Lactobacillus reuteri DSM 17938 as a Novel Topical Cosmetic Ingredient: A Proof of Concept Clinical Study in Adults with Atopic Dermatitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Investigational Products
2.3. Patient Recruitment and Sampling
2.4. Appraisal of the Cutaneous Acceptability by the Dermatologist Investigator and Cosmetic Acceptability
2.5. Clinical Evaluation by the Dermatologist Investigator
2.6. Determination of Number of Subject’s Sample Size
2.7. Statistical Analysis
2.8. Adverse Events
3. Results
3.1. Subject Characteristics
3.2. Cutaneous Acceptability of the Investigational Products
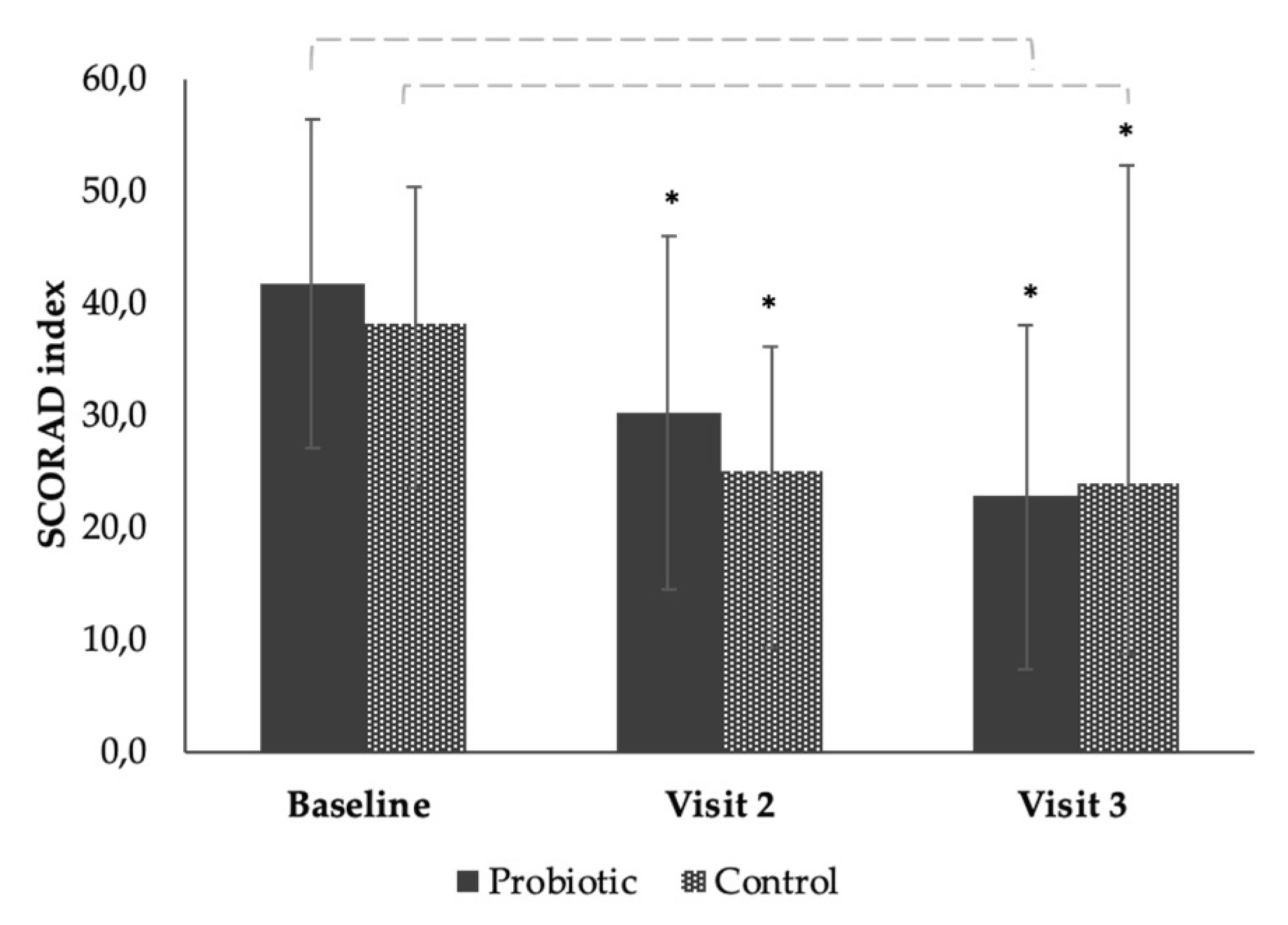
3.3. The Effect of the Products on SCORAD from Baseline to End of Study (Time Effect)
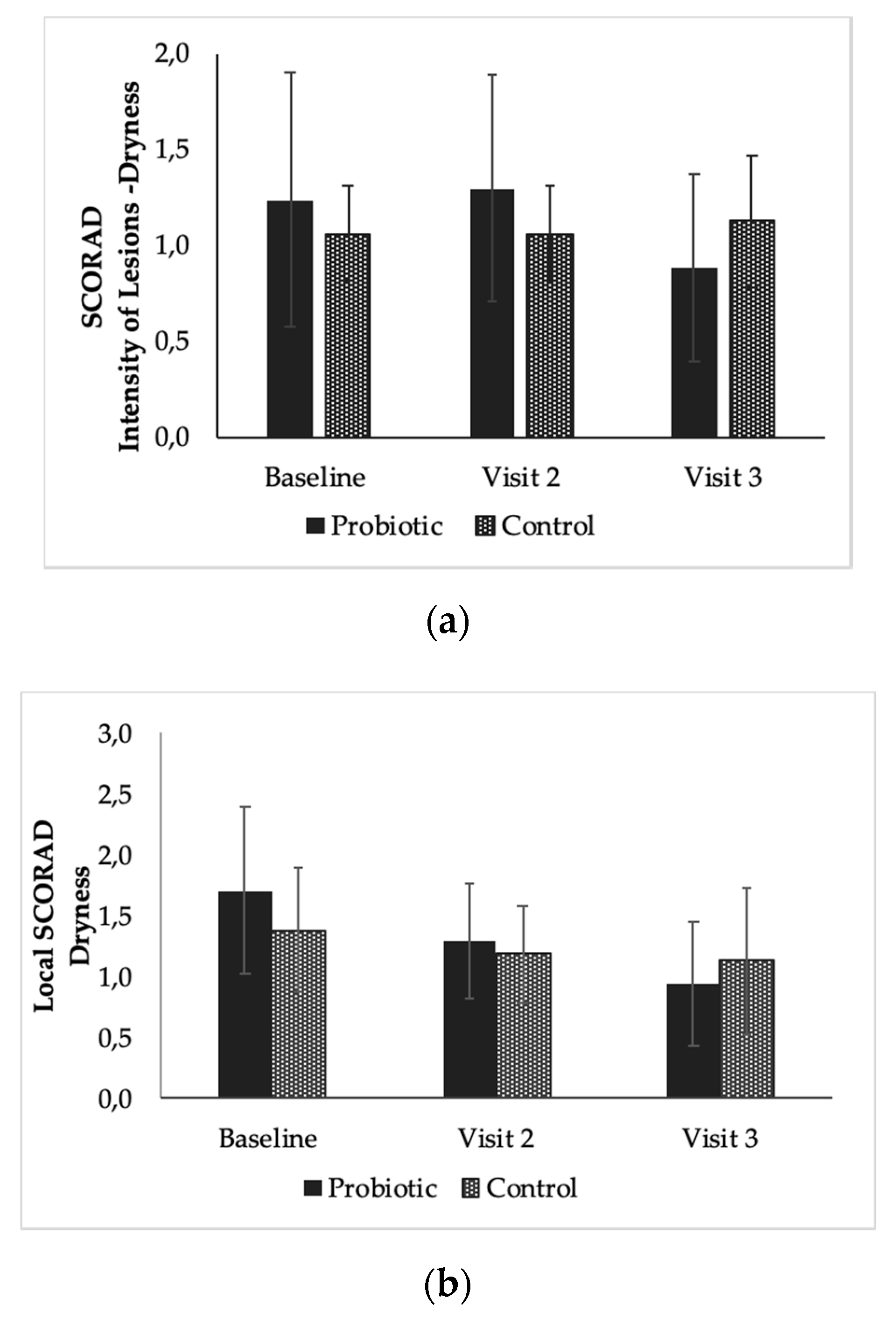
3.4. The Effect of the Products on Local SCORAD from Baseline to End of Study (Time Effect)
3.5. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Frequency of the Reactions | Number of Subjects with Reactions | |
|---|---|---|
| Probiotic (n = 17) | Control (n = 17) | |
| Reactions during the whole study | 7 | 5 |
| Reactions observed by the dermatologist | 3 | 1 |
| Reactions observed by the subject | ||
| (per type of reaction) | ||
| - Discomfort | 7 | 5 |
| - Irritation | 3 | 2 |
| - Edema | 0 | 0 |
| - Dryness | 2 | 3 |
| - Desquamation | 0 | 0 |
| - “small pimples” | 0 | 2 |
| Reactions that needed to modify the application modalities significantly | 0 | 0 |
| Reactions which can be considered as relevant * | 0 | 0 |
| Reactions considered as adverse events related to the investigational products | 0 | 0 |
| Reactions considered as serious adverse events linked to the investigational products | 0 | 0 |
| Cosmetic acceptability by the dermatologist investigator Very good—good | 17 | 17 |
| Cosmetic acceptability by the subjects | ||
| Very good-good | 11 | 9 |
| Rather good -mediocre | 4 | 6 |
