Intracellular Presence of Helicobacter pylori and Its Virulence-Associated Genotypes within the Vaginal Yeast of Term Pregnant Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Patients
2.3. Sample Processing
2.4. Search for Bacteria-Like Bodies (BLBs) within Vaginal Yeasts
2.5. Amplification of H. pylori Specific Genes from the DNA of Yeasts
2.6. Detection of H. pylori by Immunofluorescence
2.7. Amplification and Sequencing of 16S rDNA of H. pylori
2.8. Statistical Analysis
3. Results
3.1. Patients
3.2. Isolation and Identification of Yeasts and H. pylori
3.3. Detection of BLBs and H. pylori by Optical Microscopy
3.4. Relationship of Intracellular H. pylori with Age or Sexual Practices of Participants
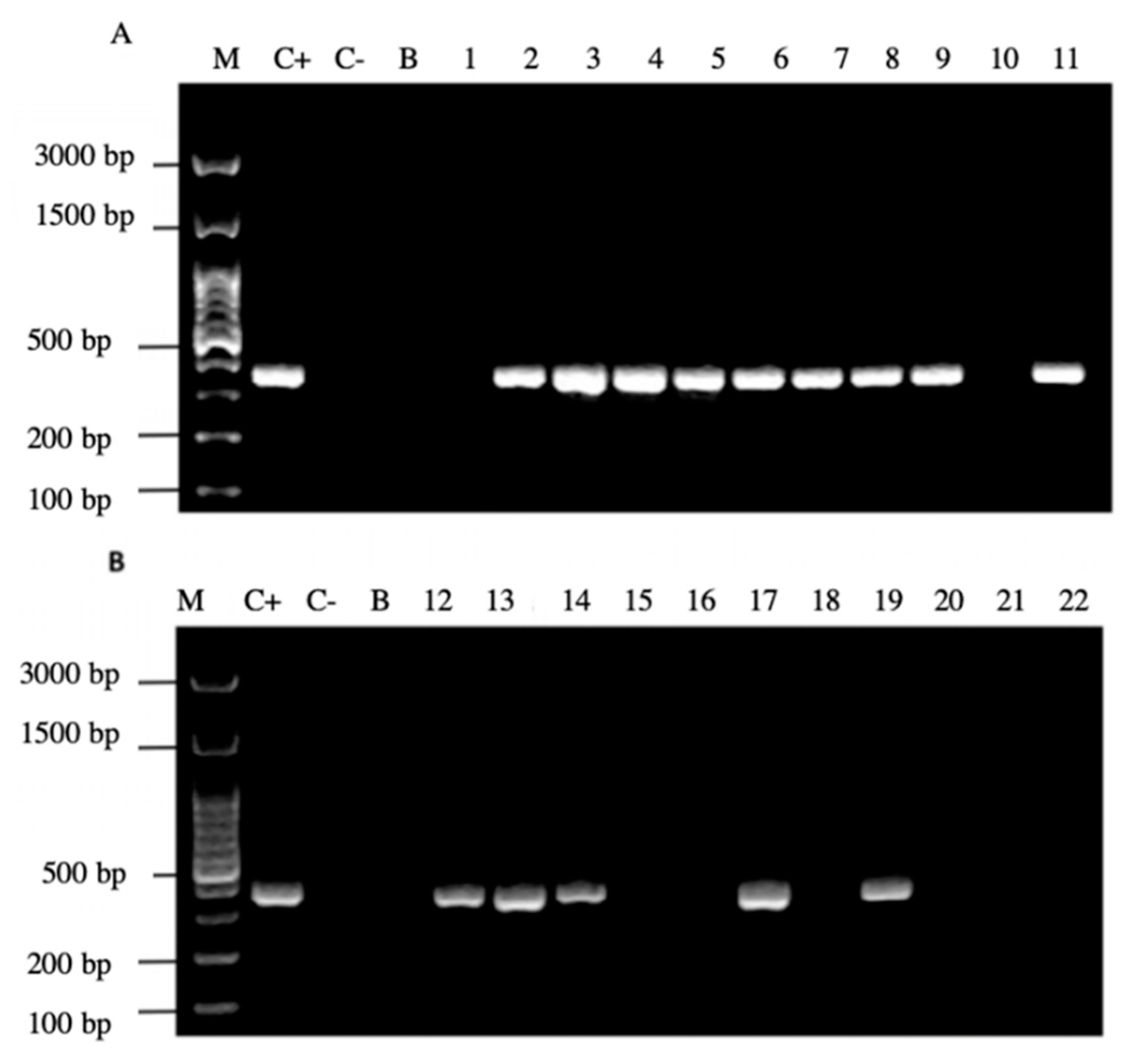
3.5. Amplification of H. pylori Specific Genes in Yeasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hemmatinezhad, B.; Momtaz, H.; Rahimi, E. VacA, cagA, iceA and oipA genotypes status and antimicrobial resistance properties of Helicobacter pylori isolated from various types of ready to eat foods. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M. Structure and function of Helicobacter pylori CagA, the first-identified bacterial protein involved in human cancer. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 196–219. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Sarkar, A.; Karmakar, B.C.; Ganguly, M.; Paul, S.; Mukhopadhyay, A.K. Novel virulence factor dupA of Helicobacter pylori as an important risk determinant for disease manifestation: An overview. World J. Gastroenterol. 2020, 26, 4739–4752. [Google Scholar] [CrossRef]
- Gilani, A.; Razavilar, V.; Rokni, N.; Rahimi, E. vacA and cagA genotypes status and antimicrobial resistance properties of Helicobacter pylori strains isolated from meat products in Isfahan province, Iran. Iran J. Vet. Res. 2017, 18, 97–102. [Google Scholar] [PubMed]
- Paredes-Osses, E.; Sáez, K.; Sanhueza, E.; Hebel, S.; González, C.; Briceño, C.; García Cancino, A. Association between cagA, vacAi, and dupA genes of Helicobacter pylori and gastroduodenal pathologies in Chilean patients. Folia Microbiol. 2017, 62, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Muhsen, K.; Cohen, D. Helicobacter pylori infection and iron stores: A systematic review and meta-analysis. Helicobacter 2008, 13, 323–340. [Google Scholar] [CrossRef]
- Gravina, A.; Federico, A.; Ruocco, E.; Lo Schiavo, A.; Masarone, M.; Tuccillo, C.; Peccerillo, F.; Miranda, A.; Romano, L.; de Sio, C.; et al. Helicobacter pylori infection but not small intestinal bacterial overgrowth may play a pathogenic role in rosacea. United Eur. Gastroenterol. J. 2015, 3, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Li, Y.; Huang, L.Y.; Guan, Q.K.; Xu, D.W.; Zhou, W.K.; Zhang, X.Z. Helicobacter pylori infection contributes to high risk of ischemic stroke: Evidence from a meta-analysis. J. Neurol. 2012, 259, 2527–2537. [Google Scholar] [CrossRef] [PubMed]
