Impact of Ozone, Sex, and Gonadal Hormones on Bronchoalveolar Lavage Characteristics and Survival in SP-A KO Mice Infected with Klebsiella pneumoniae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Bacteria
2.3. Exposure of Mice to Ozone and K. pneumoniae Bacterial Infection
2.4. BAL Analyses
2.5. Gonadectomy and Hormone Treatment
2.6. Phagocytosis Assay
2.7. Statistical Analysis
3. Results
3.1. BAL Content
3.1.1. Percentage of Polymorphonuclear Leukocytes (%PMNs)
3.1.2. Percentage of Monocytes
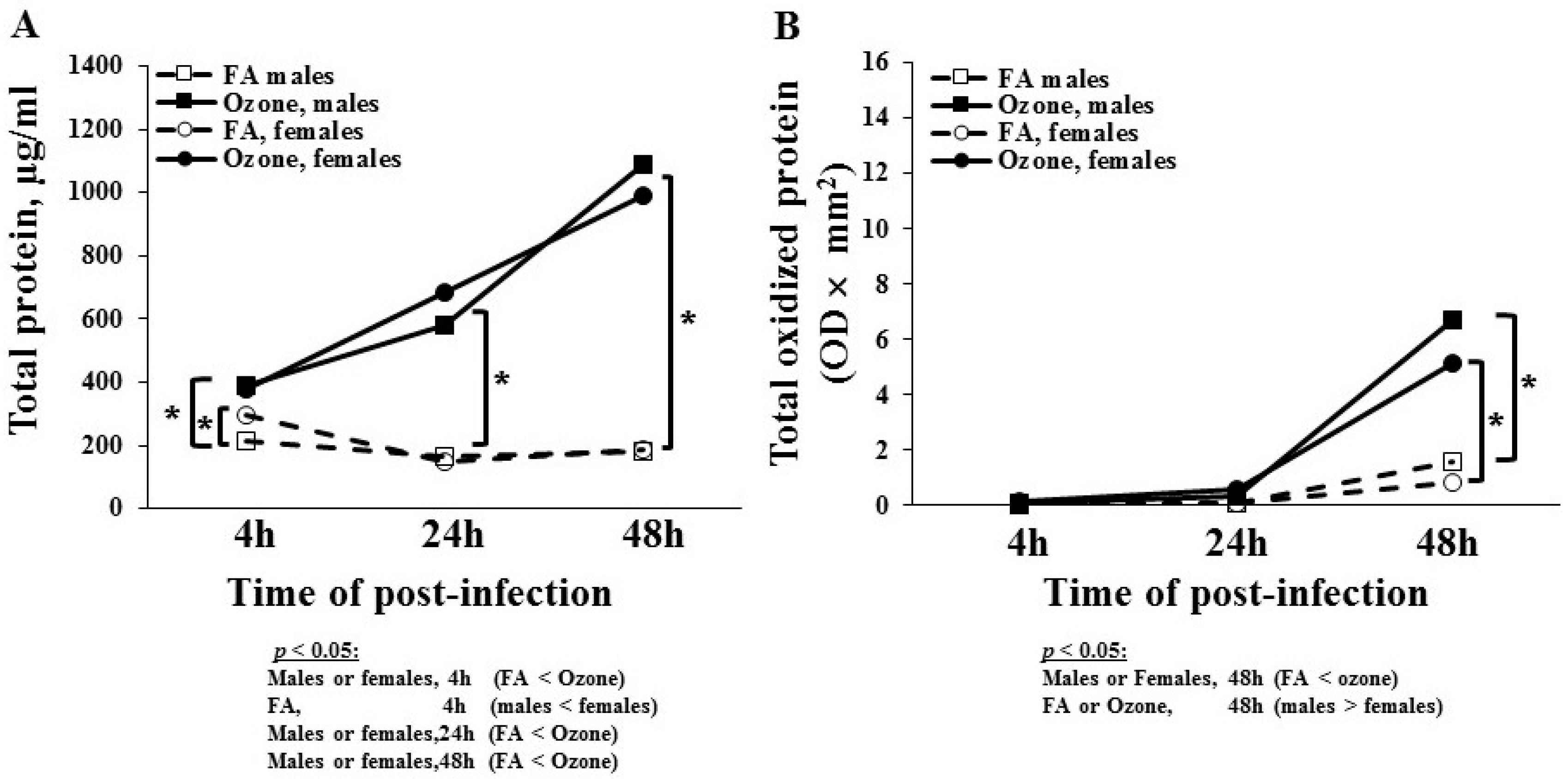
3.1.3. Total BAL Protein
3.1.4. Total Oxidized BAL Protein
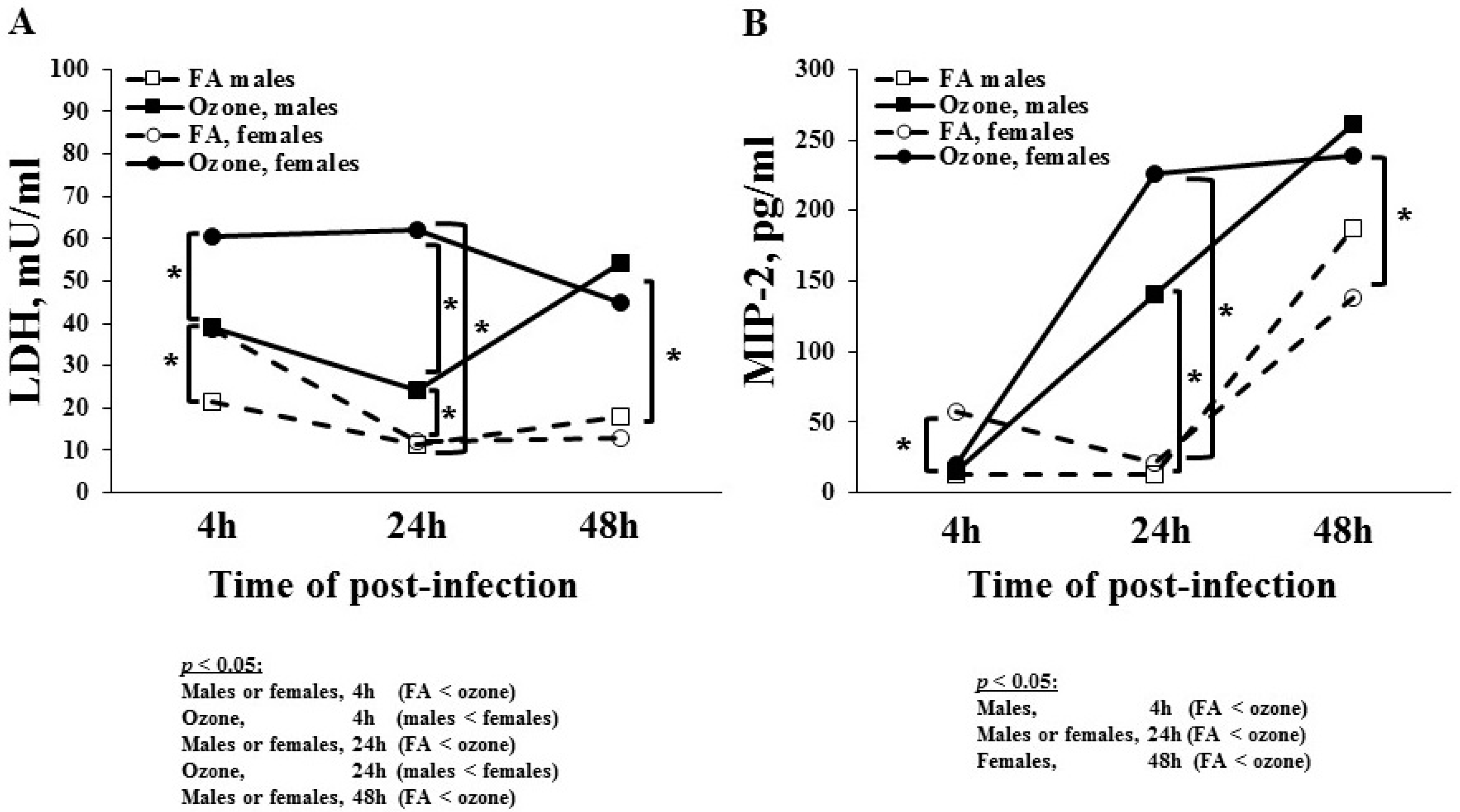
3.1.5. Lactate Dehydrogenase (LDH) Levels
3.1.6. MIP-2 Levels
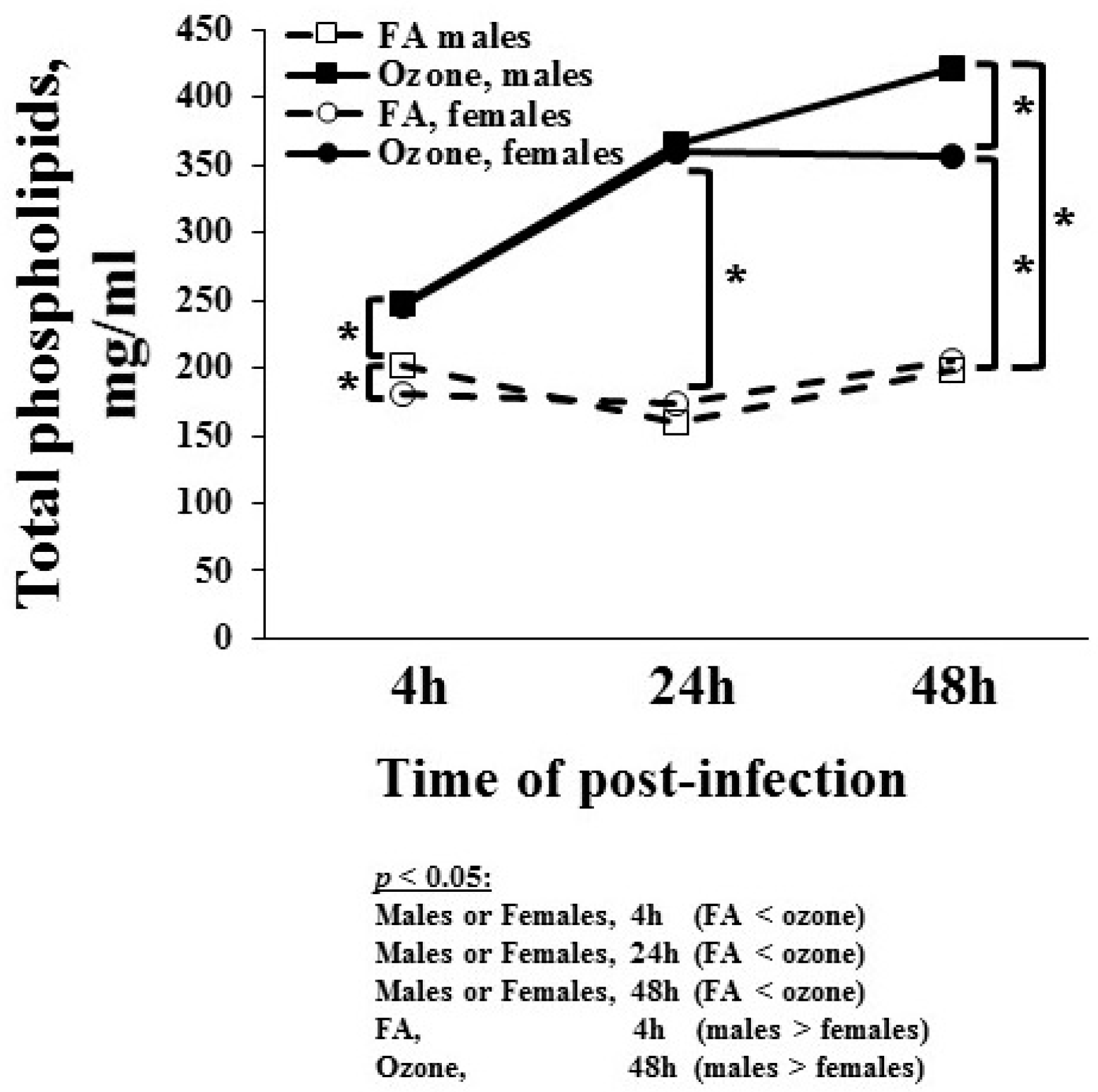
3.1.7. Total Phospholipids Level
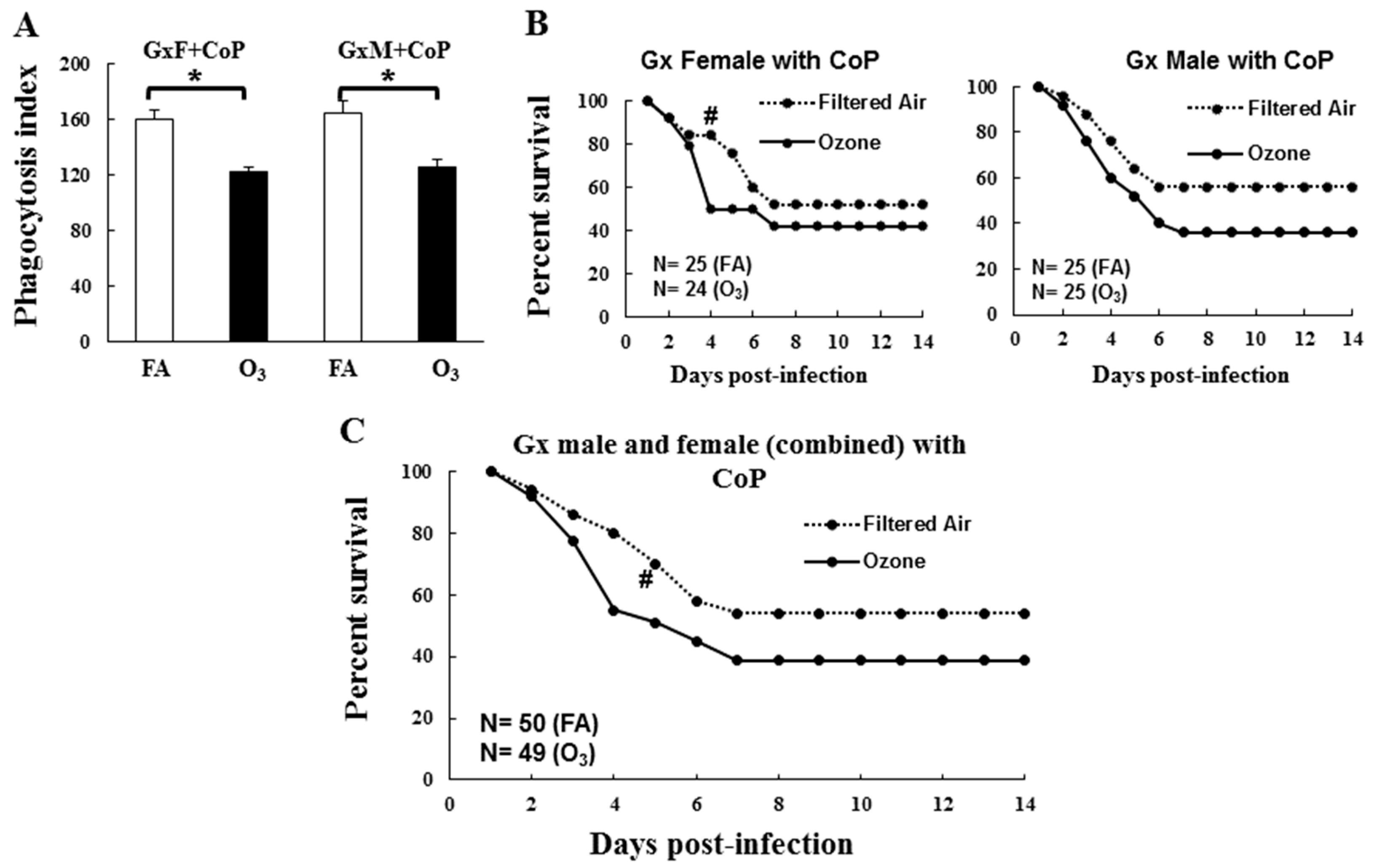
3.2. In Vivo Phagocytosis of K. pneumoniae by AMs
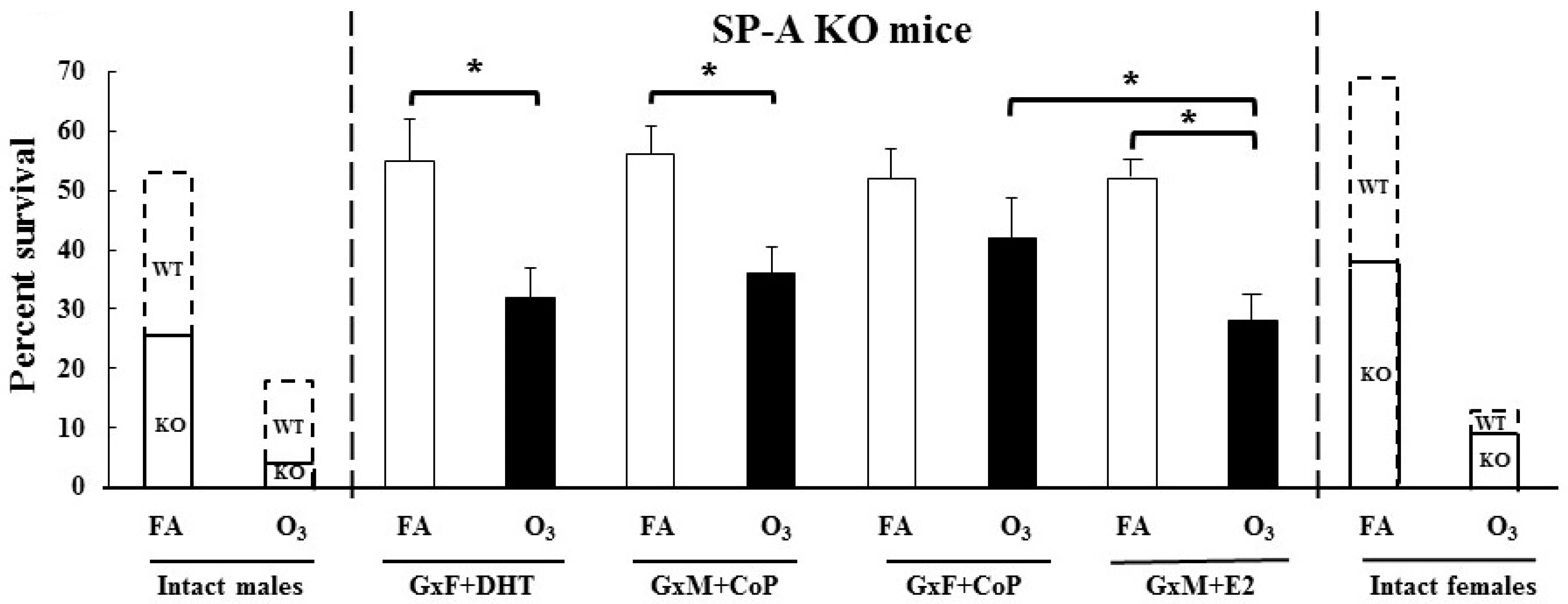
3.3. Impact of Gonadal Hormones on Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Pneumococcal vaccines: WHO position paper—2012. Wkly. Epidemiol. Rec. Relev. Épidémiologique Hebd. 2012, 87, 129–144. [Google Scholar]
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Swartz, S.; Fullman, N.; Mosser, J.; Thompson, R.L.; Reiner, R.C., Jr. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 1133–1161. [Google Scholar] [CrossRef] [Green Version]
- Paganin, F.; Lilienthal, F.; Bourdin, A.; Lugagne, N.; Tixier, F.; Genin, R.; Yvin, J. Severe community-acquired pneumonia: Assessment of microbial aetiology as mortality factor. Eur. Respir. J. 2004, 24, 779–785. [Google Scholar] [CrossRef]
- Mikerov, A.N.; Haque, R.; Gan, X.; Guo, X.; Phelps, D.S.; Floros, J. Ablation of SP-A has a negative impact on the susceptibility of mice to Klebsiella pneumoniae infection after ozone exposure: Sex differences. Respir. Res. 2008, 9, 77. [Google Scholar] [CrossRef] [Green Version]
- Mikerov, A.N.; Gan, X.; Umstead, T.M.; Miller, L.; Chinchilli, V.M.; Phelps, D.S.; Floros, J. Sex differences in the impact of ozone on survival and alveolar macrophage function of mice after Klebsiella pneumoniae infection. Respir. Res. 2008, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Baleeiro, C.E.; Wilcoxen, S.E.; Morris, S.B.; Standiford, T.J.; Paine, R. Sublethal hyperoxia impairs pulmonary innate immunity. J. Immunol. 2003, 171, 955–963. [Google Scholar] [CrossRef]
