Impact of Probiotic Combination in InR[E19]/TM2 Drosophila melanogaster on Longevity, Related Gene Expression, and Intestinal Microbiota: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probiotic and Drosophila Husbandry
2.2. Lifespan Assay
2.3. Body Weight
2.4. Quantitative Real-Time PCR (qRT-PCR)
2.5. High-Throughput Sequencing of 16S rDNA Amplicons
2.6. Statistics
3. Results
3.1. The Probiotic Combination Decreases the Mean and Median Lifespan of Male InR[E19]/TM2 D. melanogaster
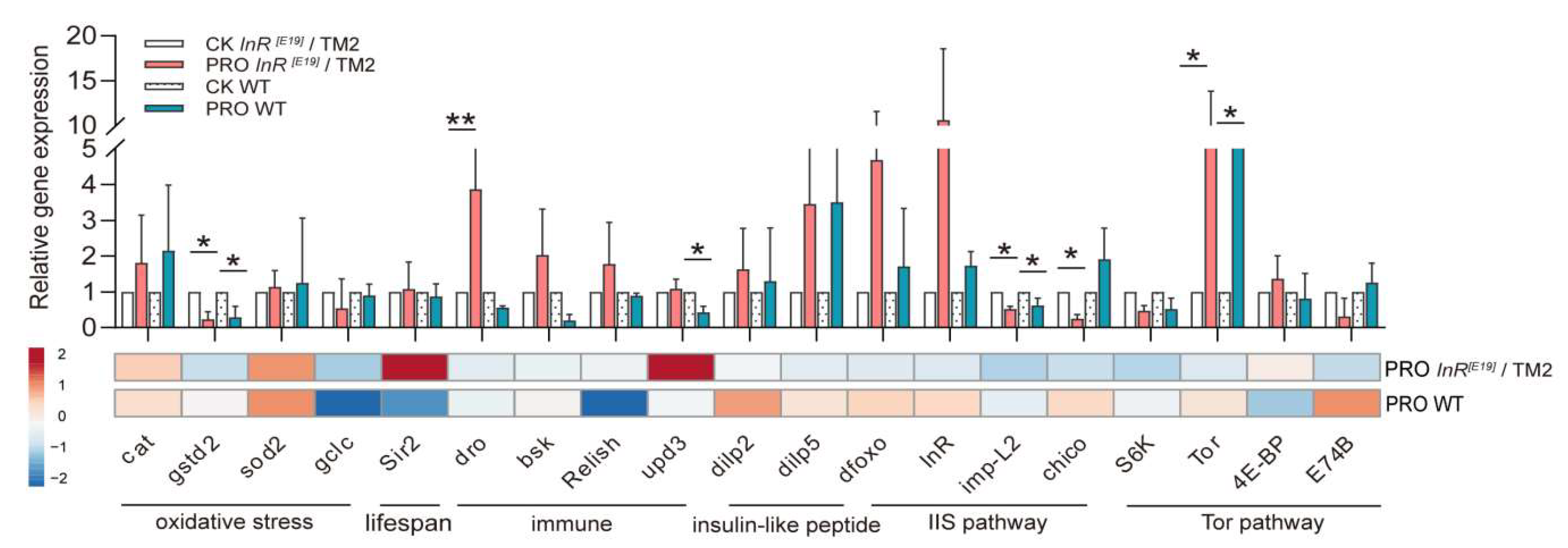
3.2. Effect of the Probiotic Combination on Expression of Age and Insulin-like Signaling-Related Genes
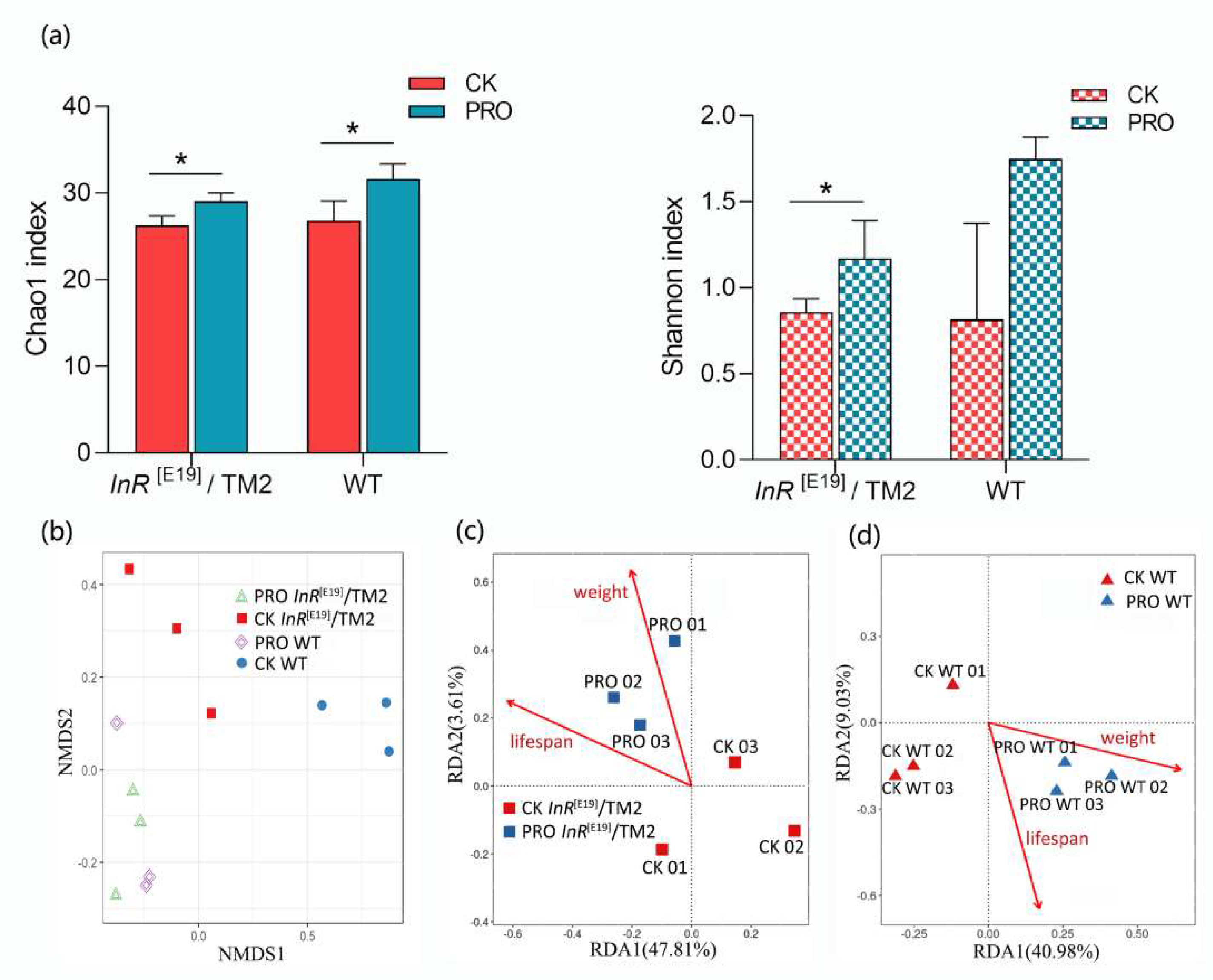
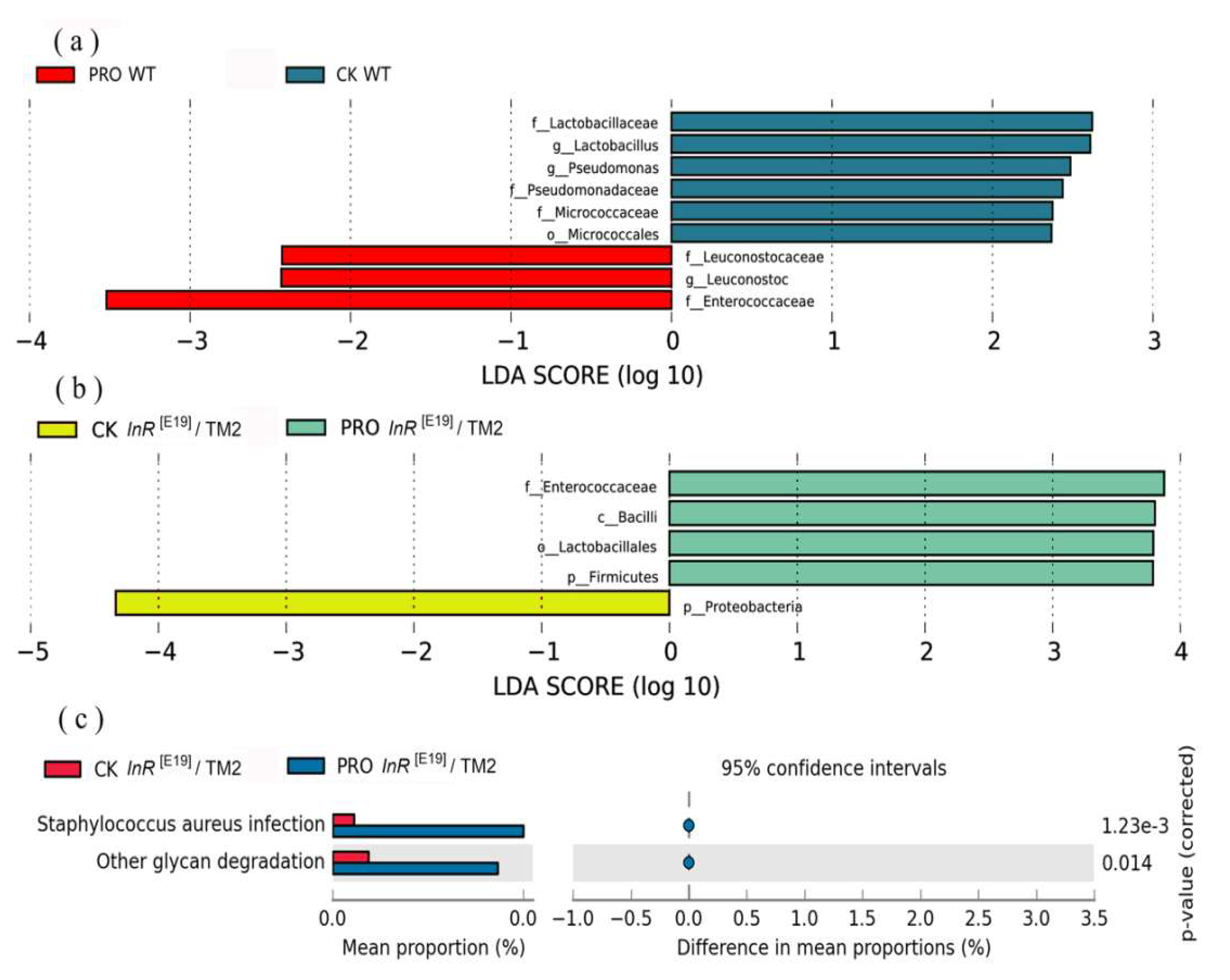
3.3. Intestinal Bacterial Community Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rolfe, R.D. Interactions among microorganisms of the indigenous intestinal flora and their influence on the host. Rev. Infec. Dis. 1984, 6, S73–S79. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, G.F.; David, A. Innate lymphoid cell interactions with microbiota: Implications for intestinal health and disease. Immunity 2012, 37, 601–610. [Google Scholar] [CrossRef] [Green Version]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life. Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Lomis, N.; Prakash, S. A novel polyphenolic prebiotic and probiotic formulation have synergistic effects on the gut microbiota influencing Drosophila melanogaster physiology. Artif. Cells Nanomed. Biotechnol. 2018, 46, 441–455. [Google Scholar] [CrossRef] [Green Version]
- Nagao-Kitamoto, H.; Shreiner, A.B.; Gillilland, M.G.; Kitamoto, S.; Ishii, C.; Hirayama, A.; Kuffa, P.; El-Zaatari, M.; Grasberger, H.; Seekatz, A.M.; et al. Functional characterization of inflammatory bowel disease-associated gut dysbiosis in gnotobiotic mice. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 468–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boursier, J.; Diehl, A.M. Implication of gut microbiota in nonalcoholic fatty liver disease. PLoS Pathog. 2015, 11, e1004559. [Google Scholar] [CrossRef] [Green Version]
- Nava, G.M.; Bielke, L.R.; Callaway, T.R.; Castaneda, M.P. Probiotic alternatives to reduce gastrointestinal infections: The poultry experience. Anim. Health Res. Rev. 2005, 6, 105–118. [Google Scholar] [CrossRef]
- Westfall, S.; Lomis, N.; Prakash, S. A polyphenol-rich prebiotic in combination with a novel probiotic formulation alleviates markers of obesity and diabetes in Drosophila. J. Funct. Foods 2018, 48, 374–386. [Google Scholar] [CrossRef]