- Cardaropoli, S.; Rolfo, A.; Todros, T. Helicobacter pylori and pregnancy-related disorders. World J. Gastroenterol. 2014, 20, 654–664. [Google Scholar] [CrossRef]
- Abo-Amer, Y.E.-E.; Sabal, A.; Ahmed, R.; Hasan, N.F.E.; Refaie, R.; Mostafa, S.M.; Mohamed, A.A.; Khalil, M.; Elagawy, W.; Abd-Elsalam, S. Relationship between Helicobacter pylori Infection and Nonalcoholic Fatty Liver Disease (NAFLD) in a Developing Country: A Cross-Sectional Study. Diabetes Metab. Syndr. Obes. 2020, 13, 619–625. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, H.; Liu, X.; Ding, C. The Relationship between Helicobacter pylori Infection and Open-Angle Glaucoma: A Meta-Analysis. Invesig. Ophthalmol. Vis. Sci. 2015, 56, 5238–5245. [Google Scholar] [CrossRef] [PubMed]
- Farhadkhani, M.; Nikaeen, M.; Hassanzadeh, A.; Nikmanesh, B. Potential transmission sources of Helicobacter pylori infection: Detection of H. pylori in various environmental samples. J. Environ. Health Sci. Eng. 2019, 17, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Siavoshi, F.; Saniee, P. Vacuoles of Candida yeast as a specialized niche for Helicobacter pylori. World J. Gastroenterol. 2014, 20, 5263–5273. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Bonfante, P. Identification of a Putative P-Transporter Operon in the Genome of a Burkholderia Strain Living inside the Arbuscular Mycorrhizal Fungus Gigaspora margarita. J. Bacteriol. 1999, 181, 4106–4109. [Google Scholar] [CrossRef]
- Dubois, A.; Borén, T. Helicobacter pylori is invasive and it may be a facultative intracellular organism. Cell Microbiol. 2007, 9, 1108–1116. [Google Scholar] [CrossRef]
- Petersen, A.M.; Krogfelt, K.A. Helicobacter pylori: An invading microorganism? A review. FEMS Immunol. Med. Microbiol. 2003, 36, 117–126. [Google Scholar] [CrossRef]
- Noach, L.A.; Rolf, T.M.; Tytgat, G.N. Electron microscopic study of association between Helicobacter pylori and gastric and duodenal mucosa. J. Clin. Pathol. 1994, 47, 699–704. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Wu, J.-J.; Lei, H.-Y. The autophagic induction in Helicobacter pylori-infected macrophage. Exp. Biol. Med. 2009, 234, 171–180. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Gorvel, J.-P.; Chu, Y.-T.; Wu, J.-J.; Lei, H.-Y. Helicobacter pylori impairs murine dendritic cell responses to infection. PLoS ONE 2010, 5, e10844. [Google Scholar] [CrossRef]