- Laichalk, L.L.; Kunkel, S.L.; Strieter, R.M.; Danforth, J.M.; Bailie, M.B.; Standiford, T.J. Tumor necrosis factor mediates lung antibacterial host defense in murine Klebsiella pneumonia. Infect. Immun. 1996, 64, 5211–5218. [Google Scholar] [CrossRef] [Green Version]
- Markart, P.; Korfhagen, T.R.; Weaver, T.E.; Akinbi, H.T. Mouse lysozyme M is important in pulmonary host defense against Klebsiella pneumoniae infection. Am. J. Respir. Crit. Care Med. 2004, 169, 454–458. [Google Scholar] [CrossRef]
- Cogen, A.L.; Moore, T.A. β2-Microglobulin-Dependent Bacterial Clearance and Survival during Murine Klebsiella pneumoniae Bacteremia. Infect. Immun. 2009, 77, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Ketko, A.K.; Donn, S.M. Surfactant-associated proteins: Structure, function and clinical implications. Curr. Pediatr. Rev. 2014, 10, 162–167. [Google Scholar] [CrossRef]
- Crouch, E.; Hartshorn, K.; Ofek, I. Collectins and pulmonary innate immunity. Immunol. Rev. 2000, 173, 52–65. [Google Scholar] [CrossRef]
- Nathan, N.; Taytard, J.; Duquesnoy, P.; Thouvenin, G.; Corvol, H.; Amselem, S.; Clement, A. Surfactant protein A: A key player in lung homeostasis. Int. J. Biochem. Cell Biol. 2016, 81, 151–155. [Google Scholar] [CrossRef]
- Phelps, D.S. Surfactant regulation of host defense function in the lung: A question of balance. Pediatric Pathol. Mol. Med. 2001, 20, 269–292. [Google Scholar] [CrossRef]
- Brinker, K.G.; Garner, H.; Wright, J.R. Surfactant protein A modulates the differentiation of murine bone marrow-derived dendritic cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L232–L241. [Google Scholar] [CrossRef] [Green Version]
- Kremlev, S.G.; Phelps, D.S. Effect of SP-A and surfactant lipids on expression of cell surface markers in the THP-1 monocytic cell line. Am. J. Physiol. Lung Cell. Mol. Physiol. 1997, 272, L1070–L1077. [Google Scholar] [CrossRef]
- Bridges, J.P.; Davis, H.W.; Damodarasamy, M.; Kuroki, Y.; Howles, G.; Hui, D.Y.; McCormack, F.X. Pulmonary surfactant proteins A and D are potent endogenous inhibitors of lipid peroxidation and oxidative cellular injury. J. Biol. Chem. 2000, 275, 38848–38855. [Google Scholar] [CrossRef] [Green Version]
- Crowther, J.E.; Kutala, V.K.; Kuppusamy, P.; Ferguson, J.S.; Beharka, A.A.; Zweier, J.L.; McCormack, F.X.; Schlesinger, L.S. Pulmonary surfactant protein a inhibits macrophage reactive oxygen intermediate production in response to stimuli by reducing NADPH oxidase activity. J. Immunol. 2004, 172, 6866–6874. [Google Scholar] [CrossRef] [Green Version]