- Thomas, D.W.; Greer, F.R. Probiotics and prebiotics in pediatrics. Pediatrics 2010, 126, 1217–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberfroid, M.B. Prebiotics and probiotics: Are they functional foods? Am. J. Clin. Nutr. 2000, 71, 1682S–1687S. [Google Scholar] [CrossRef] [PubMed]
- Clydesdale, F.M. A Proposal for the establishment of scientific criteria for health claims for functional foods. Nutr. Rev. 1997, 55, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.C.; Black, L.I.; Stussman, B.J.; Barnes, P.M.; Nahin, R.L. Trends in the Use of Complementary Health Approaches Among Adults: United States. Natl. Health Stat. Rep. 2015, 79, 1–16. [Google Scholar]
- Rijkers, G.T.; de Vos, W.M.; Brummer, R.J.; Morelli, L.; Corthier, G.; Marteau, P. Health benefits and health claims of probiotics: Bridging science and marketing. Br. J. Nutr. 2011, 106, 1291–1296. [Google Scholar] [CrossRef] [Green Version]
- Saldanha, L.G. US Food and Drug Administration regulations governing label claims for food products, including probiotics. Clin. Infect. Dis. 2008, 46, S119–S121. [Google Scholar] [CrossRef] [PubMed]
- Degnan, F.H. Clinical studies involving probiotics: When FDA’s investigational new drug rubric applies-and when it may not. Gut Microbes 2012, 3, 485–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Bermudo, M.D.; Gebel, L.; Palacios, I.M. DrosAfrica: Building an African biomedical research community using Drosophila. Semin. Cell. Dev. Biol. 2017, 70, 58–64. [Google Scholar] [CrossRef]
- Trinder, M.; Daisley, B.A.; Dube, J.S.; Reid, G. Drosophila melanogaster as a high-throughput model for host-microbiota interactions. Front. Microbiol. 2017, 8, 751. [Google Scholar] [CrossRef]
- Apidianakis, Y.; Rahme, L.G. Drosophila melanogaster as a model for human intestinal infection and pathology. Dis. Model. Mech. 2011, 4, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Apidianakis, Y.; Rahme, L.G. Drosophila melanogaster as a model host for studying Pseudomonas aeruginosa infection. Nat. Protoc. 2009, 4, 1285–1294. [Google Scholar] [CrossRef]
- Kohl, K.D.; Brun, A.; Magallanes, M.; Brinkerhoff, J.; Laspiur, A.; Acosta, J.C.; Caviedes-Vidal, E.; Bordenstein, S.R. Gut microbial ecology of lizards: Insights into diversity in the wild, effects of captivity, variation across gut regions and transmission. Mol. Ecol. 2017, 26, 1175–1189. [Google Scholar] [CrossRef]