- Wyle, F.; Tarnawski, A.; Schulman, D.; Dąbroś, W. Evidence for Gastric Mucosal Cell Invasion by C. pylori: An Ultrastructural Study. J. Clin. Gastroenterol. 1990, 92–98. [Google Scholar] [CrossRef]
- Chu, Y.-T.; Wang, Y.-H.; Wu, J.-J.; Lei, H.-Y. Invasion and Multiplication of Helicobacter pylori in Gastric Epithelial Cells and Implications for Antibiotic Resistance. Infect. Immun. 2010, 78, 4157–4165. [Google Scholar] [CrossRef] [PubMed]
- Gasch, A.P.; Werner-Washburne, M. The genomics of yeast responses to environmental stress and starvation. Funct. Integr. Genom. 2002, 2, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Soll, D.R. Candida commensalism and virulence: The evolution of phenotypic plasticity. Acta Trop. 2002, 81, 101–110. [Google Scholar] [CrossRef]
- Odds, F.C. Candida infections: An overview. Crit. Rev. Microbiol. 1987, 15, 1–5. [Google Scholar] [CrossRef]
- Soll, D.R.; Galask, R.; Schmid, J.; Hanna, C.; Mac, K.; Morrow, B. Genetic dissimilarity of commensal strains of Candida spp. carried in different anatomical locations of the same healthy women. J. Clin. Microbiol. 1991, 29, 1702–1710. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.K.; Whitehead, R. Campylobacter-like organisms and Candida in peptic ulcers and similar lesions of the upper gastrointestinal tract: A study of 247 cases. J. Clin. Pathol. 1988, 41, 1093–1098. [Google Scholar] [CrossRef]
- Ansorg, R.; Schmid, E.N. Adhesion of Helicobacter pylori to yeast cells. Zent. Bakteriol. 1998, 288, 501–508. [Google Scholar] [CrossRef]
- Porras, C.; Nodora, J.; Sexton, R.; Ferreccio, C.; Jimenez, S.; Dominguez, R.L.; Cook, P.; Anderson, G.; Morgan, D.R.; Baker, L.H.; et al. Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701). Cancer Causes Control. 2013, 24, 209–215. [Google Scholar] [CrossRef]
- Payão, S.L.M.; Rasmussen, L.T. Helicobacter pylori and its reservoirs: A correlation with the gastric infection. World J. Gastrointest. Pharm. Ther. 2016, 7, 126–132. [Google Scholar] [CrossRef]
- Siavoshi, F.; Taghikhani, A.; Malekzadeh, R.; Sarrafnejad, A.; Kashanian, M.; Jamal, A.S.; Saniee, P.; Sadeghi, S.; Sharifi, A.H. The role of mother’s oral and vaginal yeasts in transmission of Helicobacter pylori to neonates. Arch. Iran. Med. 2013, 16, 288–294. [Google Scholar]
- Burucoa, C.; Axon, A. Epidemiology of Helicobacter pylori infection. Helicobacter 2017, 22 (Suppl. 1). [Google Scholar] [CrossRef] [PubMed]