- Hickman-Davis, J.M.; Gibbs-Erwin, J.; Lindsey, J.R.; Matalon, S. Role of surfactant protein-A in nitric oxide production and mycoplasma killing in congenic C57BL/6 mice. Am. J. Respir. Cell Mol. Biol. 2004, 30, 319–325. [Google Scholar] [CrossRef]
- LeVine, A.M.; Kurak, K.E.; Bruno, M.D.; Stark, J.M.; Whitsett, J.A.; Korfhagen, T.R. Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am. J. Respir. Cell Mol. Biol. 1998, 19, 700–708. [Google Scholar] [CrossRef] [Green Version]
- LeVine, A.M.; Whitsett, J.A.; Gwozdz, J.A.; Richardson, T.R.; Fisher, J.H.; Burhans, M.S.; Korfhagen, T.R. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J. Immunol. 2000, 165, 3934–3940. [Google Scholar] [CrossRef] [Green Version]
- Card, J.W.; Zeldin, D.C. Hormonal influences on lung function and response to environmental agents: Lessons from animal models of respiratory disease. Proc. Am. Thorac. Soc. 2009, 6, 588–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorenoor, N.; Umstead, T.M.; Zhang, X.; Phelps, D.S.; Floros, J. Survival of Surfactant Protein-A1 and SP-A2 Transgenic Mice After Klebsiella pneumoniae Infection, Exhibits Sex-, Gene-, and Variant Specific Differences; Treatment with Surfactant Protein Improves Survival. Front. Immunol. 2018, 9, 2404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorenoor, N.; Zhang, X.; Umstead, T.M.; Scott Halstead, E.; Phelps, D.S.; Floros, J. Differential effects of innate immune variants of surfactant protein-A1 (SFTPA1) and SP-A2 (SFTPA2) in airway function after Klebsiella pneumoniae infection and sex differences. Respir. Res. 2018, 19, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durrani, F.; Phelps, D.S.; Weisz, J.; Silveyra, P.; Hu, S.; Mikerov, A.N.; Floros, J. Gonadal hormones and oxidative stress interaction differentially affects survival of male and female mice after lung Klebsiella pneumoniae infection. Exp. Lung Res. 2012, 38, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikerov, A.N.; Hu, S.; Durrani, F.; Gan, X.; Wang, G.; Umstead, T.M.; Phelps, D.S.; Floros, J. Impact of sex and ozone exposure on the course of pneumonia in wild type and SP-A (-/-) mice. Microb. Pathog. 2012, 52, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, V.; Angus, D.C.; Griffin, M.F.; Clermont, G.; Scott Watson, R.; Linde-Zwirble, W.T. Hospitalized community-acquired pneumonia in the elderly: Age-and sex-related patterns of care and outcome in the United States. Am. J. Respir. Crit. Care Med. 2002, 165, 766–772. [Google Scholar] [CrossRef]
