Characterization of Pediococcus acidilactici PFC69 and Lactococcus lactis PFC77 Bacteriocins and Their Antimicrobial Activities in Tarhana Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Growth Conditions
2.2. Determination of the Antimicrobial Spectrums of Lactic Acid Bacteria (LAB) Isolates
2.3. Determination of pH, Heat, and Enzyme Sensitivity of Antimicrobial Activity of Supernatants
2.4. Production and Purification of Bacteriocins from LAB Strains
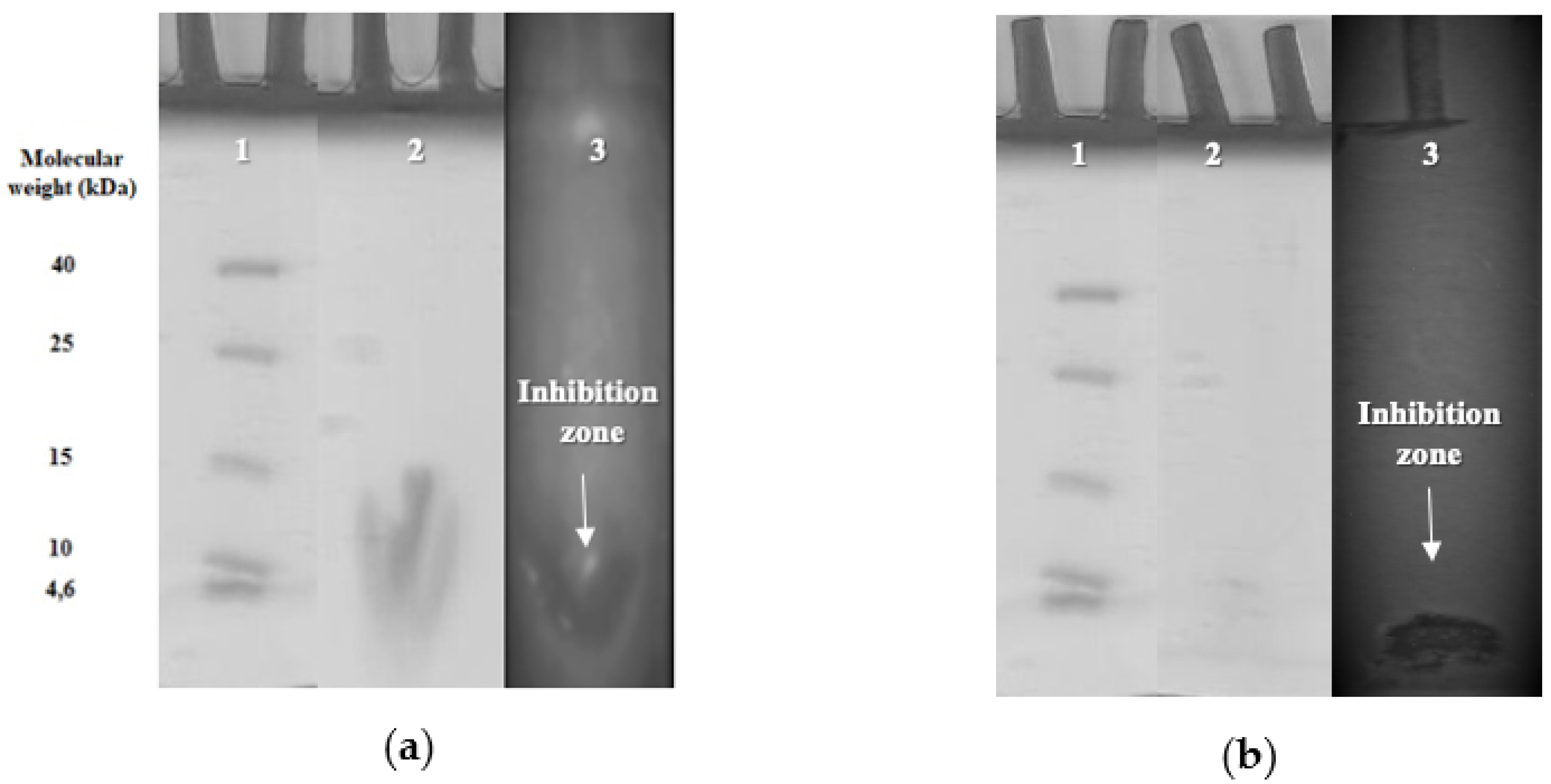
2.5. Determination of Molecular Size of Bacteriocins
2.6. Screening Bacteriocin Genes in Bacteriocin Producers
2.7. Tarhana Production, Microbiological, and Chemical Analyses in Tarhana Dough Fermentation
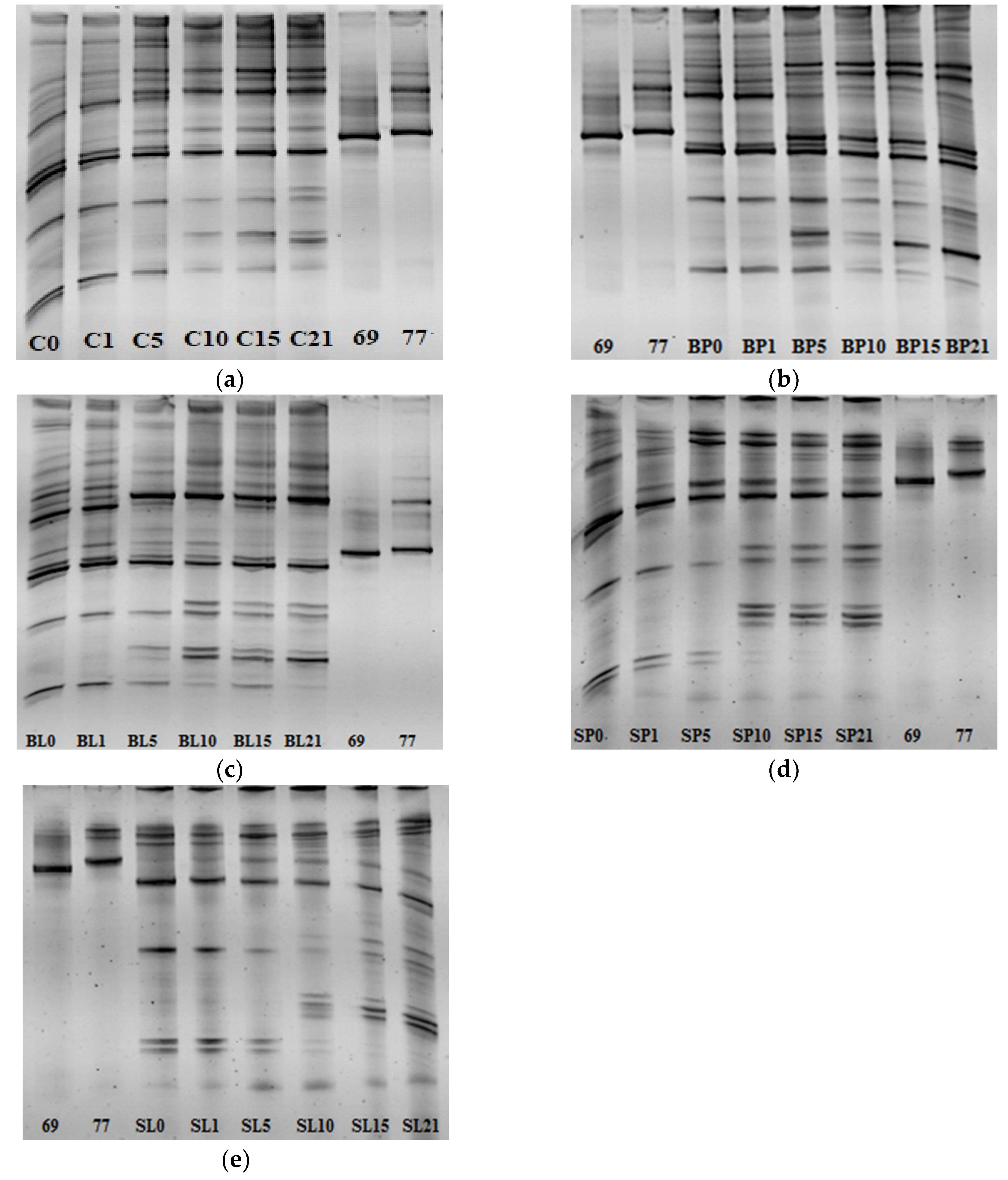
2.8. Monitoring the LAB Diversity in Tarhana Dough Fermented with Bacteriocin Producers
2.9. Statistical Analysis
3. Results
3.1. Antimicrobial Activity Spectrum of P. acidilactici PFC69 and L. lactis PFC77
3.2. The pH, Heat, and Enzyme Sensitivity Characteristics of Bacteriocin-Like Substances (BLS) Produced by P. acidilactici PFC69 and L. lactis PFC77
3.3. Purification and Molecular Sizes of PFC69 and PFC77 Bacteriocins
3.4. Partial DNA Sequences of Bacteriocin Production Encoding Genes in the Genome of P. acidilactici PFC69 and L. lactis PFC77
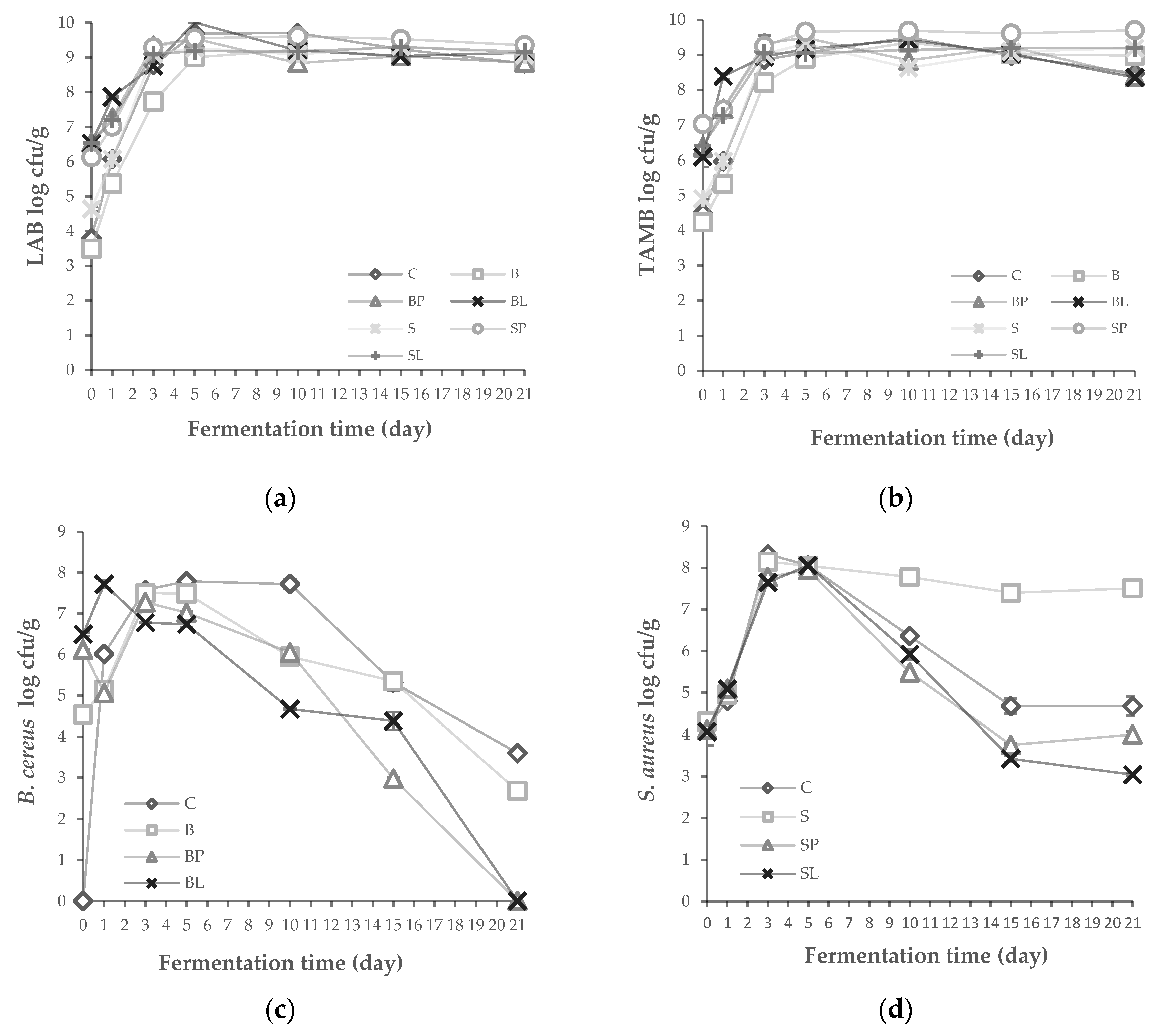
3.5. Microbiological and Chemical Properties of Tarhana Dough Produced with Bacteriocin Producers
3.6. The LAB Diversity of Tarhana Fermentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dağlıoğlu, O. Tarhana as a traditional Turkish fermented cereal food. its recipe, production and composition. Nahrung 2000, 44, 85–88. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.W. An introduction to the traditional fermented foods and beverages of Turkey. Crit. Rev. Food Sci. Nutr. 2011, 51, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Şengün, İ.Y.; Nielsen, D.S.; Karapınar, M.; Jakopsen, M. Identification of lactic acid bacteria isolated from tarhana, a traditional Turkish fermented food. Int. J. Food Microbiol. 