Expansion of Necrosis Depending on Hybrid Motor-Driven Motility of Aeromonas hydrophila in a Murine Wound Infection Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria
2.2. Bioinformatics
2.3. Mutants Construction and Complementation
2.4. Cytotoxicity Assay
2.5. Motility Assay
2.6. Mice
2.7. Histopathological examination
2.8. Evaluation of Biomarkers
2.9. Bacterial Counts in Muscles and Spleen
2.10. Mortality Rate
2.11. Statistical Analysis
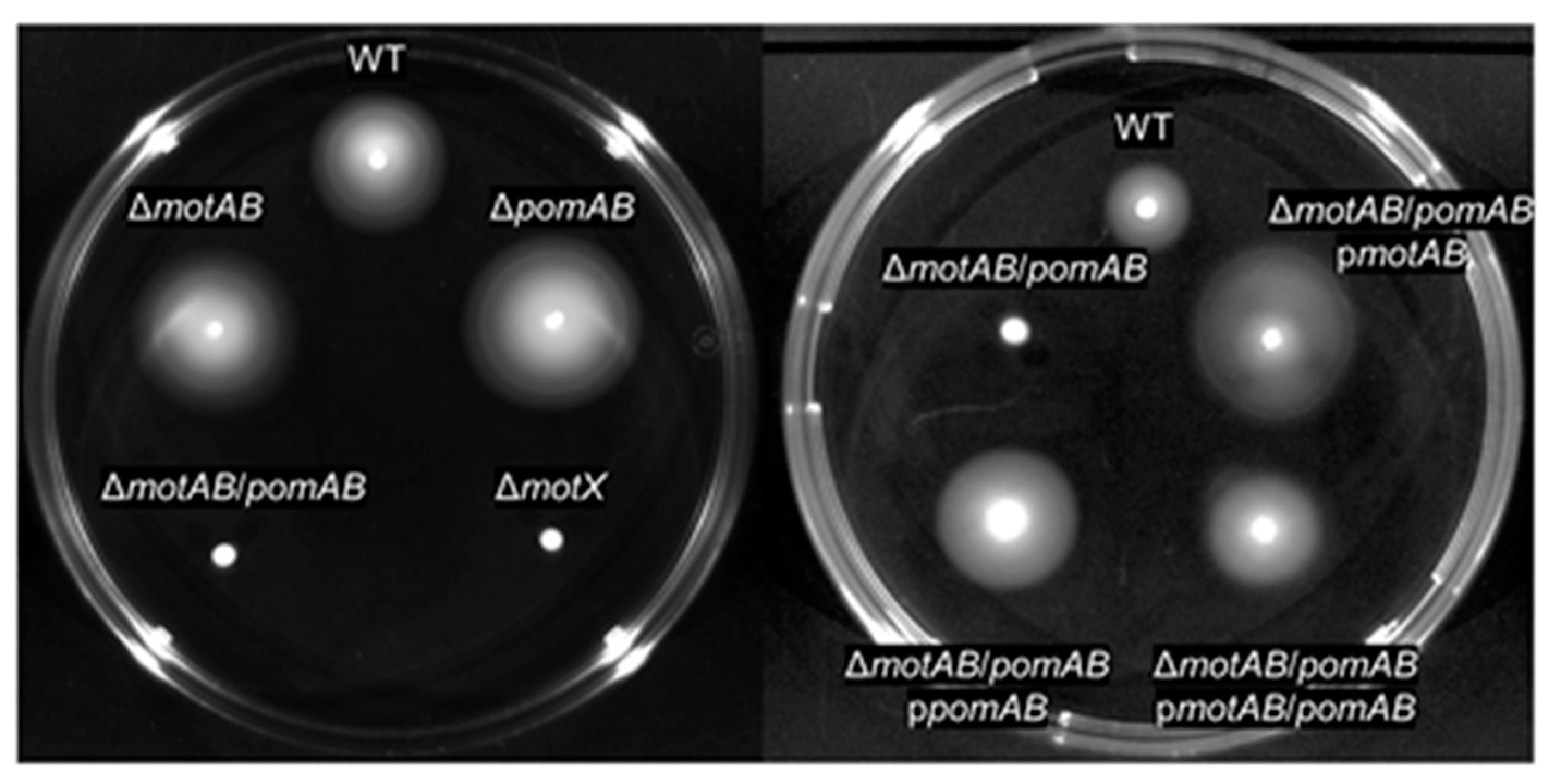
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernández-Bravo, A.; Figueras, M.J. An update on the genus Aeromonas: Taxonomy, epidemiology, and pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef]
- Parker, J.L.; Shaw, J.G. Aeromonas spp. clinical microbiology and disease. J. Infect. 2011, 62, 109–118. [Google Scholar] [CrossRef]
- Jahid, I.K.; Mizan, M.F.R.; Ha, A.J.; Ha, S.-D. Effect of salinity and incubation time of planktonic cells on biofilm formation, motility, exoprotease production, and quorum sensing of Aeromonas hydrophila. Food Microbiol. 2015, 49, 142–151. [Google Scholar] [CrossRef]
- Tomás, J.M. The main Aeromonas pathogenic factors. ISRN Microbiol. 2012, 256261. [Google Scholar] [CrossRef]
- Romero, A.; Saraceni, P.R.; Merino, S.; Figueras, A.; Tomás, J.M.; Novoa, B. The animal model determines the results of Aeromonas virulence factors. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, D.; Kozlova, E.V.; Sha, J.; Erova, T.E.; Azar, S.R.; Fitts, E.C.; Kirtley, M.L.; Tiner, B.L.; Andersson, J.A.; Grim, C.J.; et al. Cross-talk among flesh-eating Aeromonas hydrophila strains in mixed infection leading to necrotizing fasciitis. Proc. Natl. Acad. Sci. USA 2016, 113, 722. [Google Scholar] [CrossRef] [PubMed]
- Grim, C.J.; Kozlova, E.V.; Ponnusamy, D.; Fitts, E.C.; Sha, J.; Kirtley, M.L.; van Lier, C.J.; Tiner, B.L.; Erova, T.E.; Joseph, S.J.; et al. Functional genomic characterization of virulence factors from necrotizing fasciitis-causing strains of Aeromonas hydrophila. Appl. Environ. Microbiol. 2014, 80, 4162–4183. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-Y.; Peng, K.-T.; Hsu, W.-H.; Hung, C.-H.; Chuang, F.-Y.; Tsai, Y.-H. Independent Predictors of Mortality for Aeromonas Necrotizing Fasciitis of Limbs: An 18-year Retrospective Study. Sci. Rep. 2020, 10, 7716. [Google Scholar] [CrossRef] [PubMed]
- Lamy, B.; Kodjo, A.; colBVH Study Group; Laurent, F. Prospective Nationwide Study of Aeromonas Infections in France. J. Clin. Microbiol. 2009, 47, 1234–1237. [Google Scholar] [CrossRef]
- Hiransuthikul, N.; Tantisiriwat, W.; Lertutsahakul, K.; Vibhagool, A.; Boonma, P. Skin and Soft-Tissue Infections among Tsunami Survivors in Southern Thailand. Clin. Infect. Dis 2005, 41, e93–e96. [Google Scholar] [CrossRef] [PubMed]