- Matamala-Valdés, L.; Sánchez-Alonzo, K.; Parra, C.; Sáez, K.; Aguayo-Reyes, A.; García, A. Detection of intracellular Helicobacter pylori in Candida. spp from neonate oral swabs. Rev. Assoc. Med. Bras. 2018, 64, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alonzo, K.; Parra-Sepúlveda, C.; Vega, S.; Bernasconi, H.; Campos, V.L.; Smith, C.T.; Sáez, K.; García-Cancino, A. In Vitro Incorporation of Helicobacter pylori into Candida albicans Caused by Acidic pH Stress. Pathogens 2020, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Yarza, P.; Richter, M.; Peplies, J.; Euzeby, J.; Amann, R.; Schleifer, K.-H.; Ludwig, W.; Glöckner, F.O.; Rosselló-Móra, R. The All-Species Living Tree project: A 16S rRNA-based phylogenetic tree of all sequenced type strains. Syst. Appl. Microbiol. 2008, 31, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, W.; Strunk, O.; Westram, R.; Richter, L.; Meier, H.; Yadhukumar; Buchner, A.; Lai, T.; Steppi, S.; Jobb, G.; et al. ARB: A software environment for sequence data. Nucleic Acids Res. 2004, 32, 1363–1371. [Google Scholar] [CrossRef]
- Ferreccio, C.; Rollán, A.; Harris, P.R.; Serrano, C.; Gederlini, A.; Margozzini, P.; Gonzalez, C.; Aguilera, X.; Venegas, A.; Jara, A. Gastric cancer is related to early Helicobacter pylori infection in a high-prevalence country. Cancer Epidemiol. Biomark. Prev. 2007, 16, 662–667. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Gerhard, M.; Gao, J.-J.; Mejias-Luque, R.; Zhang, L.; Vieth, M.; Ma, J.-L.; Bajbouj, M.; Suchanek, S.; et al. Effect of Helicobacter pylori on gastrointestinal microbiota: A population-based study in Linqu, a high-risk area of gastric cancer. Gut 2020, 69, 1598–1607. [Google Scholar] [CrossRef]
- Rodrigues, M.F.; Guerra, M.R.; de Alvarenga, A.V.R.; de Oliveira Souza, D.Z.; e Silva Costa, R.A.V.; Cupolilo, S.M.N. Helicobacter pylori infection and gastric cancer precursor lesions: Prevalence and associated factors in a reference laboratory in southeastern brazil. Arq. Gastroenterol. 2019, 56, 419–424. [Google Scholar] [CrossRef]
- Agin, M.; Batun, I.; Ozdemir, S.; Doran, F.; Tumgor, G. Prevalence of Helicobacter pylori in Turkish children with celiac disease and its effect on clinical, histopathological, and laboratory parameters. Arch. Med. Sci. 2019, 15, 1475–1481. [Google Scholar] [CrossRef]
- Okushin, K.; Tsutsumi, T.; Ikeuchi, K.; Kado, A.; Enooku, K.; Fujinaga, H.; Moriya, K.; Yotsuyanagi, H.; Koike, K. Helicobacter pylori infection and liver diseases: Epidemiology and insights into pathogenesis. World J. Gastroenterol. 2018, 24, 3617–3625. [Google Scholar] [CrossRef]