- Gannon, C.J.; Pasquale, M.; Tracy, J.K.; McCarter, R.J.; Napolitano, L.M. Male gender is associated with increased risk for postinjury pneumonia. Shock 2004, 21, 410–414. [Google Scholar] [CrossRef] [Green Version]
- Card, J.W.; Carey, M.A.; Bradbury, J.A.; DeGraff, L.M.; Morgan, D.L.; Moorman, M.P.; Flake, G.P.; Zeldin, D.C. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J. Immunol. 2006, 177, 621–630. [Google Scholar] [CrossRef]
- Mikerov, A.N.; Cooper, T.K.; Wang, G.; Hu, S.; Umstead, T.M.; Phelps, D.S.; Floros, J. Histopathologic evaluation of lung and extrapulmonary tissues show sex differences in Klebsiella pneumoniae-infected mice under different exposure conditions. Int. J. Physiol. Pathophysiol. Pharmacol. 2011, 3, 176–190. [Google Scholar]
- Mikerov, A.N.; Phelps, D.S.; Gan, X.; Umstead, T.M.; Haque, R.; Wang, G.; Floros, J. Effect of ozone exposure and infection on bronchoalveolar lavage: Sex differences in response patterns. Toxicol. Lett. 2014, 230, 333–344. [Google Scholar] [CrossRef] [Green Version]
- Dye, J.A.; Gibbs-Flournoy, E.A.; Richards, J.H.; Norwood, J.; Kraft, K.; Hatch, G.E. Neonatal rat age, sex and strain modify acute antioxidant response to ozone. Inhal. Toxicol. 2017, 29, 291–303. [Google Scholar] [CrossRef]
- Ikegami, M.; Elhalwagi, B.M.; Palaniyar, N.; Dienger, K.; Korfhagen, T.; Whitsett, J.A.; McCormack, F.X. The Collagen-like Region of Surfactant Protein A (SP-A) Is Required for Correction of Surfactant Structural and Functional Defects in the SP-A Null Mouse. J. Biol. Chem. 2001, 276, 38542–38548. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Guo, X.; Diangelo, S.; Thomas, N.J.; Floros, J. Humanized SFTPA1 and SFTPA2 transgenic mice reveal functional divergence of SP-A1 and SP-A2: Formation of tubular myelin in vivo requires both gene products. J. Biol. Chem. 2010, 285, 11998–12010. [Google Scholar] [CrossRef] [Green Version]
- Schmiedl, A.; Vieten, G.; Mühlfeld, C.; Bernhard, W. Distribution of intracellular and secreted surfactant during postnatal rat lung development. Pediatric Pulmonol. 2007, 42, 548–562. [Google Scholar] [CrossRef]