- Presley, S.M.; Rainwater, T.R.; Austin, G.P.; Platt, S.G.; Zak, J.C.; Cobb, G.P.; Marsland, E.J.; Tian, K.; Zhang, B.; Anderson, T.A.; et al. Assessment of Pathogens and Toxicants in New Orleans, LA Following Hurricane Katrina. Environ. Sci. Technol. 2006, 40, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, T.W.; Kopari, N.M.; Pham, T.N.; Evans, H.L. Necrotizing soft tissue infections: Review and current concepts in treatment, systems of care, and outcomes. Curr. Probl. Surg. 2014, 51, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bryant, A.E. Necrotizing soft-tissue infections. N Engl. J. Med. 2017, 377, 2253–2265. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bravo, A.; Kilgore, P.B.; Andersson, J.A.; Blears, E.; Figueras, M.J.; Hasan, N.A.; Colwell, R.R.; Sha, J.; Chopra, A.K. T6SS and ExoA of flesh-eating Aeromonas hydrophila in peritonitis and necrotizing fasciitis during mono- and polymicrobial infections. Proc. Natl. Acad. Sci. USA 2019, 116, 24084–24092. [Google Scholar] [CrossRef]
- Terashima, H.; Kojima, S.; Homma, M. Chapter 2 Flagellar Motility in Bacteria: Structure and Function of Flagellar Motor. Int. Rev. Cell Mol. Biol. 2008, 270, 39–85. [Google Scholar]
- Berg, H.C. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 2003, 72, 19–54. [Google Scholar] [CrossRef]
- Minamino, T.; Imada, K.; Namba, K. Molecular motors of the bacterial flagella. Curr. Opin. Struct. Biol. 2008, 18, 693–701. [Google Scholar] [CrossRef]
- Nakamura, S.; Minamino, T. Flagella-driven motility of bacteria. Biomolecules 2019, 9. [Google Scholar] [CrossRef]
- Josenhans, C.; Suerbaum, S. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 2002, 291, 605–614. [Google Scholar] [CrossRef]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Kirov, S.M.; Castrisios, M.; Shaw, J.G. Aeromonas flagella (polar and lateral) are enterocyte adhesins that contribute to biofilm formation on surfaces. Infect. Immun. 2004, 72, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Rubires, X.; Aguilar, A.; Tomás, J.M. The role of flagella and motility in the adherence and invasion to fish cell lines by Aeromonas hydrophila serogroup O:34 strains. FEMS Microbiol Lett 1997, 151, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, Y.; Kitao, A. Gate-controlled proton diffusion and protonation-induced ratchet motion in the stator of the bacterial flagellar motor. Proc. Natl. Acad. Sci. USA 2015. [Google Scholar] [CrossRef]
- Yorimitsu, T.; Sato, K.; Asai, Y.; Kawagishi, I.; Homma, M. Functional Interaction between PomA and PomB, the Na+-driven flagellar motor components of Vibrio alginolyticus. J. Bacteriol. 1999, 181, 5103–5106. [Google Scholar] [CrossRef]
- Yorimitsu, T.; Homma, M. Na+-driven flagellar motor of Vibrio. Biochim. Biophys. Acta BBA Bioenerg. 2001, 1505, 82–93. [Google Scholar] [CrossRef]