2009, 135, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Settanni, L.; Tanguler, H.; Moschetti, G.; Reale, S.; Gargano, V.; Erten, H. Evolution of fermenting microbiota in tarhana produced under controlled technological conditions. Food Microbiol. 2011, 28, 1367–1373. [Google Scholar] [CrossRef]
- Şimşek, Ö.; Özel, S.; Çon, A.H. Comparison of lactic acid bacteria diversity during the fermentation of tarhana produced at home and on a commercial scale. Food Sci. Biotechnol. 2017, 26, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, A.; Abriouel, H.; López, R.L.; Omar, N.B. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef]
- Delves-Broughton, J.; Blackburn, P.; Evans, R.J.; Hugenholtz, J. Applications of the bacteriocin, nisin. Anton. Leeuw. Int. J. G. 1996, 69, 193–202. [Google Scholar] [CrossRef]
- Drider, D.; Fimland, G.; Hechard, Y.; McMullen, L.M.; Prevost, H. The continuing story of class IIa bacteriocins. Microbiol. Mol. Biol. R. 2006, 70, 564–582. [Google Scholar] [CrossRef]
- Anastasiadou, S.; Papagianni, M.; Filiousis, G.; Ambrosiadis, I.; Koidis, P. Pediocin SA-1, an antimicrobial peptide from Pediococcus acidilactici NRRL B5627: Production conditions, purification and characterization. Bioresour. Technol. 2008, 99, 5384–5390. [Google Scholar] [CrossRef]
- Becker, H.; Schaller, G.; von Wiese, W.; Terplan, G. Bacillus cereus in infant foods and dried milk products. Int. J. Food Microbiol. 1994, 23, 1–15. [Google Scholar] [CrossRef]
- Halpin-Dohnalek, M.I.; Marth, E.H. Staphylococcus aureus: Production of extracellular compounds and behavior in foods-a review. J. Food Prot. 1989, 52, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Turantaş, F.; Kemahlıoğlu, K. Fate of some pathogenic bacteria and molds in Turkish tarhana during fermentation and storage period. J. Food Sci. Technol. 2012, 49, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Van Belkum, M.J.; Hayema, B.J.; Geis, A.; Kok, J.; Venema, G. Cloning of two bacteriocin genes from a lactococcal bacteriocin plasmid. Appl. Environ. Microbiol. 1989, 55, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Tagg, J.R.; McGiven, A.R. Assay system for bacteriocins. Appl. Microbiol. 1971, 21, 943. [Google Scholar] [CrossRef]