- Wongphutorn, P.; Chomvarin, C.; Sripa, B.; Namwat, W.; Faksri, K. Detection and genotyping of Helicobacter pylori in saliva versus stool samples from asymptomatic individuals in Northeastern Thailand reveals intra-host tissue-specific H. pylori subtypes. BMC Microbiol. 2018, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Batioglu-Karaaltin, A.; Saatci, O.; Akpinar, M.; Celik, M.O.; Develioglu, O.; Yigit, O.; Külekçi, M.; Akarsubaşı, A.T. Helicobacter pylori in lacrimal secretions. Ear Nose Throat J. 2016, 95, E8–E11. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.; Bernabe, L.; Castaneda, C.A.; Chavez, I.; Ruiz, E.; Barreda, F.; Valdivia, D.; Suarez, N.; Nieves, J.; Dias-Neto, E.; et al. Helicobacter pylori Detected in Tap Water of Peruvian Patients with Gastric Cancer. Asian Pac. J. Cancer Prev. 2019, 20, 3193–3196. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, V.; Recordati, C.; Borella, L.; Gualdi, V.; Scanziani, E.; Selvatico, E.; Luini, M. Helicobacteraceae in Bulk Tank Milk of Dairy Herds from Northern Italy. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Gheisari, E.; Dehkordi, F.S. Genotyping of vacA alleles of Helicobacter pylori strains recovered from some Iranian food items. Trop. J. Pharm. Res. 2016, 15, 1631–1636. [Google Scholar] [CrossRef]
- O’Ryan, M.L.; Lucero, Y.; Rabello, M.; Mamani, N.; Salinas, A.M.; Peña, A.; Torres-Torreti, J.P.; Mejías, A.; Ramilo, O.; Suarez, N.; et al. Persistent and transient Helicobacter pylori infections in early childhood. Clin. Infect. Dis. 2015, 61, 211–218. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Nimrichter, L.; Oliveira, D.L.; Frases, S.; Miranda, K.; Zaragoza, O.; Alvarez, M.; Nakouzi, A.; Feldmesser, M.; Casadevall, A. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot. Cell 2007, 6, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.; Khanam, R.A.; Basher, M.S.; Azam, M.S.; Hossain, M.A.; Mirza, T.T.; Banu, K.A.; Karmoker, R.K. Helicobacter pylori Stool Antigen Assay in Hyperemesis Gravidarum. Mymensingh Med. J. 2017, 26, 250–255. [Google Scholar]
- Poveda, G.F.; Carrillo, K.S.; Monje, M.E.; Cruz, C.A.; Cancino, A.G. Helicobacter pylori infection and gastrointestinal symptoms on Chilean pregnant women. Rev. Assoc. Med. Bras. 2014, 60, 306–310. [Google Scholar] [CrossRef][Green Version]
- Grooten, I.J.; Den Hollander, W.J.; Roseboom, T.J.; Kuipers, E.J.; Jaddoe, V.W.; Gaillard, R.; Painter, R.C. Helicobacter pylori infection: A predictor of vomiting severity in pregnancy and adverse birth outcome. Am. J. Obstet. Gynecol. 2017, 216, 512. [Google Scholar] [CrossRef]
- Mubarak, N.; Gasim, G.I.; Khalafalla, K.E.; Ali, N.I.; Adam, I. Helicobacter pylori, anemia, iron deficiency and thrombocytopenia among pregnant women at Khartoum, Sudan. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Sekhar Goud, E.V.S.; Kannan, R.; Rao, U.K.; Joshua, E.; Tavaraja, R.; Jain, Y. Identification of Helicobacter pylori in Saliva of Patients with and without Gastritis by Polymerase Chain Reaction. J. Pharm. Bioallied Sci. 2019, 11, S523–S529. [Google Scholar] [CrossRef] [PubMed]