- Manev, H.; Dimitrijevic, N. Drosophila model for in vivo pharmacological analgesia research. Eur. J. Pharmacol. 2004, 491, 207–208. [Google Scholar] [CrossRef]
- Giacomotto, J.; Segalat, L. High-throughput screening and small animal models, where are we? Br. J. Pharmacol. 2010, 160, 204–216. [Google Scholar] [CrossRef] [Green Version]
- Choi, N.H.; Lucchetta, E.; Ohlstein, B. Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 18702–18707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keely, S.J. Decoding host-microbiota communication in the gut—Now we’re flying! J. Physiol. 2017, 595, 417–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, J.E.; Fischer, C.N.; Miles, J.; Handelsman, J. Frequent replenishment sustains the beneficial microbiome of Drosophila melanogaster. mBio 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemaitre, B.; Miguel-Aliaga, I. The digestive tract of Drosophila melanogaster. Annu. Rev. Genet. 2013, 47, 377–404. [Google Scholar] [CrossRef] [Green Version]
- Nikbakht, E.; Khalesi, S.; Singh, I.; Williams, L.T.; West, N.P.; Colson, N. Effect of probiotics and synbiotics on blood glucose: A systematic review and meta-analysis of controlled trials. Eur. J. Nutr. 2018, 57, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Andrzej, O.; Maria, B. Preliminary characteristics of lactobacillus and bifidobacterium strains as probiotic candidates. Polish J. Food Nutr. Sci. 2006, 15, 269–275. [Google Scholar]
- Neel, J.S.; Swami, O.C. Role of probiotics in diabetes: A review of their rational and efficacy. Eur. Med. J. 2017, 5, 104–110. [Google Scholar]
- Khalesi, S.; Bellissimo, N.; Vandelanotte, C.; Williams, S.; Stanley, D.; Irwin, C. A review of probiotic supplementation in healthy adults: Helpful or hype? Eur. J. Clin. Nutr. 2019, 73, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Razmpoosh, E.; Javadi, A.; Ejtahed, H.S.; Mirmiran, P.; Javadi, M.; Yousefinejad, A. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: A randomized placebo controlled trial. Diabetes. Metab. Syndr. 2019, 13, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Omar, J.M.; Chan, Y.M.; Jones, M.L.; Prakash, S.; Jones, P.J.H. LactoBacillus fermentum and LactoBacillus amylovorus as probiotics alter body adiposity and gut microflora in healthy persons. J. Funct. Foods 2013, 5, 116–123. [Google Scholar] [CrossRef]