- Franz, C.M.A.P.; Du Toit, M.; von Holy, A.; Schillinger, U.; Holzapfel, W.H. Production of nisin-like bacteriocins by Lactococcus lactis strains isolated from vegetables. J. Basic Microbiol. 1997, 37, 196–197. [Google Scholar] [CrossRef]
- Schagger, H.; von Jagow, G. Tricine-sodium dodecyl slphate-poly-acrylamide gel electrophoresis for the separation of protein in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Macwana, S.J.; Muriana, P.M. A bacteriocin PCR array for identification of bacteriocin-related structural genes in lactic acid bacteria. J. Microbiol. Meth. 2012, 88, 197–204. [Google Scholar] [CrossRef]
- Anonymous. TS 2282 Tarhana Standard; Institute of Turkish Standards: Ankara, Turkey, 2004. [Google Scholar]
- Pitcher, D.G.; Saunders, N.A.; Owen, R.J. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 1989, 8, 151–156. [Google Scholar] [CrossRef]
- Meroth, C.B.; Walter, J.; Hertel, C.; Brandt, M.J.; Hammes, W.P. Monitoring the bacterial population dynamics in sourdough fermentation processes by using PCR-Denaturing Gradient Gel Electrophoresis. Appl. Environ. Microbiol. 2003, 69, 475–482. [Google Scholar] [CrossRef]
- Miambi, E.; Guyot, J.P.; Ampe, F. Identification, isolation and quantification of representative bacteria from fermented cassava dough using an integrated approach of culture-dependent and culture independent methods. Int. J. Food. Microbiol. 2003, 82, 111–120. [Google Scholar] [CrossRef]
- Millette, M.; Dupont, C.; Archambault, D.; Lacroix, M. Partial characterization of bacteriocins produced by human Lactococcus lactis and Pediococccus acidilactici isolates. J. Appl. Microbiol. 