| Bad | 2 | 1 |
| Conclusion on Cutaneous Acceptability | GOOD | GOOD |
| Means and Standard Deviations (SD) | ||||
|---|---|---|---|---|
| n | Probiotic | n | Control | |
| Baseline (day 0) | 17 | 41.74 ± 14.66 | 16 | 36.69 ± 10.75 |
| Visit 2 (day 28) | 17 | 30.20 ± 15.83 | 16 | 24.98 ± 11.06 |
| Visit 3 (day 56) | 17 | 22.68 ± 15.38 | 16 | 23.96 ± 9.58 |
| Means and Standard Deviations (SD) | ||||
|---|---|---|---|---|
| n | Probiotic | n | Control | |
| Baseline (day 0) | 17 | 9.00 ± 3.95 | 16 | 8.19 ± 3.56 |
| Visit 2 (day 28) | 17 | 6.76 ± 4.07 | 16 | 5.38 ± 2.92 |
| Visit 3 (day 56) | 17 | 4.94 ± 4.25 | 16 | 4.94 ± 2.82 |

References
- McPherson, T. Current understanding in pathogenesis of atopic dermatitis. Indian J. Dermatol. 2016, 61, 649–655. [Google Scholar] [CrossRef]
- Asher, M.I.; Montefort, S.; Bjorksten, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Silverberg, J.I. Healthcare Utilization, Patient Costs, and Access to Care in US Adults with Eczema. JAMA Dermatol. 2015, 151, 743–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbarot, S.; Auziere, S.; Gadkari, A.; Girolomoni, G.; Puig, L.; Simpson, E.L.; Margolis, D.J.; Bruin-Weller, M.; Eckert, L.; De Bruin-Weller, M. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy 2018, 73, 1284–1293. [Google Scholar] [CrossRef] [Green Version]
- Novak, N. Immune mechanisms leading to atopic dermatitis. J. Allergy Clin. Immunol. 2003, 112, S128–S139. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, E.; Banderali, G.; Barberi, S.; Gualandri, L.; Pietra, B.; Riva, E.; Cerri, A. Atopic dermatitis: Recent insight on pathogenesis and novel therapeutic target. Asian Pac. J. Allergy Immunol. 2016, 34, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; Gallo, R.L. Evidence that Human Skin Microbiome Dysbiosis Promotes Atopic Dermatitis. J. Investig. Dermatol. 