- Oosting, R.; Van Greevenbroek, M.; Verhoef, J.; Van Golde, L.; Haagsman, H. Structural and functional changes of surfactant protein A induced by ozone. Am. J. Physiol. Lung Cell. Mol. Physiol. 1991, 261, L77–L83. [Google Scholar] [CrossRef]
- Wang, G.; Bates-Kenney, S.R.; Tao, J.Q.; Phelps, D.S.; Floros, J. Differences in biochemical properties and in biological function between human SP-A1 and SP-A2 variants, and the impact of ozone-induced oxidation. Biochemistry 2004, 43, 4227–4239. [Google Scholar] [CrossRef]
- Wang, G.; Umstead, T.M.; Phelps, D.S.; Al-Mondhiry, H.; Floros, J. The effect of ozone exposure on the ability of human surfactant protein a variants to stimulate cytokine production. Environ. Health Perspect. 2002, 110, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Wang, G.; Phelps, D.S.; Al-Mondhiry, H.; Floros, J. Human SP-A genetic variants and bleomycin-induced cytokine production by THP-1 cells: Effect of ozone-induced SP-A oxidation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L546–L553. [Google Scholar] [CrossRef]
- Oosting, R.S.; Iwaarden, J.F.V.; Bree, L.V.; Verhoef, J.; Golde, L.M.V.; Haagsman, H.P. Exposure of surfactant protein A to ozone in vitro and in vivo impairs its interactions with alveolar cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 1992, 262, L63–L68. [Google Scholar] [CrossRef]
- Mikerov, A.N.; Umstead, T.M.; Gan, X.; Huang, W.; Guo, X.; Wang, G.; Phelps, D.S.; Floros, J. Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L121–L130. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Myers, C.; Mikerov, A.; Floros, J. Effect of cysteine 85 on biochemical properties and biological function of human surfactant protein A variants. Biochemistry 2007, 46, 8425–8435. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Roche, L.; Riera-Romo, M.; Mesta, F.; Hernández-Matos, Y.; Barrios, J.M.; Martínez-Sánchez, G.; Al-Dalaien, S.M. Medical ozone promotes Nrf2 phosphorylation reducing oxidative stress and pro-inflammatory cytokines in multiple sclerosis patients. Eur. J. Pharm. 2017, 811, 148–154. [Google Scholar] [CrossRef]