- Yorimitsu, T.; Sowa, Y.; Ishijima, A.; Yakushi, T.; Homma, M. The systematic substitutions around the conserved charged residues of the cytoplasmic loop of Na+-driven flagellar motor component PomA. J. Mol. Biol. 2002, 320, 403–413. [Google Scholar] [CrossRef]
- Fukuoka, H.; Yakushi, T.; Homma, M. Concerted effects of amino acid substitutions in conserved charged residues and other residues in the cytoplasmic domain of PomA, a stator component of Na+-driven flagella. J. Bacteriol. 2004, 186, 6749–6758. [Google Scholar] [CrossRef]
- Wilhelms, M.; Vilches, S.; Molero, R.; Shaw, J.G.; Tomás, J.M.; Merino, S. Two redundant sodium-driven stator motor proteins are involved in Aeromonas hydrophila polar flagellum rotation. J. Bacteriol. 2009, 191, 2206–2217. [Google Scholar] [CrossRef]
- Simossis, V.A.; Heringa, J. PRALINE: A multiple sequence alignment toolbox that integrates homology-extended and secondary structure information. Nucleic Acids Res. 2005, 33, W289–W294. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kashimoto, T.; Kado, T.; Akeda, Y.; Yoshioka, K.; Kodama, T.; Yamamoto, M.; Okamura, M.; Kakuda, T.; Ueno, S. Chemotactic invasion in deep soft tissue by Vibrio vulnificus is essential for the progression of necrotic lesions. Virulence 2020, 11, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Paulick, A.; Delalez, N.J.; Brenzinger, S.; Steel, B.C.; Berry, R.M.; Armitage, J.P.; Thormann, K.M. Dual stator dynamics in the Shewanella oneidensis MR-1 flagellar motor. Mol. Microbiol. 2015, 96, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Paulick, A.; Koerdt, A.; Lassak, J.; Huntley, S.; Wilms, I.; Narberhaus, F.; Thormann, K.M. Two different stator systems drive a single polar flagellum in Shewanella oneidensis MR-1. Mol. Microbiol. 2009, 71, 836–850. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, T.; Terashima, H.; Kojima, S.; Homma, M. A conserved residue, PomB-F22, in the transmembrane segment of the flagellar stator complex, has a critical role in conducting ions and generating torque. Microbiol. Res. 2011, 157, 2422–2432. [Google Scholar] [CrossRef] [PubMed]
- Molero, R.; Wilhelms, M.; Infanzón, B.; Tomás, J.M.; Merino, S. Aeromonas hydrophila motY is essential for polar flagellum function, and requires coordinate expression of motX and Pom proteins. Microbiology 2011, 157, 2772–2784. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.J.; Swift, S.; Kirke, D.F.; Keevil, C.W.; Dodd, C.E.R.; Williams, P. The regulation of biofilm development by quorum sensing in Aeromonas hydrophila. Environ. Microbiol. 2002, 4, 18–28. [Google Scholar] [CrossRef]
- Kozlova, E.V.; Khajanchi, B.K.; Sha, J.; Chopra, A.K. Quorum sensing and c-di-GMP-dependent alterations in gene transcripts and virulence-associated phenotypes in a clinical isolate of Aeromonas hydrophila. Microb. Pathog. 2011, 50, 213–223. [Google Scholar] [CrossRef]