2017, 137, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; Gallo, R.L. The Role of the Skin Microbiome in Atopic Dermatitis. Curr. Allergy Asthma Rep. 2015, 15, 65. [Google Scholar] [CrossRef]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; Murray, P.R.; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef] [Green Version]
- Madison, K.C. Barrier Function of the Skin: “La Raison d’Être” of the Epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Gooderham, M.; Hong, H.C.-H.; Eshtiaghi, P.; Papp, K. Dupilumab: A review of its use in the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2018, 78, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- A Kiken, D.; Silverberg, N.B. Atopic dermatitis in children, part 2: Treatment options. Cutis 2006, 78, 401–406. [Google Scholar] [PubMed]
- Eichenfield, L.F.; Tom, K.L.; Chamlin, S.L.; Feldman, S.R.; Hanifin, J.M.; Simpson, E.L.; Berger, T.G.; Bergman, J.N.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 1. Diagnosis and assessment of atopic dermatitis. J. Am. Acad. Dermatol. 2013, 70, 338–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodén, M. Role of Topical Emollients and Moisturizers in the Treatment of Dry Skin Barrier Disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788. [Google Scholar] [CrossRef]
- Hon, K.L.; Kung, J.S.C.; Ng, W.G.G.; Leung, T.F. Emollient treatment of atopic dermatitis: Latest evidence and clinical considerations. Drugs Context 2018, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Isolauri, E.; Arvola, T.; Sutas, Y.; Moilanen, E.; Salminen, S. Probiotics in the management of atopic eczema. Clin. Exp. Allergy 2000, 30, 1605–1610. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Navarro-López, V.; Ramirez-Boscá, A.; Ramón-Vidal, D.; Ruzafa-Costas, B.; Genovés-Martínez, S.; Chenoll-Cuadros, E.; Carrion-Gutierrez, M.; De La Parte, J.H.; Prieto-Merino, D.; Codoñer-Cortés, F.M. Effect of Oral Administration of a Mixture of Probiotic Strains on SCORAD Index and Use of Topical Steroids in Young Patients with Moderate Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2018, 154, 37–43. [Google Scholar] [CrossRef]
