Evaluation of Antiviral, Antibacterial and Antiproliferative Activities of the Endophytic Fungus Curvularia papendorfii, and Isolation of a New Polyhydroxyacid †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Endophytic Fungus: Isolation and Taxonomic Characterization
2.3. Cultivation of the Fungus and Extraction of the Metabolites
2.4. Biological Assays
2.4.1. Cytotoxicity Tests: Cells, Media and Protocols
2.4.2. Antiviral Assay: Media, Viruses and Protocols
Immunofluorescence Analysis (IFA)
2.4.3. Antibacterial Assay: Cells, Media and Protocols
2.4.4. Antiproliferative Activity: Cells, Media and Protocol
2.5. Analytical and Spectroscopic Analysis
2.6. Purification and Identification of Metabolites from C. papendorfii Crude Extract
3. Results and Discussion
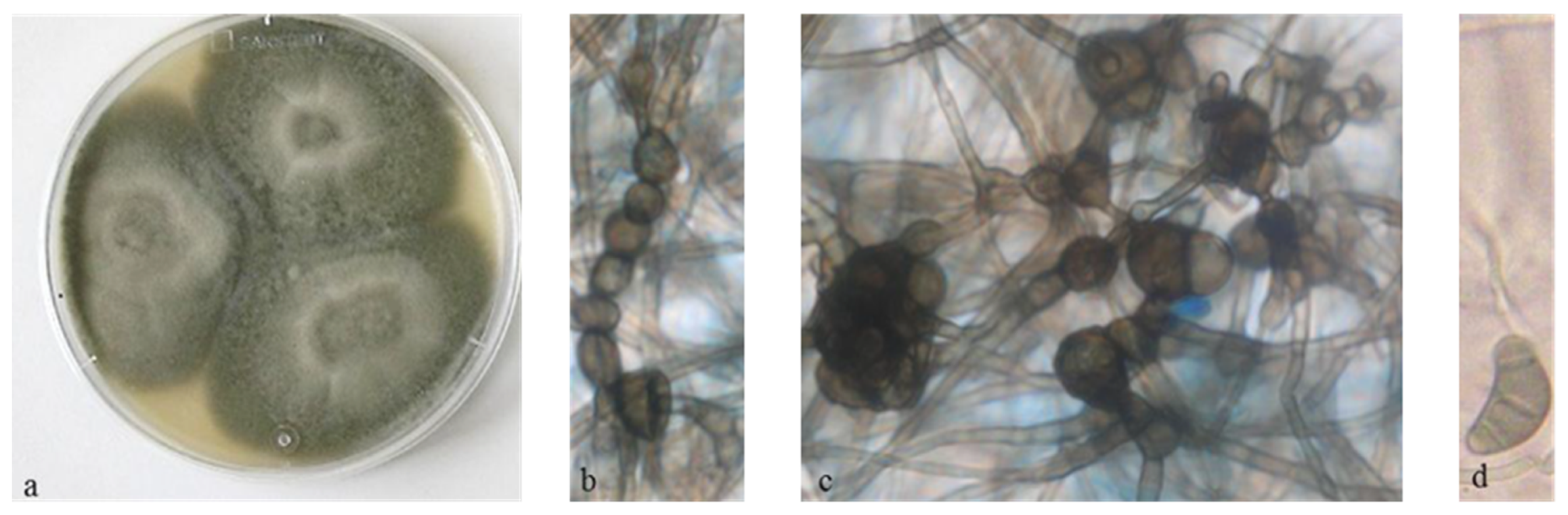
3.1. Isolation and Taxonomic Characterization of the Fungal Strain
3.2. Screening of Biological Activities of C. papendorfii Crude Extract
3.2.1. Cytotoxic Effects
3.2.2. Antiviral Activity of Crude Extract
3.2.3. Antibacterial Activity of Crude Extract
3.2.4. Antiproliferative Activity of Crude Extract
3.3. Phytochemical Analysis
3.4. Biological Activities of Identified Compounds and Kheiric Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pene, F.; Merlat, A.; Vabret, A.; Rozenberg, F.; Buzyn, A.; Dreyfus, F.; Cariou, A.; Freymuth, F.; Lebon, P. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin. Infect. Dis. 2003, 37, 929–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, Y.X.; Ng, Y.L.; Tam, J.P.; Liu, D.X. Human Coronaviruses: A Review of Virus-Host Interactions. Diseases 2016, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Lau, S.K.P.; Huang, Y.; Yuen, K.-Y. Coronavirus diversity, phylogeny and interspecies jumping. Exp. Biol. Med. 2009, 234, 1117–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peiris, J.S.M.; Guan, Y.; Yuen, K.Y. Severe acute respiratory syndrome. Nat. Med. 2004, 10, S88–S97. [Google Scholar] [CrossRef]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Bartsch, S.; Lopman, B.A.; Ozawa, S.; Hall, A.J.; Lee, B. Global economic burden of norovirus gastroenteritis. PLoS ONE 2016, 11, e0151219. [Google Scholar] [CrossRef] [Green Version]
- Glass, R.I.; Parashar, U.D.; Estes, M.K. Norovirus gastroenteritis. N. Engl. J. Med. 2009, 361, 1776–1785. [Google Scholar] [CrossRef] [Green Version]
- Karst, S.M.; Wobus, C.E.; Goodfellow, I.G.; Green, K.Y.; Virgin, H.W. Advances in norovirus biology. Cell Host Microbe 2014, 15, 668–680. [Google Scholar] [CrossRef] [Green Version]
- WHO. General Documents on Antimicrobial Resistance. Available online: http://www.who.int/antimicrobial-resistance/publications/general-documents/en/ (accessed on 22 April 2020).