- Storelli, G.; Defaye, A.; Erkosar, B.; Hols, P.; Royet, J.; Leulier, F. LactoBacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell. Metab. 2011, 14, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Fast, D.; Duggal, A.; Foley, E. Monoassociation with LactoBacillus plantarum disrupts intestinal homeostasis in adult drosophila melanogaster. mBio 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Milani, C.; Lugli, G.A.; Duranti, S.; Turroni, F.; Mancabelli, L.; Ferrario, C.; Mangifesta, M.; Hevia, A.; Viappiani, A.; Scholz, M.; et al. Bifidobacteria exhibit social behavior through carbohydrate resource sharing in the gut. Sci. Rep. 2015, 5, 15782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holowacz, S.; Guigné, C.; Chêne, G.; Mouysset, S.; Guilbot, A.; Seyrig, C.; Dubourdeau, M. A multispecies LactoBacillus- and Bifidobacterium-containing probiotic mixture attenuates body weight gain and insulin resistance after a short-term challenge with a high-fat diet in C57/BL6J mice. PharmaNutrition 2015, 3, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Franz, C.M.; Huch, M.; Abriouel, H.; Holzapfel, W.; Galvez, A. Enterococci as probiotics and their implications in food safety. Int. J. Food. Microbiol. 2011, 151, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Kathrani, A.; Larsen, J.A.; Kass, P.H.; Fascetti, A.J. Effect of short-term probiotic Enterococcus faecium SF68 dietary supplementation in overweight and obese cats without comorbidities. Vet. Rec. Open 2016, 3, 000164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, K.; Pooranachithra, M.; Balamurugan, K.; Goel, G. Multivariate analysis of increase in life span of caenorhabditis elegans through intestinal colonization by indigenous probiotic strains. Probiotics Antimicrob. Proteins 2019, 11, 865–873. [Google Scholar] [CrossRef]
- Schifano, E.; Zinno, P.; Guantario, B.; Roselli, M.; Marcoccia, S.; Devirgiliis, C.; Uccelletti, D. The foodborne strain LactoBacillus fermentum MBC2 triggers pept-1-dependent pro-longevity effects in caenorhabditis elegans. Microorganisms 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westfall, S.; Lomis, N.; Prakash, S. Longevity extension in Drosophila through gut-brain communication. Sci. Rep. 2018, 8, 8362. [Google Scholar] [CrossRef] [PubMed]
- Broughton, S.; Partridge, L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem. J. 2009, 418, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chistyakova, O.V. Signaling pathway of insulin and insulin-like growth factor 1 (IGF-1) as a potential regulator of lifespan. J. Evol. Biochem. Physiol. 2011, 44, 1–11. [Google Scholar] [CrossRef]