- Abrahamsson, T.R.; Jakobsson, T.; Böttcher, M.F.; Fredrikson, M.; Jenmalm, M.C.; Bjorksten, B.; Oldaeus, G. Probiotics in prevention of IgE-associated eczema: A double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2007, 119, 1174–1180. [Google Scholar] [CrossRef]
- Gueniche, A.; Bastien, P.; Ovigne, J.M.; Kermici, M.; Courchay, G.; Chevalier, V.; Breton, L.; Castiel-Higounenc, I. Bifidobacterium longum lysate, a new ingredient for reactive skin. Exp. Dermatol. 2009, 19, e1–e8. [Google Scholar] [CrossRef]
- Gueniche, A.; Benyacoub, J.; Philippe, D.; Bastien, P.; Kusy, N.; Breton, L.; Blum, S.; Castiel-Higounenc, I. Lactobacillus paracasei CNCM I-2116 (ST11) inhibits substance P-induced skin inflammation and accelerates skin barrier function recovery in vitro. Eur. J. Dermatol. 2010, 20, 731–737. [Google Scholar]
- Kim, J.-Y.; Park, B.-K.; Park, H.-J.; Park, Y.-H.; Kim, B.-O.; Pyo, S. Atopic dermatitis-mitigating effects of new Lactobacillus strain, Lactobacillus sakei probio 65 isolated from Kimchi. J. Appl. Microbiol. 2013, 115, 517–526. [Google Scholar] [CrossRef]
- Park, S.B.; Im, M.; Lee, Y.; Lee, J.H.; Lim, J.; Park, Y.-H.; Seo, Y.J. Effect of Emollients Containing Vegetable-DerivedLactobacillusin the Treatment of Atopic Dermatitis Symptoms: Split-Body Clinical Trial. Ann. Dermatol. 2014, 26, 150–155. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2018 March 19 -. Identifier NCT03469076, Studying the Skin Microbiome and the Potential of a Topical Probiotic Cream for Patients With Acne; 2006 February 3. Available online: https://clinicaltrials.gov/ct2/show/NCT03469076 (accessed on 10 July 2020).