- Kirienko, N.V.; Rahme, L.; Cho, Y.-H. Beyond Antimicrobials: Non-Traditional Approaches to Combating Multidrug-Resistant Bacteria; Frontiers Media SA: Lausanne, Switzerland, 2019; ISBN 9782889632565. [Google Scholar]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Khiralla, A.; Spina, R.; Saliba, S.; Laurain-Mattar, D. Diversity of natural products of the genera Curvularia and Bipolaris. Fungal Biol. Rev. 2019, 33, 101–122. [Google Scholar] [CrossRef]
- Khiralla, A.; Mohamed, I.; Thomas, J.; Mignard, B.; Spina, R.; Yagi, S.; Laurain-Mattar, D. A pilot study of antioxidant potential of endophytic fungi from some Sudanese medicinal plants. Asian Pac. J. Trop. Med. 2015, 8, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Khiralla, A. Phytochemical Study, Cytotoxic and Antibacterial Potentialities of Endophytic Fungi from Medicinal Plants from Sudan. Ph.D. Thesis, University of Lorraine, Nancy, France, 2015. [Google Scholar]
- Khiralla, A.; Mohamed, I.E.; Tzanova, T.; Schohn, H.; Slezack-Deschaumes, S.; Hehn, A.; André, P.; Carre, G.; Spina, R.; Lobstein, A.; et al. Endophytic fungi associated with Sudanese medicinal plants show cytotoxic and antibiotic potential. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Nilsson, R.H.; Alias, S.A.; Ariyawansa, H.A.; Blair, J.E.; Cai, L.; de Cock, A.W.A.M.; Dissanayake, A.J.; Glockling, S.L.; Goonasekara, I.D.; et al. One stop shop: Backbones trees for important phytopathogenic genera: I (2014). Fungal Divers. 2014, 67, 21–125. [Google Scholar] [CrossRef] [Green Version]
- Avinash, K.S.; Ashwini, H.S.; Babu, H.N.R.; Krishnamurthy, Y.L. Antimicrobial Potential of Crude Extract of Curvularia lunata, an Endophytic Fungi Isolated from Cymbopogon caesius. Available online: https://www.hindawi.com/journals/jmy/2015/185821/ (accessed on 18 April 2020).