- Minamino, T.; Terahara, N.; Kojima, S.; Namba, K. Autonomous control mechanism of stator assembly in the bacterial flagellar motor in response to changes in the environment. Mol. Microbiol. 2018, 109, 723–734. [Google Scholar] [CrossRef]
- Chakraborty, T.; Huhle, B.; Hof, H.; Bergbauer, H.; Goebel, W. Marker exchange mutagenesis of the aerolysin determinant in Aeromonas hydrophila demonstrates the role of aerolysin in A. hydrophila-associated systemic infections. Infect. Immun. 1987, 55, 2274–2280. [Google Scholar] [CrossRef]
- Chopra, A.K.; Xu, X.-J.; Ribardo, D.; Gonzalez, M.; Kuhl, K.; Peterson, J.W.; Houston, C.W. The Cytotoxic enterotoxin of Aeromonas hydrophila induces proinflammatory cytokine production and activates arachidonic acid metabolism in macrophages. Infect. Immun. 2000, 68, 2808–2818. [Google Scholar] [CrossRef]
- Fadl, A.A.; Galindo, C.L.; Sha, J.; Erova, T.E.; Houston, C.W.; Olano, J.P.; Chopra, A.K. Deletion of the genes encoding the type III secretion system and cytotoxic enterotoxin alters host responses to Aeromonas hydrophila infection. Microb. Pathog. 2006, 40, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.D. The biology of Vibrio vulnificus. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Kashimoto, T.; Morita, M.; Kado, T.; Matsuda, K.; Yamasaki, M.; Ueno, S. identification of in vivo essential genes of Vibrio vulnificus for establishment of wound infection by Signature-tagged mutagenesis. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]




| Protein | Organism | Strain | Length | E Value | Identities | Positives | Interaction with FliG | Ion Channel Pore | Ion Binding | Torque Generation |
|---|---|---|---|---|---|---|---|---|---|---|
| MotA | A. hydrophila | RIMD111065 | 252 | - | - | - | Arg86 and Glu94 | - | - | Pro150 and Pro199 |
| A. hydrophila | ATCC7966 | 252 | 0.00E+00 | 252/252(100%) | 252/252(100%) | Arg86 and Glu94 | - | - | Pro150 and Pro199 | |
| A. piscicola | AH-3 | 252 | 9E−177 | 234/251(93%) | 240/251(95%) | Arg86 and Glu94 | Phe183 | Asn194 | Pro150 and Pro199 | |
| E. coli | K12 | 295 | 4.00E−16 | 55/210(26%) | 93/210(44%) | Arg90 and Glu98 | Met206 | Tyr217 | Pro173 and Pro222 | |
| S.typhimurium | LT2 | 295 | 3.00E−17 | 59/211(28%) | 96/211(45%) | Arg90 and Glu98 | Met206 | Tyr217 | Pro173 and Pro222 | |
| S. oneidensis | MR-1 | 243 | 3.00E−134 | 174/242(72%) | 207/242(85%) | Arg86 and Glu94 | - | - | Pro150 and Pro199 | |
| MotB | A. hydrophila | RIMD111065 | 291 | - | - | - | Asp21 | Ala29 | Asp21 | - |
| A. hydrophila | ATCC7966 | 291 | 0 | 291/291(100%) | 291/291(100%) | Asp21 | Ala29 | Asp21 | ||
| A. piscicola | AH-3 | 291 | 0 | 280/291(96%) | 285/291(97%) | Asp21 | Ala29 | Asp21 | ||
| E. coli | K12 | 308 | 3.00E−16 | 73/296(25%) | 135/296(45%) | Asp32 | Ala39 | Asp32 | ||