- Broughton, S.J.; Matthew, D.W.P.; Tomoatsu, I.; Bass, T.M.; Jake, J.; Yasmine, D.; Pedro, M.; Ernst, H.; Withers, D.J.; Leevers, S.J.; et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl. Acad. Sci. USA 2005, 102, 3105–3110. [Google Scholar] [CrossRef] [Green Version]
- Hwangbo, D.S.; Gershman, B.; Tu, M.P.; Palmer, M.; Tatar, M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 2004, 429, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Dirk, B.; HeInRich, J. JNK signaling confers tolerance to oxidative stress and extends lifespan in drosophila. Dev. Cell 2003, 5, 811–816. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.C.; Bohmann, D.; Jasper, H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 2005, 121, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broughton, S.; Alic, N.; Slack, C.; Bass, T.; Ikeya, T.; Vinti, G.; Tommasi, A.M.; Driege, Y.; Hafen, E.; Partridge, L. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS ONE 2008, 3, e3721. [Google Scholar] [CrossRef] [Green Version]
- Kapahi, P.; Chen, D.; Rogers, A.N.; Katewa, S.D.; Li, P.W.; Thomas, E.L.; Kockel, L. With TOR, less is more: A key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010, 11, 453–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broughton, S.J.; Slack, C.; Alic, N.; Metaxakis, A.; Bass, T.M.; Driege, Y.; Partridge, L. DILP-producing median neurosecretory cells in the Drosophila brain mediate the response of lifespan to nutrition. Aging Cell 2010, 9, 336–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greer, E.L.; Brunet, A. Signaling networks in aging. J. Cell. Sci. 2008, 121, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.W.; Wang, H.D.; Bai, H.; Wu, M.S.; Yen, J.H.; Tatar, M.; Fu, T.F.; Wang, P.Y. Tequila regulates insulin-like signaling and extends life span in Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1461–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelle, Z.; Joost, V.D.H.; Bastiaan, J.Z.; Matthew, D.W.P. Both overlapping and independent mechanisms determine how diet and insulin-ligand knockouts extend lifespan of Drosophila melanogaster. npj Aging Mech. Dis. 2017, 3, 1–4. [Google Scholar]