- Campos, F.F.; Rosa, L.H.; Cota, B.B.; Caligiorne, R.B.; Rabello, A.L.T.; Alves, T.M.A.; Rosa, C.A.; Zani, C.L. Leishmanicidal metabolites from Cochliobolus sp., an endophytic fungus isolated from Piptadenia adiantoides (Fabaceae). PLoS Negl. Trop. Dis. 2008, 2, e348. [Google Scholar] [CrossRef]
- Wells, J.M.; Cole, R.J.; Cutler, H.C.; Spalding, D.H. Curvularia lunata, a New Source of Cytochalasin B. Appl. Environ. Microbiol. 1981, 41, 967–971. [Google Scholar] [CrossRef] [Green Version]
- Han, W.B.; Lu, Y.H.; Zhang, A.H.; Zhang, G.F.; Mei, Y.N.; Jiang, N.; Lei, X.; Song, Y.C.; Ng, S.W.; Tan, R.X. Curvulamine, a new antibacterial alkaloid incorporating two undescribed units from a Curvularia species. Org. Lett. 2014, 16, 5366–5369. [Google Scholar] [CrossRef]
- Han, W.B.; Zhang, A.H.; Deng, X.Z.; Lei, X.; Tan, R.X. Curindolizine, an Anti-Inflammatory Agent Assembled via Michael Addition of Pyrrole Alkaloids Inside Fungal Cells. Org. Lett. 2016, 18, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Greve, H.; Schupp, P.J.; Eguereva, E.; Kehraus, S.; König, G.M. Ten-Membered Lactones from the Marine-Derived Fungus Curvularia sp. J. Nat. Prod. 2008, 71, 1651–1653. [Google Scholar] [CrossRef] [PubMed]
- Greve, H.; Schupp, P.J.; Eguereva, E.; Kehraus, S.; Kelter, G.; Maier, A.; Fiebig, H.-H.; König, G.M. Apralactone A and a New Stereochemical Class of Curvularins from the Marine-Derived Fungus Curvularia sp. Eur. J. Org. Chem. 2008, 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.-A.; Shao, C.-L.; Gu, Y.-C.; Blum, M.; Gan, L.-S.; Wang, K.-L.; Chen, M.; Wang, C.-Y. Antifouling and Fungicidal Resorcylic Acid Lactones from the Sea Anemone-Derived Fungus Cochliobolus lunatus. J. Agric. Food Chem. 2014, 62, 3183–3191. [Google Scholar] [CrossRef]
- Jadulco, R.; Brauers, G.; Edrada, R.A.; Ebel, R.; Wray, V.; Sudarsono; Proksch, P. New Metabolites from Sponge-Derived Fungi Curvularia lunata and Cladosporium herbarum. J. Nat. Prod. 2002, 65, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, G.W.; Roeymans, H.J. Cynodontin, the tetrahydroxyanthraquinone of Curvularia and Drechslera species. Experientia 1977, 33, 1283–1284. [Google Scholar] [CrossRef]
- Bills, G.F.; Peláez, F.; Polishook, J.D.; Diez-Matas, M.T.; Harris, G.H.; Clapp, W.H.; Dufresne, C.; Byrne, K.M.; Nallin-Omstead, M.; Jenkins, R.G.; et al. Distribution of zaragozic acids (squalestatins) among filamentous ascomycetes. Mycol. Res. 1994, 98, 733–739. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H.; Muench, H.A.; Reed, L.I.; Reed, L.I.; Reed, L.J. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Thabti, I.; Albert, Q.; Philippot, S.; Dupire, F.; Westerhuis, B.; Fontanay, S.; Risler, A.; Kassab, T.; Elfalleh, W.; Aferchichi, A.; et al. Advances on Antiviral Activity of Morus spp. Plant Extracts: Human Coronavirus and Virus-Related Respiratory Tract Infections in the Spotlight. Molecules 2020, 25, 1876. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016. [Google Scholar] [CrossRef]
- Elmi, A.; Spina, R.; Abdoul-Latif, F.; Yagi, S.; Fontanay, S.; Risler, A.; Duval, R.E.; Laurain-Mattar, D. Rapid screening for bioactive natural compounds in Indigofera caerulea Rox fruits. Ind. Crops Prod. 2018, 125, 123–130. [Google Scholar] [CrossRef]
- Elmi, A.; Spina, R.; Risler, A.; Philippot, S.; Mérito, A.; Duval, R.E.; Abdoul-latif, F.M.; Laurain-Mattar, D. Evaluation of Antioxidant and Antibacterial Activities, Cytotoxicity of Acacia seyal Del Bark Extracts and Isolated Compounds. Molecules 2020, 25, 2392. [Google Scholar] [CrossRef]