- Nash, A.K.; Auchtung, T.A.; Wong, M.C.; Smith, D.P.; Gesell, J.R.; Ross, M.C.; Stewart, C.J.; Metcalf, G.A.; Muzny, D.M.; Gibbs, R.A.; et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.P.; Siwicki, A.K. Basic Hematology and Serology for Fish Health Programs; Asian Fisheries Society: Manila, Philippines, 1995; pp. 185–202. [Google Scholar]
- Pich, O.Q.; Merrell, D.S. The ferric uptake regulator of Helicobacter pylori: A critical player in the battle for iron and colonization of the stomach. Future Microbiol. 2013, 8, 725–738. [Google Scholar] [CrossRef]
- Basmaciyan, L.; Bon, F.; Paradis, T.; Lapaquette, P.; Dalle, F. Candida albicans Interactions with the Host: Crossing the Intestinal Epithelial Barrier. Tissue Barriers 2019, 7, 1612661. [Google Scholar] [CrossRef]
- Prince, A.L.; Chu, D.M.; Seferovic, M.D.; Antony, K.M.; Ma, J.; Aagaard, K.M. The Perinatal Microbiome and Pregnancy: Moving Beyond the Vaginal Microbiome. Cold Spring Harb. Perspect. Med. 2015, 5, a023051. [Google Scholar] [CrossRef]
- Dimitriadi, D. Helicobacter pylori: A sexually transmitted bacterium? Cent. Eur. J. Urol. 2014, 67, 407–409. [Google Scholar] [CrossRef]
- Eslick, G. Helicobacter pylori infection transmitted sexually via oral-genital contact: A hypothetical model. Sex. Transm. Infect. 2000, 76, 489–492. [Google Scholar] [CrossRef]
- Minakami, H.; Hayashi, M.; Sato, I. Does Helicobacter pylori colonize the vagina of pregnant women? J. Infect. 2000, 41, 112–113. [Google Scholar] [CrossRef]
- Shimoyama, T.; Fukuda, S.; Tanaka, M.; Mikami, T.; Munakata, A.; Crabtree, J.E. CagA seropositivity associated with development of gastric cancer in a Japanese population. J. Clin. Pathol. 1998, 51, 225–228. [Google Scholar] [CrossRef]
- Best, A.; Price, C.; Ozanic, M.; Santic, M.; Jones, S.; Kwaik, Y.A. A Legionella pneumophila amylase is essential for intracellular replication in human macrophages and amoebae. Sci. Rep. 2018, 8, 6340. [Google Scholar] [CrossRef] [PubMed]
- Ogiwara, H.; Sugimoto, M.; Ohno, T.; Vilaichone, R.-K.; Mahachai, V.; Graham, D.; Yamaoka, Y. Role of deletion located between the intermediate and middle regions of the Helicobacter pylori vacA Gene in Cases of Gastroduodenal Diseases. J. Clin. Microbiol. 2009, 47, 3493–3500. [Google Scholar] [CrossRef] [PubMed]
- Atherton, J.C.; Cao, P.; Peek, R.M.; Tummuru, M.K.; Blaser, M.J.; Cover, T.L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 1995, 270, 17771–17777. [Google Scholar] [CrossRef] [PubMed]