- Wang, G.; Umstead, T.M.; Hu, S.; Mikerov, A.N.; Phelps, D.S.; Floros, J. Differential Effects of Human SP-A1 and SP-A2 on the BAL Proteome and Signaling Pathways in Response to Klebsiella pneumoniae and Ozone Exposure. Front. Immunol. 2019, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Haque, R.; Umstead, T.M.; Ponnuru, P.; Guo, X.; Hawgood, S.; Phelps, D.S.; Floros, J. Role of surfactant protein-A (SP-A) in lung injury in response to acute ozone exposure of SP-A deficient mice. Toxicol. Appl. Pharm. 2007, 220, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Mikerov, A.N.; Umstead, T.M.; Huang, W.; Liu, W.; Phelps, D.S.; Floros, J. SP-A1 and SP-A2 variants differentially enhance association of Pseudomonas aeruginosa with rat alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L150–L158. [Google Scholar] [CrossRef] [Green Version]
- Mikerov, A.N.; Wang, G.; Umstead, T.M.; Zacharatos, M.; Thomas, N.J.; Phelps, D.S.; Floros, J. Surfactant protein A2 (SP-A2) variants expressed in CHO cells stimulate phagocytosis of Pseudomonas aeruginosa more than do SP-A1 variants. Infect. Immun. 2007, 75, 1403–1412. [Google Scholar] [CrossRef] [Green Version]
- LeVine, A.M.; Hartshorn, K.; Elliott, J.; Whitsett, J.; Korfhagen, T. Absence of SP-A modulates innate and adaptive defense responses to pulmonary influenza infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L563–L572. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Siddiqui, J.; Hendry, M.; Akiyama, J.; Edmondson, J.; Brown, C.; Allen, L.; Levitt, S.; Poulain, F.; Hawgood, S. Surfactant Protein-A–Deficient Mice Display an Exaggerated Early Inflammatory Response to a β -Resistant Strain of Influenza A Virus. Am. J. Respir. Cell Mol. Biol. 2002, 26, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Balamayooran, G.; Batra, S.; Fessler, M.B.; Happel, K.I.; Jeyaseelan, S. Mechanisms of neutrophil accumulation in the lungs against bacteria. Am. J. Respir. Cell Mol. Biol. 2010, 43, 5–16. [Google Scholar] [CrossRef]
- Floros, J.; Phelps, D. Surfactant-update of intensive care medicine. In Update of Intensive Care Medicine; Nakos, G., Lekka, M., Eds.; University of Ioannina: Ioannina, Greece, 2002; Volume 6, pp. 87–102. [Google Scholar]
- LeVine, A.M.; Kurak, K.E.; Wright, J.R.; Watford, W.T.; Bruno, M.D.; Ross, G.F.; Whitsett, J.A.; Korfhagen, T.R. Surfactant Protein-A Binds Group B Streptococcus Enhancing Phagocytosis and Clearance from Lungs of Surfactant Protein-A–Deficient Mice. Am. J. Respir. Cell Mol. Biol. 1999, 20, 279–286. [Google Scholar] [CrossRef] [Green Version]