- Alic, N.; Hoddinott, M.P.; Vinti, G.; Partridge, L. Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumour suppressor. Aging Cell 2011, 10, 137–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roed, N.K.; Viola, C.M.; Kristensen, O.; Schluckebier, G.; Norrman, M.; Sajid, W.; Wade, J.D.; Andersen, A.S.; Kristensen, C.; Ganderton, T.R.; et al. Structures of insect Imp-L2 suggest an alternative strategy for regulating the bioavailability of insulin-like hormones. Nat. Commun. 2018, 9, 3860. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, J.; Wang, D.S.; Yang, Q.; Feng, B.; Kang, W.B.; Yang, L.; Liu, G.; Zhao, M.G. Sinomenine attenuates chronic inflammatory pain in mice. Metab. Brain Dis. 2017, 32, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Katewa, S.D.; Kapahi, P. Role of TOR signaling in aging and related biological processes in Drosophila melanogaster. Exp. Gerontol. 2011, 46, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Miron, M.; Lasko, P.; Sonenberg, N. Signaling from Akt to FRAP/TOR targets both 4E-BP and S6K in Drosophila melanogaster. Mol. Cell. Biol. 2003, 23, 9117–9126. [Google Scholar] [CrossRef] [Green Version]
- Zid, B.M.; Rogers, A.N.; Katewa, S.D.; Vargas, M.A.; Kolipinski, M.C.; Lu, T.A.; Benzer, S.; Kapahi, P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell 2009, 139, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Felix, R.; Hugo, S.; George, T.; Ernst, H. PDK1 regulates growth through Akt and S6K in Drosophila. Proc. Natl. Acad. Sci. USA 2001, 98, 15020–15025. [Google Scholar]
- Griswold, A.J.; Chang, K.T.; Runko, A.P.; Knight, M.A.; Min, K.T. Sir2 mediates apoptosis through JNK-dependent pathways in Drosophila. Proc. Natl. Acad. Sci. USA 2008, 105, 8673–8678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajindar, S.S.; Richard, W. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar]
- Peng, C.; Chan, H.Y.E.; Huang, Y.; Yu, H.; Chen, Z.Y. Apple polyphenols extend the mean lifespan of Drosophila melanogaster. J. Agric. Food Chem. 2011, 59, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wheeler, C.T.; Alberico, T.; Sun, X.; Seeberger, J.; Laslo, M.; Spangler, E.; Kern, B.; de Cabo, R.; Zou, S. The effect of resveratrol on lifespan depends on both gender and dietary nutrient composition in Drosophila melanogaster. Age 2013, 35, 69–81. [Google Scholar] [CrossRef]
- Rachel, W.; Shakeela, F.; Reika, M.; Lucas, B.; Mark, H.; Jason, G.W.; Stephen, L.H. Increased expression of Drosophila Sir2 extends life span in a dosedependent manner. Aging 2013, 5, 682–691. [Google Scholar]