2006, 102, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M.; Anastasiadou, S. Pediocins: The bacteriocins of Pediococci. sources, production, properties and applications. Microbial. Cell Fact. 2009, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, L.; Zhao, X.; Zhou, Z. Partial characteristics and antimicrobial mode of pediocin produced by Pediococcus acidilactici PA003. Ann. Microbiol. 2015, 65, 1753–1762. [Google Scholar] [CrossRef]
- Porto, M.C.; Kuniyoshi, T.M.; Azevedo, P.O.; Vitolo, M.; Oliveira, R.P. Pediococcus spp.: An important genus of lactic acid bacteria and pediocin producers. Biotechnol. Adv. 2017, 35, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Capita, R.; Alonso-Calleja, C. Antibiotic-resistant bacteria: A challenge for the food industry. Crit. Rev. Food Sci. Nut. 2013, 53, 11–48. [Google Scholar] [CrossRef]
- Mirkovic, N.; Polovic, N.; Vukotic, G.; Jovcic, B.; Miljkovic, M.; Radulovic, Z.; Diep, D.B.; Kojic, M. Lactococcus lactis LMG2081 produces two bacteriocins, a nonlantibiotic and a novel lantibiotic. Appl. Environ. Microbiol. 2016, 82, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Dalgic, A.C.; Belibagli, K.B. Hazard analysis critical control points implementation in traditional foods: A case study of tarhana processing. Int. J. Food Sci. Technol. 2008, 43, 1352–1360. [Google Scholar] [CrossRef]
- Bravo, D.; Rodríguez, E.; Medina, M. Nisin and lacticin 481 coproduction by Lactococcus lactis strains isolated from raw ewes’ milk. J. Dairy Sci. 2009, 92, 4805–4811. [Google Scholar] [CrossRef]
- Sawa, N.; Koga, S.; Okamura, K.; Ishibashi, N.; Zendo, T.; Sonomoto, K. Identification and characterization of novel multiple bacteriocins produced by Lactobacillus sakei D98. J. Appl. Microbiol. 2013, 115, 61–69. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial production of bacteriocins: Latest research development and applications. Biotechnol. Adv. 2018, 36, 2187–2200. [Google Scholar] [CrossRef]