- LeVine, A.M.; Gwozdz, J.; Stark, J.; Bruno, M.; Whitsett, J.; Korfhagen, T. Surfactant protein-A enhances respiratory syncytial virus clearance in vivo. J. Clin. Investig. 1999, 103, 1015–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koptides, M.; Umstead, T.M.; Floros, J.; Phelps, D.S. Surfactant protein A activates NF-kappa B in the THP-1 monocytic cell line. Am. J. Physiol. Lung Cell. Mol. Physiol. 1997, 273, L382–L388. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Phelps, D.S. Comparison of SP-A and LPS effects on the THP-1 monocytic cell line. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L110–L117. [Google Scholar] [CrossRef] [PubMed]
- Floros, J.; Wang, G.; Mikerov, A.N. Genetic complexity of the human innate host defense molecules, surfactant protein A1 (SP-A1) and SP-A2--impact on function. Crit. Rev. Eukaryot. Gene Exp. 2009, 19, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Quintero, O.A.; Korfhagen, T.R.; Wright, J.R. Surfactant protein A regulates surfactant phospholipid clearance after LPS-induced injury in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L76–L85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Gil, J. Structure of pulmonary surfactant membranes and films: The role of proteins and lipid–protein interactions. Biochim. Et Biophys. Acta (BBA) Biomembr. 2008, 1778, 1676–1695. [Google Scholar] [CrossRef] [Green Version]
- Tonks, A.; Parton, J.; Tonks, A.J.; Morris, R.H.K.; Finall, A.; Jones, K.P.; Jackson, S.K. Surfactant phospholipid DPPC downregulates monocyte respiratory burst via modulation of PKC. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L1070–L1080. [Google Scholar] [CrossRef] [Green Version]
- Frump, A.L.; Lahm, T. Sex hormone signaling in the lung in health and disease: Airways, parenchyma, and pulmonary vasculature. In Gender, Sex Hormones and Respiratory Disease; Springer: Berlin, Germany, 2016; pp. 27–62. [Google Scholar]
- Taneja, V. Sex Hormones Determine Immune Response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Zurfluh, S.; Nickler, M.; Ottiger, M.; Steuer, C.; Kutz, A.; Christ-Crain, M.; Zimmerli, W.; Thomann, R.; Hoess, C.; Henzen, C.; et al. Dihydrotestosterone is a predictor for mortality in males with community-acquired pneumonia: Results of a 6-year follow-up study. Respir. Res. 2018, 19, 240. [Google Scholar] [CrossRef]
- Lauritzen, S.K.; Adams, W.C. Ozone inhalation effects consequent to continuous exercise in females: Comparison to males. J. Appl. Physiol. 1985, 59, 1601–1606. [Google Scholar] [CrossRef]