- Gamboa, M.A.; Bayman, P. Communities of Endophytic Fungi in Leaves of a Tropical Timber Tree (Guarea guidonia: Meliaceae). Biotropica 2001, 33, 352–360. [Google Scholar] [CrossRef]
- Busi, S.; Peddikotla, P.; Yenamandra, V. Secondary Metabolites of Curvularia oryzae MTCC 2605. Rec. Nat. Prod. 2009, 3, 204–208. [Google Scholar]
- Kharwar, R.N.; Verma, V.C.; Strobel, G.; Ezra, D. The endophytic fungal complex of Catharanthus roseus (L.) G. Don. Curr. Sci. 2008, 95, 228–233. [Google Scholar]
- Shen, X.; Cheng, Y.-L.; Cai, C.; Fan, L.; Gao, J.; Hou, C.-L. Diversity and Antimicrobial Activity of Culturable Endophytic Fungi Isolated from Moso Bamboo Seeds. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanthi, K.A.U.; Wickramaarachchi, S.; Wijeratne, E.M.K.; Paranagama, P.A. Two new antioxidant active polyketides from Penicillium citrinum, an endolichenic fungus isolated from Parmotreama species in Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2015, 43, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Geetha, V.; Venkatachalam, A.; Suryanarayanan, T.S.; Doble, M. Isolation and Characterization of New Antioxidant and Antibacterial Compounds from Algicolous Marine Fungus Curvularia Tuberculata. In Proceedings of the International Conference on Bioscience, Biochemistry and Bioinformatics (IPCBEE), Hyderabad, India, 16−18 December 2011; IACSIT Press: Singapore, 2011; Volume 5. [Google Scholar]
- Bradburne, A.F. An investigation of the replication of coronaviruses in suspension cultures of L132 cells. Arch. Gesamte Virusforsch. 1972, 37, 297–307. [Google Scholar] [CrossRef]
- Robilotti, E.; Deresinski, S.; Pinsky, B.A. Norovirus. Clin. Microbiol. Rev. 2015, 28, 134–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linnakoski, R.; Reshamwala, D.; Veteli, P.; Cortina-Escribano, M.; Vanhanen, H.; Marjomäki, V. Antiviral Agents from Fungi: Diversity, Mechanisms and Potential Applications. Front. Microbiol. 2018, 9, 2325. [Google Scholar] [CrossRef] [Green Version]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef] [Green Version]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Phongpaichit, S.; Nikom, J.; Rungjindamai, N.; Sakayaroj, J.; Hutadilok-Towatana, N.; Rukachaisirikul, V.; Kirtikara, K. Biological activities of extracts from endophytic fungi isolated from Garcinia plants. FEMS Immunol. Med. Microbiol. 2007, 51, 517–525. [Google Scholar] [CrossRef] [Green Version]
- CASFM. EUCAST 2019; La Société Française de Microbiologie: Paris, France, 2019. [Google Scholar]
- Rani, R.; Sharma, D.; Chaturvedi, M.; Parkash Yadav, J. Antibacterial Activity of Twenty Different Endophytic Fungi Isolated from Calotropis procera and Time Kill Assay. Clin. Microbiol. Open Access 2017, 6. [Google Scholar] [CrossRef]
- Kaaniche, F.; Hamed, A.; Abdel-Razek, A.S.; Wibberg, D.; Abdissa, N.; Euch, I.Z.E.; Allouche, N.; Mellouli, L.; Shaaban, M.; Sewald, N. Bioactive secondary metabolites from new endophytic fungus Curvularia. sp isolated from Rauwolfia macrophylla. PLoS ONE 2019, 14, e0217627. [Google Scholar] [CrossRef] [Green Version]
- Vélëz, H.; Glassbrook, N.J.; Daub, M.E. Mannitol metabolism in the phytopathogenic fungus Alternaria alternata. Fungal Genet. Biol. 2007, 44, 258–268. [Google Scholar] [CrossRef]
- Patel, T.K.; Williamson, J.D. Mannitol in Plants, Fungi, and Plant-Fungal Interactions. Trends Plant Sci. 2016, 21, 486–497. [Google Scholar] [CrossRef]
- Song, S.H.; Vieille, C. Recent advances in the biological production of mannitol. Appl. Microbiol. Biotechnol. 2009, 84, 55–62. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 17 August 2020).