- Prince, T.; McBain, A.J.; O’Neill, C.A. Lactobacillus reuteri Protects Epidermal Keratinocytes from Staphylococcus aureus-Induced Cell Death by Competitive Exclusion. Appl. Environ. Microbiol. 2012, 78, 5119–5126. [Google Scholar] [CrossRef] [Green Version]
- Mohammedsaeed, W.; McBain, A.J.; Cruickshank, S.M.; O’Neill, C.A. Lactobacillus rhamnosus GG Inhibits the Toxic Effects of Staphylococcus aureus on Epidermal Keratinocytes. Appl. Environ. Microbiol. 2014, 80, 5773–5781. [Google Scholar] [CrossRef] [Green Version]
- Mohammedsaeed, W.; Cruickshank, S.; McBain, A.J.; O’Neill, C.A. Lactobacillus rhamnosus GG Lysate Increases Re-Epithelialization of Keratinocyte Scratch Assays by Promoting Migration. Sci. Rep. 2015, 5, 16147. [Google Scholar] [CrossRef] [Green Version]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Sinkiewicz, G.; Ljunggren, L. Occurrence of Lactobacillus reuteri in human breast milk. Microb. Ecol. Health Dis. 2008, 20. [Google Scholar] [CrossRef]
- Xu, M.; Wang, J.; Wang, N.; Sun, F.; Wang, L.; Liu, X.-H. The Efficacy and Safety of the Probiotic Bacterium Lactobacillus reuteri DSM 17938 for Infantile Colic: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2015, 10, e0141445. [Google Scholar] [CrossRef] [Green Version]
- Urbanska, M.; Gieruszczak-Białek, D.; Szajewska, H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment. Pharmacol. Ther. 2016, 43, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Mangalat, N.; Liu, Y.; Fatheree, N.Y.; Ferris, M.J.; Van Arsdall, M.R.; Chen, Z.; Rahbar, M.H.; Gleason, W.A.; Norori, J.; Tran, D.Q.; et al. Safety and Tolerability of Lactobacillus reuteri DSM 17938 and Effects on Biomarkers in Healthy Adults: Results from a Randomized Masked Trial. PLoS ONE 2012, 7, e43910. [Google Scholar] [CrossRef] [Green Version]
- Rosander, A.; Connolly, E.; Roos, S. Removal of Antibiotic Resistance Gene-Carrying Plasmids from Lactobacillus reuteri ATCC 55730 and Characterization of the Resulting Daughter Strain, L. reuteri DSM 17938. Appl. Environ. Microbiol. 2008, 74, 6032–6040. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, L.; Auchtung, T.A.; Hermans, K.E.; Whitehead, D.; Borhan, B.; Britton, R.A. The antimicrobial compound reuterin (3-hydroxypropionaldehyde) induces oxidative stress via interaction with thiol groups. Microbiology 2010, 156, 1589–1599. [Google Scholar] [CrossRef] [Green Version]
- Spinler, J.K.; Auchtung, J.; Brown, A.; Boonma, P.; Oezguen, N.; Ross, C.L.; Luna, R.A.; Runge, J.; Versalovic, J.; Peniche, A.; et al. Next-Generation Probiotics Targeting Clostridium difficile through Precursor-Directed Antimicrobial Biosynthesis. Infect. Immun. 2017, 85, e00303-17. [Google Scholar] [CrossRef] [Green Version]
- Khmaladze, I.; Butler, É.; Fabre, S.; Gillbro, J.M. Lactobacillus reuteri DSM 17938-A comparative study on the effect of probiotics and lysates on human skin. Exp. Dermatol. 2019, 28, 822–828. [Google Scholar] [CrossRef]
- Jones, S.E.; Versalovic, J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Fatheree, N.Y.; Dingle, B.M.; Tran, D.Q.; Rhoads, J.M. Lactobacillus reuteri DSM 17938 Changes the Frequency of Foxp3+ Regulatory T Cells in the Intestine and Mesenteric Lymph Node in Experimental Necrotizing Enterocolitis. PLoS ONE 2013, 8, e56547. [Google Scholar] [CrossRef]