- Di, Q.; Wang, Y.; Zanobetti, A.; Wang, Y.; Koutrakis, P.; Choirat, C.; Dominici, F.; Schwartz, J.D. Air Pollution and Mortality in the Medicare Population. N. Engl. J. Med. 2017, 376, 2513–2522. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.P.; Sternberg, R.I.; Hull, W.; Buchsbaum, J.A.; Whitsett, J. Decreased surfactant protein A in patients with bacterial pneumonia. Am. Rev. Respir. Dis. 1993, 147, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Park, Y.R.; Kim, H.R.; Kim, H.-C.; Choi, C.-M. Prolonged effect of air pollution on pneumonia: A nationwide cohort study. Eur. Respir. J. 2017, 50, OA467. [Google Scholar] [CrossRef]
- Croft, D.P.; Zhang, W.; Lin, S.; Thurston, S.W.; Hopke, P.K.; Masiol, M.; Squizzato, S.; Van Wijngaarden, E.; Utell, M.J.; Rich, D.Q. The Association between Respiratory Infection and Air Pollution in the Setting of Air Quality Policy and Economic Change. Ann. Am. Thorac. Soc. 2019, 16, 321–330. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhi, C.K.; Mikerov, A.N.; Durrani, F.; Umstead, T.M.; Hu, S.; Wang, G.; Phelps, D.S.; Floros, J. Impact of Ozone, Sex, and Gonadal Hormones on Bronchoalveolar Lavage Characteristics and Survival in SP-A KO Mice Infected with Klebsiella pneumoniae. Microorganisms 2020, 8, 1354. https://doi.org/10.3390/microorganisms8091354
Gandhi CK, Mikerov AN, Durrani F, Umstead TM, Hu S, Wang G, Phelps DS, Floros J. Impact of Ozone, Sex, and Gonadal Hormones on Bronchoalveolar Lavage Characteristics and Survival in SP-A KO Mice Infected with Klebsiella pneumoniae. Microorganisms. 2020; 8(9):1354. https://doi.org/10.3390/microorganisms8091354
Chicago/Turabian StyleGandhi, Chintan K., Anatoly N. Mikerov, Faryal Durrani, Todd M. Umstead, Sanmei Hu, Guirong Wang, David S. Phelps, and Joanna Floros. 2020. "Impact of Ozone, Sex, and Gonadal Hormones on Bronchoalveolar Lavage Characteristics and Survival in SP-A KO Mice Infected with Klebsiella pneumoniae" Microorganisms 8, no. 9: 1354. https://doi.org/10.3390/microorganisms8091354
APA StyleGandhi, C. K., Mikerov, A. N., Durrani, F., Umstead, T. M., Hu, S., Wang, G., Phelps, D. S., & Floros, J. (2020). Impact of Ozone, Sex, and Gonadal Hormones on Bronchoalveolar Lavage Characteristics and Survival in SP-A KO Mice Infected with Klebsiella pneumoniae. Microorganisms, 8(9), 1354. https://doi.org/10.3390/microorganisms8091354