- Lenart, J.; Pikuła, S. 10-Undecynoic acid, an inhibitor of cytochrome P450 4A1, inhibits ethanolamine-specific phospholipid base exchange reaction in rat liver microsomes. Acta Biochim. Pol. 1999, 46, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.B.; George, B.; Ebersole, J.L. Antimicrobial activity of n-6, n-7 and n-9 fatty acids and their esters for oral microorganisms. Arch. Oral Biol. 2010, 55, 555–560. [Google Scholar] [CrossRef] [Green Version]
- Dadwal, V.; Agrawal, H.; Sonkhla, K.; Joshi, R.; Gupta, M. Characterization of phenolics, amino acids, fatty acids and antioxidant activity in pulp and seeds of high altitude Himalayan crab apple fruits (Malus baccata). J. Food Sci. Technol. 2018, 55, 2160–2169. [Google Scholar] [CrossRef]
- Degenhardt, R.T.; Farias, I.V.; Grassi, L.T.; Franchi, G.C.; Nowill, A.E.; Bittencourt, C.M.D.S.; Wagner, T.M.; de Souza, M.M.; Cruz, A.B.; Malheiros, A. Characterization and evaluation of the cytotoxic potential of the essential oil of Chenopodium ambrosioides. Rev. Bras. Farmacogn. 2016, 26, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Devys, M.; Férézou, J.-P.; Topgi, R.S.; Barbier, M.; Bousquet, J.-F.; Kollmann, A. Structure and biosynthesis of phomenoic acid, an antifungal compound isolated from Phoma lingam Tode. J. Chem. Soc. Perkin Trans. 1984, 1, 2133–2137. [Google Scholar] [CrossRef]
- Topgi, R.S.; Devys, M.; Bousquet, J.F.; Kollmann, A.; Barbier, M. Phomenoic acid and phomenolactone, antifungal substances from Phoma lingam (Tode) Desm.: Kinetics of their biosynthesis, with an optimization of the isolation procedures. Appl. Environ. Microbiol. 1987, 53, 966–968. [Google Scholar] [CrossRef] [Green Version]
- Bloor, S. Arthrinic Acid, a Novel Antifungal Polyhydroxyacid from Arthrinium phaeospermum. J. Antibiot. 2008, 61, 515–517. [Google Scholar] [CrossRef] [Green Version]







| Bacterial Strains | Inhibition Zone (mm) with Ethyl Acetate Extract |
|---|---|
| P. aeruginosa | 6 |
| E. coli | 6 |
| Salmonella Abony | 6 |
| Staphylococcus aureus | 13 |
| MRSA | 13 |
| S. arlettae | 9 |
| S. lentus | 10 |
| S. epidermidis | 12 |
| S. haemolyticus | 10 |
| S. xylosus | 10 |
| S. sciuri | 10 |
| S. warneri | 10 |
| S. capitis | 12 |
| S. lugdunensis | 10 |
| E. faecalis | 9 |
| E. faecium | 6 |
| B. cereus | 6 |
| K. sedentarius | 9 |
| Position | Methanol d4 | Pyridine d5 | ||
|---|---|---|---|---|
| 13C δC | 1H δH (J in Hz) | 13C δC | 1H δH (J in Hz) | |