- Hoffmann, J.; Romey, R.; Fink, C.; Yong, L.; Roeder, T. Overexpression of Sir2 in the adult fat body is sufficient to extend lifespan of male and female Drosophila. Aging 2013, 5, 316–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogina, B.; Helfand, S. Helfand. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. USA 2004, 101, 15998–16003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsden, S.; Cheung, Y.Y.; Seroude, L. Functional analysis of the Drosophila immune response during aging. Aging Cell 2008, 7, 225–236. [Google Scholar] [CrossRef]
- Wright, V.M.; Vogt, K.L.; Smythe, E.; Zeidler, M.P. Differential activities of the Drosophila JAK/STAT pathway ligands Upd, Upd2 and Upd3. Cell Signal. 2011, 23, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Clancy, D.J.; Gems, D.; Harshman, L.G.; Oldham, S.; Stocker, H.; Hafen, E.; Leevers, S.J.; Partridge, L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 2001, 292, 104–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloch, E. Mini-review: Probiotics and disease prevention in different host systems. Br. Microbiol. Res. J. 2013, 3, 42–57. [Google Scholar] [CrossRef]
- Barengolts, E. Gut microbiota, prebiotics, probiotics, and synbiotics in management of obesity and prediabetes: Review of randomized controlled trials. Endocr. Pract. 2016, 22, 1224–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, E.A.; Roy, T.; D’Adamo, C.R.; Wieland, L.S. Probiotics and gastrointestinal conditions: An overview of evidence from the Cochrane Collaboration. Nutrition 2018, 45, 125–134. [Google Scholar] [CrossRef]
- Sanap, D.; Garje, M.; Godge, G. Probiotics, their health benefits and applications for development of human health: A review. J. Drug Deliv. Ther. 2019, 9, 631–640. [Google Scholar]
- Sudun, S.; Liu, S.; Xiao, C.; Peng, C.; Liang, L.; He, X.; Zhao, S.; Zhang, G. Probiotic strains improve high-fat diet-induced hypercholesterolemia through modulating gut microbiota in ways different from atorvastatin. Food Funct. 2019, 10, 6098–6109. [Google Scholar] [CrossRef]
- Moser, A.M.; Spindelboeck, W.; Halwachs, B.; Strohmaier, H.; Kump, P.; Gorkiewicz, G.; Hogenauer, C. Effects of an oral synbiotic on the gastrointestinal immune system and microbiota in patients with diarrhea-predominant irritable bowel syndrome. Eur. J. Nutr. 2019, 58, 2767–2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonathan, M.L.; Carla, M.D.A. Elton revisited: A review of evidence linking diversity and invasibili. OIKOS 1999, 87, 15–26. [Google Scholar]