| S.typhimurium | LT2 | 309 | 3.00E−15 | 72/293(25%) | 124/293(42%) | Asp33 | Ala40 | Asp33 | ||
| S. oneidensis | MR-1 | 275 | 1.00E−95 | 133/289(46%) | 196/289(67%) | Asp21 | Ala28 | Asp21 | ||
| PomA | A. hydrophila | RIMD111065 | 252 | - | - | - | Arg88 and Glu96 | Leu183 | Asn194 | Pro151 and Pro199 |
| A. hydrophila | ATCC7966 | 252 | 0 | 252/252(100%) | 252/252(100%) | Arg88 and Glu96 | Leu183 | Asn194 | Pro151 and Pro199 | |
| A. piscicola | AH-3 | 245 | 8E−35 | 69/241(29%) | 127/241(52%) | Arg85 and Glu93 | - | Asn193 | pro150 and pro198 | |
| V. cholerae | O395 | 254 | 1.00E−116 | 164/247(66%) | 199/247(80%) | Arg88 and Glu96 | Leu183 | Asn194 | Pro151 and Pro199 | |
| V.vulnificus | CMCP6 | 253 | 3.00E−122 | 163/247(66%) | 200/247(80%) | Arg88 and Glu96 | Leu183 | Asn194 | Pro151 and Pro199 | |
| S. oneidensis | MR-1 | 255 | 6.00E−121 | 159/251(63%) | 201/251(80%) | Arg88 and Glu96 | Leu183 | Asn194 | Pro151 and Pro199 | |
| PomB | A. hydrophila | RIMD111065 | 299 | - | - | - | Asp20 | Cys27 | Asp20 | Phe18 |
| A. hydrophila | ATCC7966 | 299 | 0 | 299/299(100%) | 299/299(100%) | Asp20 | Cys27 | Asp20 | Phe18 | |
| A. piscicola | AH-3 | 303 | 2E−22 | 70/305(23%) | 124/305(40%) | Asp24 | - | Asp24 | - | |
| V. cholerae | O395 | 318 | 1.00E−134 | 182/302(60%) | 231/302(76%) | Asp23 | Cys30 | Asp23 | Phe21 | |
| V.vulnificus | CMCP6 | 318 | 4.00E−137 | 187/308(61%) | 234/308(75%) | Asp24 | Cys31 | Asp24 | Phe22 | |
| S. oneidensis | MR-1 | 308 | 9.00E−132 | 182/302(60%) | 222/302(73%) | Asp20 | Cys27 | Asp20 | Phe18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazaki, K.; Kashimoto, T.; Niwano, A.; Yamasaki, M.; Nomura, M.; Akeda, Y.; Ueno, S. Expansion of Necrosis Depending on Hybrid Motor-Driven Motility of Aeromonas hydrophila in a Murine Wound Infection Model. Microorganisms 2021, 9, 10. https://doi.org/10.3390/microorganisms9010010
Yamazaki K, Kashimoto T, Niwano A, Yamasaki M, Nomura M, Akeda Y, Ueno S. Expansion of Necrosis Depending on Hybrid Motor-Driven Motility of Aeromonas hydrophila in a Murine Wound Infection Model. Microorganisms. 2021; 9(1):10. https://doi.org/10.3390/microorganisms9010010
Chicago/Turabian StyleYamazaki, Kohei, Takashige Kashimoto, Ayuha Niwano, Moeko Yamasaki, Mayu Nomura, Yukihiro Akeda, and Shunji Ueno. 2021. "Expansion of Necrosis Depending on Hybrid Motor-Driven Motility of Aeromonas hydrophila in a Murine Wound Infection Model" Microorganisms 9, no. 1: 10. https://doi.org/10.3390/microorganisms9010010
APA StyleYamazaki, K., Kashimoto, T., Niwano, A., Yamasaki, M., Nomura, M., Akeda, Y., & Ueno, S. (2021). Expansion of Necrosis Depending on Hybrid Motor-Driven Motility of Aeromonas hydrophila in a Murine Wound Infection Model. Microorganisms, 9(1), 10. https://doi.org/10.3390/microorganisms9010010