- Yadegar, A.; Mohabati Mobarez, A.; Zali, M.R. Genetic diversity and amino acid sequence polymorphism in Helicobacter pylori CagL hypervariable motif and its association with virulence markers and gastroduodenal diseases. Cancer Med. 2019, 8, 1619–1632. [Google Scholar] [CrossRef]
- Ricci, V.; Sommi, P.; Boquet, P. 19—Helicobacter pylori vacuolating toxin. In The Comprehensive Sourcebook of Bacterial Protein Toxins, 4th ed.; Alouf, J., Ladant, D., Popoff, M.R., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 515–557. ISBN 978-0-12-800188-2. [Google Scholar]
- Capurro, M.I.; Greenfield, L.K.; Prashar, A.; Xia, S.; Abdullah, M.; Wong, H.; Zhong, X.Z.; Bertaux-Skeirik, N.; Chakrabarti, J.; Siddiqui, I.; et al. VacA generates a protective intracellular reservoir for Helicobacter pylori that is eliminated by activation of the lysosomal calcium channel TRPML1. Nat. Microbiol. 2019, 4, 1411–1423. [Google Scholar] [CrossRef]
- Satin, B.; Norais, N.; Telford, J.; Rappuoli, R.; Murgia, M.; Montecucco, C.; Papini, E. Effect of Helicobacter pylori vacuolating toxin on maturation and extracellular release of procathepsin D and on epidermal growth factor degradation. J. Biol. Chem. 1997, 272, 25022–25028. [Google Scholar] [CrossRef]
- Tavakolian, A.; Siavoshi, F.; Eftekhar, F. Candida albicans release intracellular bacteria when treated with Amphotericin B. Arch. Iran. Med. 2018, 21, 191–198. [Google Scholar]
- Siavoshi, F.; Sahraee, M.; Ebrahimi, H.; Sarrafnejad, A.; Saniee, P. Natural fruits, flowers, honey, and honeybees harbor Helicobacter pylori-positive yeasts. Helicobacter 2018, 23, e12471. [Google Scholar] [CrossRef]
- Saniee, P.; Siavoshi, F.; Nikbakht Broujeni, G.; Khormali, M.; Sarrafnejad, A.; Malekzadeh, R. Localization of H. pylori within the vacuole of Candida yeast by direct immunofluorescence technique. Arch. Iran. Med. 2013, 16, 705–710. [Google Scholar]
- Heydari, S.; Siavoshi, F.; Ebrahimi, H.; Sarrafnejad, A.; Sharifi, A.H. Excision of endosymbiotic bacteria from yeast under aging and starvation stresses. Infect. Genet. Evol. 2020, 78, 104141. [Google Scholar] [CrossRef]







| Gene | Region | Sequence | Tm °C | Base Pairs (amplicon) | Reference |
|---|---|---|---|---|---|
| 16S rRNA | F-5′CTCGAGAGACTAAGCCCTCC-3′ R-5′ATTACTGACGCTGATGTGC-3′ | 53 | 110 | [5] | |
| cagA | F-5′GATAACAGGCAAGCTTTTGAGG-3′ R-5′CTGCAAAAGATTGTTTGGCAGA-3′ | 55 | 349 | [32] | |
| vacA | s1a | F-5′-GTCAGCATCACACCGCAA-3′ R-5′-CTGCTTGAATGCGCCAAAC-3′ | 55 | 190 | [33] |
| vacA | s1b | F-5′AGCGCCATACCGCAAGAG-3′ R-5′-CTGCTTGAATGCGCCAAAC-3′ | 55 | 187 | [33] |
| vacA | s2 | F-5′-GCTAACACGCCAAATGATCC-3′ R-5′-CTGCTTGAATGCGCCAAAC-3′ | 55 | 199 | [33] |
| vacA | m1 | F-5′-GGTCAAAATGCGGTCATGG-3′ R-5′-CCATTGGTACCTGTAGAAAC-3′ | 50 | 290 | [33] |