- Liu, Y.; Tran, D.Q.; Fatheree, N.Y.; Rhoads, J.M. Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3+ regulatory T cells in a mouse model of necrotizing enterocolitis. Am. J. Physiol. Liver Physiol. 2014, 307, G177–G186. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.-S.; Oh, J.-S.; Lee, S.-W.; Lim, H.-S.; Choi, N.-K.; Kim, S. Effect of Lactobacillus reuteri on the proliferation of Propionibacterium acnes and Staphylococcus epidermidis. J. Microbiol. 2012, 50, 137–142. [Google Scholar] [CrossRef]
- Dicksved, J.; Schreiber, O.; Willing, B.; Petersson, J.; Rang, S.; Phillipson, M.; Holm, L.; Roos, S. Lactobacillus reuteri Maintains a Functional Mucosal Barrier during DSS Treatment Despite Mucus Layer Dysfunction. PLoS ONE 2012, 7, e46399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, H.C.; Jburney, P.G.; Pembroke, A.C.; Hay, R.J.; Atopic Dermatitis Diagnostic Criteria Working Party. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Brit. J. Dermatol. 1994, 131, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Snatchfold, J. Cutaneous acceptability of a moisturizing cream in subjects with sensitive skin. J. Cosmet. Dermatol. 2018, 18, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Favrel, S.; Mielewczyk, E.; Liberek, A.; Paw, E.; Chabowska, I.; Sirvent, A.; Ribet, V.; Delarue, A. A high-emollient liquid cleanser for very dry and atopic-prone skin: Results of an in-use tolerance and efficacy study conducted under dermatological, pediatric, and ophthalmological supervision. J. Cosmet. Dermatol. 2019, 19, 1155–1160. [Google Scholar] [CrossRef]
- Anonymous. Severity scoring of atopic dermatitis: The SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993, 186, 23–31. [Google Scholar] [CrossRef]
- Seité, S.; Zelenkova, H.; Martin, R. Clinical efficacy of emollients in atopic dermatitis patients—relationship with the skin microbiota modification. Clin. Cosmet. Investig. Dermatol. 2017, 10, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.; Chalmers, J.; Hanifin, J.M.; Thomas, K.S.; Cork, M.; McLean, W.I.; Brown, S.J.; Chen, Z.; Chen, Y.; Williams, H.C. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J. Allergy Clin. Immunol. 2014, 134, 818–823. [Google Scholar] [CrossRef] [Green Version]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Tong, Y.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef] [Green Version]
- Nisbet, S.J. Skin acceptability of a cosmetic moisturizer formulation in female subjects with sensitive skin. Clin. Cosmet. Investig. Dermatol. 2018, 11, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Hon, K.L.; Tsang, Y.C.; Pong, N.H.; Lee, V.W.Y.; Luk, N.M.; Chow, C.M.; Leung, T.F. Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema. Hong Kong Med. J. 2015, 21, 417–425. [Google Scholar] [CrossRef]