- Tap, J.; Furet, J.P.; Bensaada, M.; Philippe, C.; Roth, H.; Rabot, S.; Lakhdari, O.; Lombard, V.; Henrissat, B.; Corthier, G.; et al. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ. Microbiol. 2015, 17, 4954–4964. [Google Scholar] [CrossRef] [PubMed]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stecher, B.; Denzler, R.; Maier, L.; Bernet, F.; Sanders, M.J.; Pickard, D.J.; Barthel, M.; Westendorf, A.M.; Krogfelt, K.A.; Walker, A.W.; et al. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc. Natl. Acad. Sci. USA 2012, 109, 1269–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sifri, C.D.; Begun, J.; Ausubel, F.M.; Calderwood, S.B. Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect. Immun. 2003, 71, 2208–2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, K.; Hölzel, C.S.; Cui, Y.F.; Richard, M.; Wang, Y.; Richard, D.; Andrea, D.; Rupert, B.; Erwin, M.; Ding, S.Y. Probiotic Bacillus cereus strains, a potential risk for public health in China. Front. Microb. 2016, 7, 718. [Google Scholar] [CrossRef] [PubMed]

| - | Total no. of Drosophila (n) | Mean (% Change) | Median (% Change) | Log-Rank (vs. Standard Diet) |
|---|---|---|---|---|
| Standard diet InR | 100 | 36.850 | 40.238 | - |
| Probiotics InR | 113 | 31.115 (15.56%) | 30.652 (23.823%) | p = 0.005335 |
| Standard diet WT | 120 | 40.958333 | 42.000 | - |
| Probiotics WT | 113 | 44.769912 (9.306%) | 49.000 (16.667%) | p = 0.006050 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Sun, H.; Yang, W.; Gao, M.; Xu, H. Impact of Probiotic Combination in InR[E19]/TM2 Drosophila melanogaster on Longevity, Related Gene Expression, and Intestinal Microbiota: A Preliminary Study. Microorganisms 2020, 8, 1027. https://doi.org/10.3390/microorganisms8071027
Ma S, Sun H, Yang W, Gao M, Xu H. Impact of Probiotic Combination in InR[E19]/TM2 Drosophila melanogaster on Longevity, Related Gene Expression, and Intestinal Microbiota: A Preliminary Study. Microorganisms. 2020; 8(7):1027. https://doi.org/10.3390/microorganisms8071027
Chicago/Turabian StyleMa, Shuang, Hao Sun, Weichao Yang, Mingfu Gao, and Hui Xu. 2020. "Impact of Probiotic Combination in InR[E19]/TM2 Drosophila melanogaster on Longevity, Related Gene Expression, and Intestinal Microbiota: A Preliminary Study" Microorganisms 8, no. 7: 1027. https://doi.org/10.3390/microorganisms8071027
APA StyleMa, S., Sun, H., Yang, W., Gao, M., & Xu, H. (2020). Impact of Probiotic Combination in InR[E19]/TM2 Drosophila melanogaster on Longevity, Related Gene Expression, and Intestinal Microbiota: A Preliminary Study. Microorganisms, 8(7), 1027. https://doi.org/10.3390/microorganisms8071027