| vacA | m2 | F-5′-GGAGCCCCAGGAAACATTG-3′ R-5′-CATAACTAGCGCCTTGCAC-3′ | 55 | 352 | [33] |
| dupA | F-5′-ACAAGGACGATTGAGCGATGG-3′ R-5′-TGGCTAGTTTGAGGTCTTAGG-3′ | 61 | 515 | [5] |
| Negative for Intrayeast H. pylori | Positive for Intrayeast H. pylori | |
|---|---|---|
| Age (years) | % | % |
| 14–20 | 88% | 12% |
| 21–27 | 85% | 15% |
| 28–34 | 70% | 30% |
| 35–44 | 67% | 33% |
| Family Health Center | Number of Vaginal Discharge Samples | Percentage of Samples Positive for Presence of Yeasts |
|---|---|---|
| O’Higgins | 24 | 14% (6/44) |
| Tucapel | 16 | 11% (5/44) |
| Dr. Víctor Manuel Fernández | 62 | 75% (33/44) |
| Total | 102 | 100% |
| Women Negative for Intrayeast H. pylori | Women Positive for Intrayeast H. pylori | |||||
|---|---|---|---|---|---|---|
| Sexual Practice | Answer | n | % | n | % | p Value |
| Anal sex | No | 73 | 78% | 21 | 22% | 0.5159 |
| Yes | 7 | 88% | 1 | 13% | ||
| Oral sex | No | 68 | 77% | 20 | 23% | 0.4757 |
| Yes | 12 | 86% | 2 | 14% | ||
| Genotype | Frequency | Percentage (%) |
|---|---|---|
| cagA+, vacAs1a/m1, dupA− | 7 | 32 |
| cagA+, vacAs1a, dupA− | 5 | 23 |
| cagA+-, vacA−, dupA− | 1 | 5 |
| cagA+, vacAm1, dupA− | 1 | 5 |
| cagA−, vacAs1a, dupA− | 8 | 35 |
| Total | 22 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Alonzo, K.; Matamala-Valdés, L.; Parra-Sepúlveda, C.; Bernasconi, H.; Campos, V.L.; Smith, C.T.; Sáez, K.; García-Cancino, A. Intracellular Presence of Helicobacter pylori and Its Virulence-Associated Genotypes within the Vaginal Yeast of Term Pregnant Women. Microorganisms 2021, 9, 131. https://doi.org/10.3390/microorganisms9010131
Sánchez-Alonzo K, Matamala-Valdés L, Parra-Sepúlveda C, Bernasconi H, Campos VL, Smith CT, Sáez K, García-Cancino A. Intracellular Presence of Helicobacter pylori and Its Virulence-Associated Genotypes within the Vaginal Yeast of Term Pregnant Women. Microorganisms. 2021; 9(1):131. https://doi.org/10.3390/microorganisms9010131
Chicago/Turabian StyleSánchez-Alonzo, Kimberly, Lillian Matamala-Valdés, Cristian Parra-Sepúlveda, Humberto Bernasconi, Víctor L. Campos, Carlos T. Smith, Katia Sáez, and Apolinaria García-Cancino. 2021. "Intracellular Presence of Helicobacter pylori and Its Virulence-Associated Genotypes within the Vaginal Yeast of Term Pregnant Women" Microorganisms 9, no. 1: 131. https://doi.org/10.3390/microorganisms9010131
APA StyleSánchez-Alonzo, K., Matamala-Valdés, L., Parra-Sepúlveda, C., Bernasconi, H., Campos, V. L., Smith, C. T., Sáez, K., & García-Cancino, A. (2021). Intracellular Presence of Helicobacter pylori and Its Virulence-Associated Genotypes within the Vaginal Yeast of Term Pregnant Women. Microorganisms, 9(1), 131. https://doi.org/10.3390/microorganisms9010131