- Olsson, M.; Broberg, A.; Jernas, M.; Carlsson, L.; Rudemo, M.; Suurküla, M.; Svensson, P.-A.; Benson, M. Increased expression of aquaporin 3 in atopic eczema. Allergy 2006, 61, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Attarian, H.; Zee, P.; Silverberg, J.I. Burden of Sleep and Fatigue in US Adults With Atopic Dermatitis. Dermatitis 2016, 27, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Perez-Burgos, A.; Wang, L.; Neufeld, K.-A.M.; Mao, Y.-K.; Ahmadzai, M.; Janssen, L.J.; Stanisz, A.M.; Bienenstock, J.; Kunze, W.A. The TRPV1 channel in rodents is a major target for antinociceptive effect of the probioticLactobacillus reuteriDSM 17938. J. Physiol. 2015, 593, 3943–3957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonchak, J.G.; Swerlick, R.A. Emerging therapies for atopic dermatitis: TRPV1 antagonists. J. Am. Acad. Dermatol. 2018, 78, S63–S66. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Genet. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Genet. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Park, H.-Y.; Kim, C.-R.; Huh, I.-S.; Jung, M.-Y.; Seo, E.-Y.; Park, J.-H.; Lee, D.-Y.; Yang, J.-M. Staphylococcus aureusColonization in Acute and Chronic Skin Lesions of Patients with Atopic Dermatitis. Ann. Dermatol. 2013, 25, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Nakatsuji, T.; Gallo, R.L. The role of the skin microbiome in atopic dermatitis. Ann. Allergy Asthma Immunol. 2018, 122, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, B.E.; Ahn, K.; Leung, D.Y. Interactions between Atopic Dermatitis and Staphylococcus aureus Infection: Clinical Implications. Allergy Asthma Immunol. Res. 2019, 11, 593–603. [Google Scholar] [CrossRef]
- Tytgat, H.L.P.; Douillard, F.P.; Reunanen, J.; Rasinkangas, P.; Hendrickx, A.P.A.; Laine, P.; Paulin, L.; Satokari, R.; De Vos, W.M. Lactobacillus rhamnosus GG Outcompetes Enterococcus faecium via Mucus-Binding Pili: Evidence for a Novel and Heterospecific Probiotic Mechanism. Appl. Environ. Microbiol. 2016, 82, 5756–5762. [Google Scholar] [CrossRef] [Green Version]


| Parameter | Probiotic Group (n = 17) | Control Group (n = 17) |
|---|---|---|
| Age in years (range) | 40.7 (19–66) | 33.1 (19–57) |
| Female (n) | 17 | 15 |
| Ethnicity (n) | ||
| Caucasian | 7 | 6 |
| Black | 10 | 11 |
| Skin Appearance | ||
| Normal | 2 | 2 |
| Dry | 12 | 9 |
| Very Dry | 3 | 6 |
| AD severity at recruitment (SCORAD index) (x ± s.d) | 41.7 ± 14.7 | 36.7 ± 10.7 |
| Subjects with other allergies | ||
| Sinusitis | 2 | 2 |
| Hay fever, pollen, dust, pets | 3 | 6 |
| Average volume of product used during study duration (g) | 82.2 g | 62.9 g |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butler, É.; Lundqvist, C.; Axelsson, J. Lactobacillus reuteri DSM 17938 as a Novel Topical Cosmetic Ingredient: A Proof of Concept Clinical Study in Adults with Atopic Dermatitis. Microorganisms 2020, 8, 1026. https://doi.org/10.3390/microorganisms8071026
Butler É, Lundqvist C, Axelsson J. Lactobacillus reuteri DSM 17938 as a Novel Topical Cosmetic Ingredient: A Proof of Concept Clinical Study in Adults with Atopic Dermatitis. Microorganisms. 2020; 8(7):1026. https://doi.org/10.3390/microorganisms8071026
Chicago/Turabian StyleButler, Éile, Christoffer Lundqvist, and Jakob Axelsson. 2020. "Lactobacillus reuteri DSM 17938 as a Novel Topical Cosmetic Ingredient: A Proof of Concept Clinical Study in Adults with Atopic Dermatitis" Microorganisms 8, no. 7: 1026. https://doi.org/10.3390/microorganisms8071026
APA StyleButler, É., Lundqvist, C., & Axelsson, J. (2020). Lactobacillus reuteri DSM 17938 as a Novel Topical Cosmetic Ingredient: A Proof of Concept Clinical Study in Adults with Atopic Dermatitis. Microorganisms, 8(7), 1026. https://doi.org/10.3390/microorganisms8071026