- De Vuyst, L.; Leroy, F. Bacteriocins from lactic acid bacteria: Production, purification, and food applications. J. Mol. Microbiol. Biotechnol. 2007, 13, 194–199. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.M.; Kuniyoshi, T.M.; Oliveira, R.; Hill, C.; Ross, P.R.; Cotter, P.D. Antimicrobials for food and feed; a bacteriocin perspective. Curr. Opin. Biotechnol. 2020, 61, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Bali, V.; Panesar, P.S.; Bera, M.B.; Kennedy, J.F. Bacteriocins: Recent Trends and Potential Applications. Crit. Rev. Food Sci. Nut. 2016, 56, 817–834. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.İ.; Özel, B.; Şimşek, Ö. A natural way of food preservation: Bacteriocins and their applications. In Health and Safety Aspects of Food Processing Technologies; Malik, A., Erginkaya, Z., Erten, H., Eds.; Springer: Cham, Berlin, Germany, 2019. [Google Scholar]

| Indicator Strains | P. acidilactici PFC69 | L. lactis PFC77 | ||
|---|---|---|---|---|
| Agar Spot * | Well Diffusion | Agar Spot | Well Diffusion | |
| M. luteus DSM1790 | +++ | +++ | +++ | +++ |
| L. monocytogenes ATCC 7644 | +++ | +++ | +++ | +++ |
| S. aureus ATCC 29213 | ++ | + | ++ | + |
| S. aureus ATCC 25923 | +++ | + | ++ | + |
| E. coli ATCC 25922 | ++ | - | - | - |
| S. typhimurium ATCC 14028 | +++ | - | - | - |
| B. cereus ATCC 11778 | + | ++ | ++ | + |
| E. faecalis ATCC 19433 | +++ | +++ | ++ | ++ |
| VRE * | 50/50 | 48/50 | 8/50 | 0/50 |
| MRSA * | 5/50 | 0/50 | 2/50 | 0/50 |
| Isolates | PFC69 | PFC77 | |
|---|---|---|---|
| Treatments | Activity (AU) | ||
| Control | 12,800 | 12,800 | |
| pH treatments | 2 | 25,600 | 25,600 |
| 3 | 25,600 | 25,600 | |
| 4 | 25,600 | 25,600 | |
| 5 | 12,800 | 25,600 | |
| 6 | 12,800 | 12,800 | |
| 7 | 12,800 | 12,800 | |
| 8 | 6400 | 6400 | |
| 9 | 3200 | 3200 | |
| 10 | 1600 | 1600 | |
| 11 | 0 | 800 | |
| Heat treatments | 80 °C, 5 min | 12,800 | 12,800 |
| 80 °C, 10 min | 12,800 | 12,800 | |
| 80 °C, 15 min | 12,800 | 12,800 | |
| 90 °C, 5 min | 12,800 | 12,800 | |
| 90 °C, 10 min | 12,800 | 12,800 | |
| 90 °C, 15 min | 12,800 | 12,800 | |
| 100 °C, 5 min | 12,800 | 6400 | |
| 100 °C, 10 min | 12,800 | 6400 | |
| 100 °C, 15 min | 12,800 | 6400 | |