| C-1 | 175.3 | - | 175.0 | - |
| C-2 | 43.5 | 2.47 m (2H, H-2, H-2′) | 44.6 | 3.05 dd (1H, J = 15 Hz, J = 8.5 Hz, H-2′) 2.96 dd (1H, J = 15 Hz, J = 5 Hz, H-2) |
| C-3 | 70.3 | 4.47 m (1H, H-3) | 69.9 | 5.18 m (1H, H-3) |
| C-4 | 135.6 | 5.59 dd (1H, H-4, J = 6.6 Hz, 18 Hz) | 136.5 | 6.10 dd (1H, H-4, J = 6 Hz, 15 Hz) |
| C-5 | 128.9 | 5.73 dd (1H, H-5, J = 7 Hz, 15 Hz) | 128.0 | 6.32 dd (1H, H-5, J = 7 Hz, 15 Hz) |
| C-6 | 41.4 | 2.26 m (2H, H-6, H-6′) | 42.0 | 2.58 m (2H, H-6, H-6′) |
| C-7 | 73.7 | 4.05 m (1H, H-7) | 72.7 | 4.52 m (1H, H-7) |
| C-8 | 134.1 | 5.48 dd (1H, H-8, J = 7 Hz, 15 Hz) | 137.0 | 5.97 dd (1H, H-8, J = 6 Hz, 15 Hz) |
| C-9 | 136.0 | 5.64 dd (1H, H-9, J = 7 Hz, 15 Hz) | 127.9 | 6.21 dd (1H, H-9, J =7.6 Hz, 15 Hz) |
| C-10 | 41.5 | 2.23 m (2H, H-10, H-10′) | 42.1 | 2.64 m (2H, H-10, H-10′) |
| C-11 | 73.4 | 4.03 m (1H, H-11) | 72.9 | 4.51 m (1H, H-11) |
| C-12 | 136.3 | 5.54 dd (1H, H-12, J = 7 Hz, 18 Hz) | 134.8 | 5.98 dd (1H, H-12, J = 6 Hz, 15 Hz) |
| C-13 | 128.8 | 5.70 dd (1H, H-13, J = 7 Hz, 15 Hz) | 134.5 | 6.27 dd (1H, H-13, J = 7.6 Hz, 15 Hz) |
| C-14 | 41.6 | 2.35 m (1H, H-14) | 41.35 | 2.72 m (1H, H-14) |
| C-15 | 83.7 | 3.72 d (1H, H-15, J = 8.6 Hz) | 82.8 | 4.18 d (1H, J = 8 Hz, H-15) |
| C-16 | 139.0 | - | 139.5 | - |
| C-17 | 129.9 | 5.92 s (1H, H-17) | 128.9 | 6.51 bs (1H, H-17) |
| C-18 | 135.9 | - | 137.1 | - |
| C-19 | 140.0 | 5.14 d (1H, H-19, J = 10 Hz) | 138.3 | 5.40 d (1H, J = 10 Hz, H-19) |
| C-20 | 31.4 | 2.70 m (1H, H-20) | 30.7 | 2.91 m (1H, H-20) |
| C-21 | 46.7 | 1.30 m (1H, H-21) 1.05 m (1H, H-21′) | 45.9 | 1.42 m (1H, H-21′) 1.07 m (1H, H-21) |
| C-22 | 32.9 | 1.43 m (1H, H-22) | 32.0 | 1.43 m (1H, H-22) |
| C-23 | 46.8 | 1.20 m (1H, H-23) 0.95 m (1H, H-23′) | 45.9 | 1.22 m (1H, H-23′) 0.95 m (1H, H-23) |
| C-24 | 29.5 | 1.52 m (1H, H-24) | 28.7 | 1.66 m (1H, H-24) |
| C-25 | 30.5 | 1.09 m (1H, H-25) 1.39 m (1H, H-25′) | 29.9 | 1.29 m (1H, H-25′) 1.07 m (1H, H-25) |
| C-26 | 11.7 | 0.86 t (3H, CH3, H-26, J = 7 Hz) | 11.7 | 0.85 t (3H, CH3, J = 7 Hz, C-26) |
| C-27 | 17.9 | 0.89 d (3H, CH3, H-27, J = 7 Hz) | 18.4 | 1.16 d (3H, CH3, J = 6.6 Hz, C-27) |
| C-28 | 13.5 | 1.77 d (3H, CH3, H-28, J = 1.3 Hz) | 14.2 | 2.17 d (3H, CH3, J = 0.9 Hz, C-28) |
| C-29 | 61.2 | 4.16 AB system, d (1H, H-29, J = 12 Hz) 4.16 AB system, d (1H, H-29′, J = 12 Hz) | 61.0 | 4.60 d (1H, J AB = 12 Hz, H-29′) 4.58 d (1H, J AB = 12 Hz, H-29) |
