and Antioxidant Responses of Phormidium ambiguum and Microcystis aeruginosa Under Diurnally Varying Light Conditions. Microorganisms, 8(6), 890. https://doi.org/10.3390/microorganisms8060890