| 121 °C, 15 min | 12,800 | 6400 | |
| Enzyme treatments | Catalase | 12,800 | 12,800 |
| Lysozyme | 12,800 | 12,800 | |
| α Amylase | 12,800 | 12,800 | |
| Lipase | 12,800 | 12,800 | |
| Proteinase K | 0 | 0 | |
| Trypsin | 0 | 0 | |
| Pepsin | 0 | 12,800 | |
| α Chymotrypsin | 0 | 0 | |
| Isolates | Purification Steps | Volume (mL) | Antibacterial Activity (AU/mL) | Antibacterial Activity (Total AU) |
|---|---|---|---|---|
| PFC69 | Cell free-culture supernatant | 500 | 12,800 | 6.4 × 106 |
| Ammonium sulfate precipitation | 50 | 25,600 | 1.3 × 106 | |
| Solid phase extraction | 5 | 12,800 | 6.4 × 104 | |
| High-pressure liquid chromatography | 1 | 800 | 8.0 × 102 | |
| PFC77 | Cell free-culture supernatant | 500 | 12,800 | 6.4 × 106 |
| Ammonium sulfate precipitation | 50 | 25,600 | 1.3 × 106 | |
| Solid phase extraction | 5 | 800 | 4.0 × 103 | |
| High-pressure liquid chromatography | 1 | 400 | 4.0 × 102 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, H.İ.; Şimşek, Ö. Characterization of Pediococcus acidilactici PFC69 and Lactococcus lactis PFC77 Bacteriocins and Their Antimicrobial Activities in Tarhana Fermentation. Microorganisms 2020, 8, 1083. https://doi.org/10.3390/microorganisms8071083
Kaya Hİ, Şimşek Ö. Characterization of Pediococcus acidilactici PFC69 and Lactococcus lactis PFC77 Bacteriocins and Their Antimicrobial Activities in Tarhana Fermentation. Microorganisms. 2020; 8(7):1083. https://doi.org/10.3390/microorganisms8071083
Chicago/Turabian StyleKaya, Halil İbrahim, and Ömer Şimşek. 2020. "Characterization of Pediococcus acidilactici PFC69 and Lactococcus lactis PFC77 Bacteriocins and Their Antimicrobial Activities in Tarhana Fermentation" Microorganisms 8, no. 7: 1083. https://doi.org/10.3390/microorganisms8071083
APA StyleKaya, H. İ., & Şimşek, Ö. (2020). Characterization of Pediococcus acidilactici PFC69 and Lactococcus lactis PFC77 Bacteriocins and Their Antimicrobial Activities in Tarhana Fermentation. Microorganisms, 8(7), 1083. https://doi.org/10.3390/microorganisms8071083