| C-30 | 22.9 | 0.99 d (3H, CH3, H-30, J = 6.6 Hz) | 23.1 | 1.06 d (3H, CH3, J = 6.6 Hz, C-30) |
| C-31 | 20.3 | 0.84 d (3H, CH3, H-31, J = 6.6 Hz) | 20.1 | 0.84 d (3H, CH3, J = 6 Hz, C-31) |
| C-32 | 20.7 | 0.87 d (3H, CH3, H-32, J = 6.6 Hz) | 20.8 | 0.91 d (3H, CH3, J = 6.6 Hz, C-32) |
| - | 4.98 broad m (5H, 5-OH) | |||
| Fraction Name | Compound Number | Compound Name | Formula | Molecular Weight | Calculated Retention Index | Retention Time (min) | Similarity (%) |
|---|---|---|---|---|---|---|---|
| F1 | 1 | 2,4-Di-tert-butylphenol | C14H22O | 206 | 1447 | 14.21 | 96 |
| F1 | 2 | 1-Octadecene | C18H36 | 252 | 1730 | 17.610 | 96 |
| F1 | 3 | Undec-10-ynoic acid | C11H18O2 | 182 | 2262 | 22.797 | 80 |
| F1 | 4 | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl ester | C35H62O3 | 530 | 3598 | 32.063 | 87 |
| F3 | 5 | Methyl palmitoleate | C17H34O2 | 270 | 1986 | 20.257 | 94 |
| F3 | 6 | Methyl linolelaidate | C19H34O2 | 294 | 2159 | 21.887 | 94 |
| F3 | 7 | Methyl petroselinate | C19H36O2 | 296 | 2166 | 21.947 | 85 |
| F3 | 8 | Methyl stearate | C19H38O2 | 298 | 2194 | 22.187 | 92 |
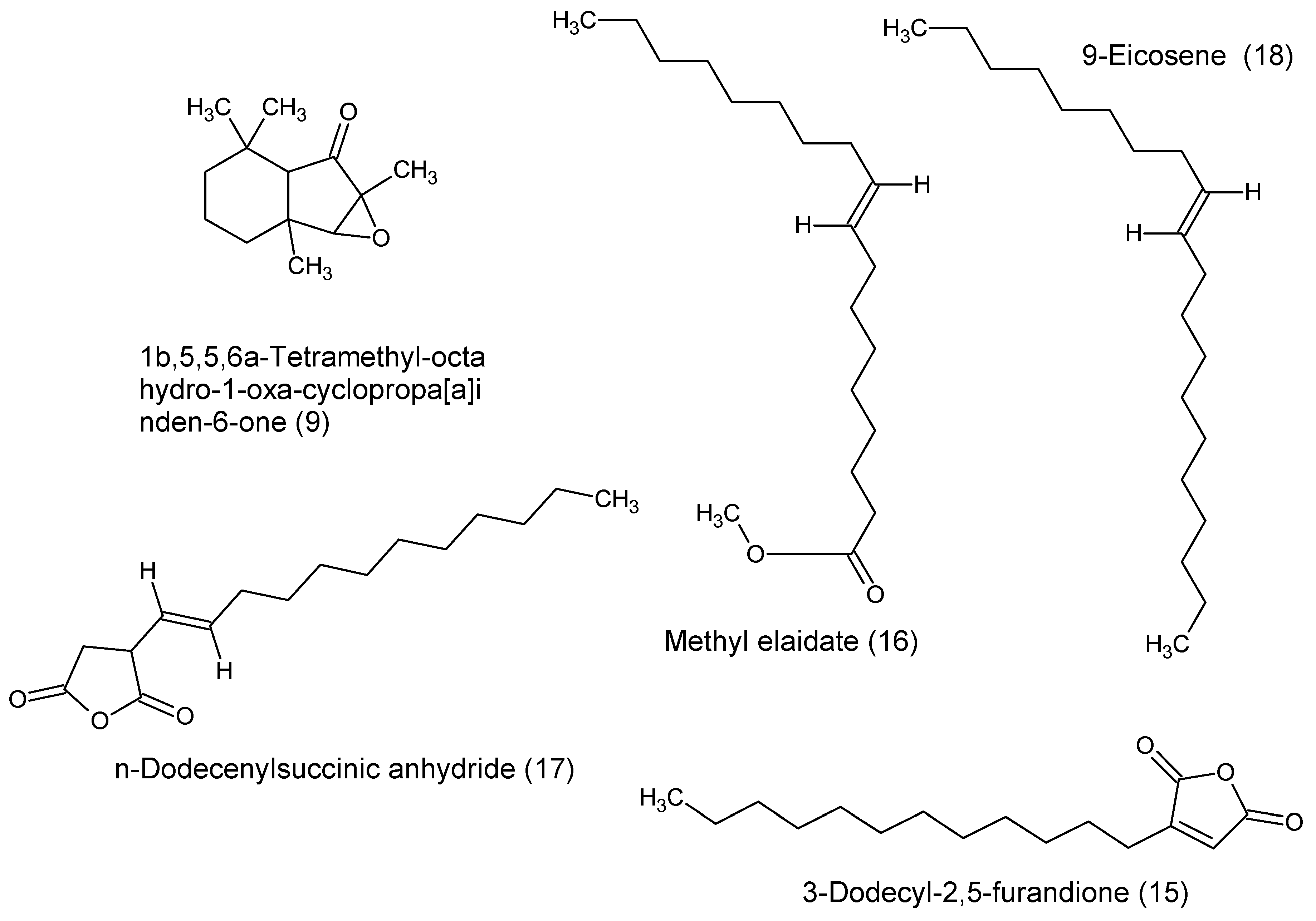
| F10.C | 9 | 1b,5,5,6a-Tetramethyl-octahydro-1-oxa-cyclopropa[a]inden-6-one | C13H20O2 | 208 | 2253 | 22.718 | 82 |
| F10.C | 10 | 4-Oxo-β-isodamascol | C13H20O2 | 208 | 2290 | 23.030 | 80 |
| F10.C | 11 | Oleamide | C18H35NO | 281 | 3188 | 29.548 | 92 |
| F10.C | 12 | 1,2-Octadecanediol | C18H38O2 | 286 | 3556 | 31.742 | 87 |
| F10.C | 13 | Cyclopentadecanol | C15H30O | 226 | 3796 | 33.665 | 94 |
| F10.D | 14 | Dicyclohexane | C12H22 | 166 | 1269 | 11.733 | 94 |
| F10.D | 1 | 2,4-Di-tert-butylphenol | C14H22O | 206 | 1450 | 14.300 | 89 |
| F10.D | 15 | 3-Dodecyl-2,5-furandione | C16H26O3 | 266 | 1825 | 18.630 | 86 |
| F10.D | 16 | Methyl elaidate | C19H36O2 | 296 | 2031 | 20.680 | 94 |
| F10.E | 1 | 2,4-Di-tert-butylphenol | C14H22O | 206 | 1450 | 14.297 | 91 |
| F10.E | 2 | 1-Octadecene | C18H36 | 252 | 1730 | 17.610 | 95 |
| F10.E | 17 | n-Dodecenylsuccinic anhydride | C16H26O3 | 266 | 1832 | 18.697 | 86 |
| F10.E | 18 | 9-Eicosene | C20H40 | 280 | 2016 | 20.543 | 89 |
| F10.E | 19 | 11,14-Eicosadienoic acid, methyl ester | C21H38O2 | 322 | 2025 | 20.623 | 88 |
| F10.E | 20 | Methyl oleate | C19H36O2 | 296 | 2031 | 20.683 | 93 |
| Precipitate | 21 | Ascaridole | C10H16O2 | 168 | 2177 | 22.042 | 82 |
| Precipitate | 9 | 1b,5,5,6a-Tetramethyl-octahydro-1-oxa-cyclopropa[a]inden-6-one | C13H20O2 | 208 | 2253 | 22.718 | 82 |
| Precipitate | 22 | 1-Eicosanol | C20H42O | 298 | 3557 | 31.750 | 90 |
| Strains | Crude Extract of C. papendorfii | Kheiric Acid |
|---|---|---|
| Staphylococcus aureus | MIC = 312 µg/mL | MIC = 62.5 µg/mL |
| MRSA | MIC = 312 µg/mL | MIC = 62.5 µg/mL |
| HCoV 229E | 15% of the reduction of the virus-induced cytopathogenic effect at MOI 0.001 | No reduction of the virus-induced cytopathogenic effect |
| FCV F9 | 40% of the reduction of the virus-induced cytopathogenic effect at lower MOI 0.0001 | No reduction of the virus-induced cytopathogenic effect |
| MCF7 | IC50 = 21.5 ± 5.9 µg/mL | IC50 > 100 µg/mL |
| HT29 | IC50 > 100 µg/mL | IC50 > 100 µg/mL |
| HCT116 | IC50 > 100 µg/mL | IC50 > 100 µg/mL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khiralla, A.; Spina, R.; Varbanov, M.; Philippot, S.; Lemiere, P.; Slezack-Deschaumes, S.; André, P.; Mohamed, I.; Yagi, S.M.; Laurain-Mattar, D. Evaluation of Antiviral, Antibacterial and Antiproliferative Activities of the Endophytic Fungus Curvularia papendorfii, and Isolation of a New Polyhydroxyacid. Microorganisms 2020, 8, 1353. https://doi.org/10.3390/microorganisms8091353
Khiralla A, Spina R, Varbanov M, Philippot S, Lemiere P, Slezack-Deschaumes S, André P, Mohamed I, Yagi SM, Laurain-Mattar D. Evaluation of Antiviral, Antibacterial and Antiproliferative Activities of the Endophytic Fungus Curvularia papendorfii, and Isolation of a New Polyhydroxyacid. Microorganisms. 2020; 8(9):1353. https://doi.org/10.3390/microorganisms8091353
Chicago/Turabian StyleKhiralla, Afra, Rosella Spina, Mihayl Varbanov, Stéphanie Philippot, Pascal Lemiere, Sophie Slezack-Deschaumes, Philippe André, Ietidal Mohamed, Sakina Mohamed Yagi, and Dominique Laurain-Mattar. 2020. "Evaluation of Antiviral, Antibacterial and Antiproliferative Activities of the Endophytic Fungus Curvularia papendorfii, and Isolation of a New Polyhydroxyacid" Microorganisms 8, no. 9: 1353. https://doi.org/10.3390/microorganisms8091353
APA StyleKhiralla, A., Spina, R., Varbanov, M., Philippot, S., Lemiere, P., Slezack-Deschaumes, S., André, P., Mohamed, I., Yagi, S. M., & Laurain-Mattar, D. (2020). Evaluation of Antiviral, Antibacterial and Antiproliferative Activities of the Endophytic Fungus Curvularia papendorfii, and Isolation of a New Polyhydroxyacid. Microorganisms, 8(9), 1353. https://doi.org/10.3390/microorganisms8091353







