Not Just Transporters: Alternative Functions of ABC Transporters in Bacillus subtilis and Listeria monocytogenes
Abstract
:1. Introduction
2. ABC Transporters Involved in Drug Export, Drug Sensing and Detoxification
2.1. The Multidrug Transporters BmrA and BmrCD
2.2. BceAB-BceRS Systems in B. subtilis and L. monocytogenes
3. ABC Transporters Affecting Cell Wall Biosynthesis and Remodeling
3.1. The YtrBCDEF Transporter of B. subtilis
3.2. The Putative ABC Transporter EslABC of L. monocytogenes
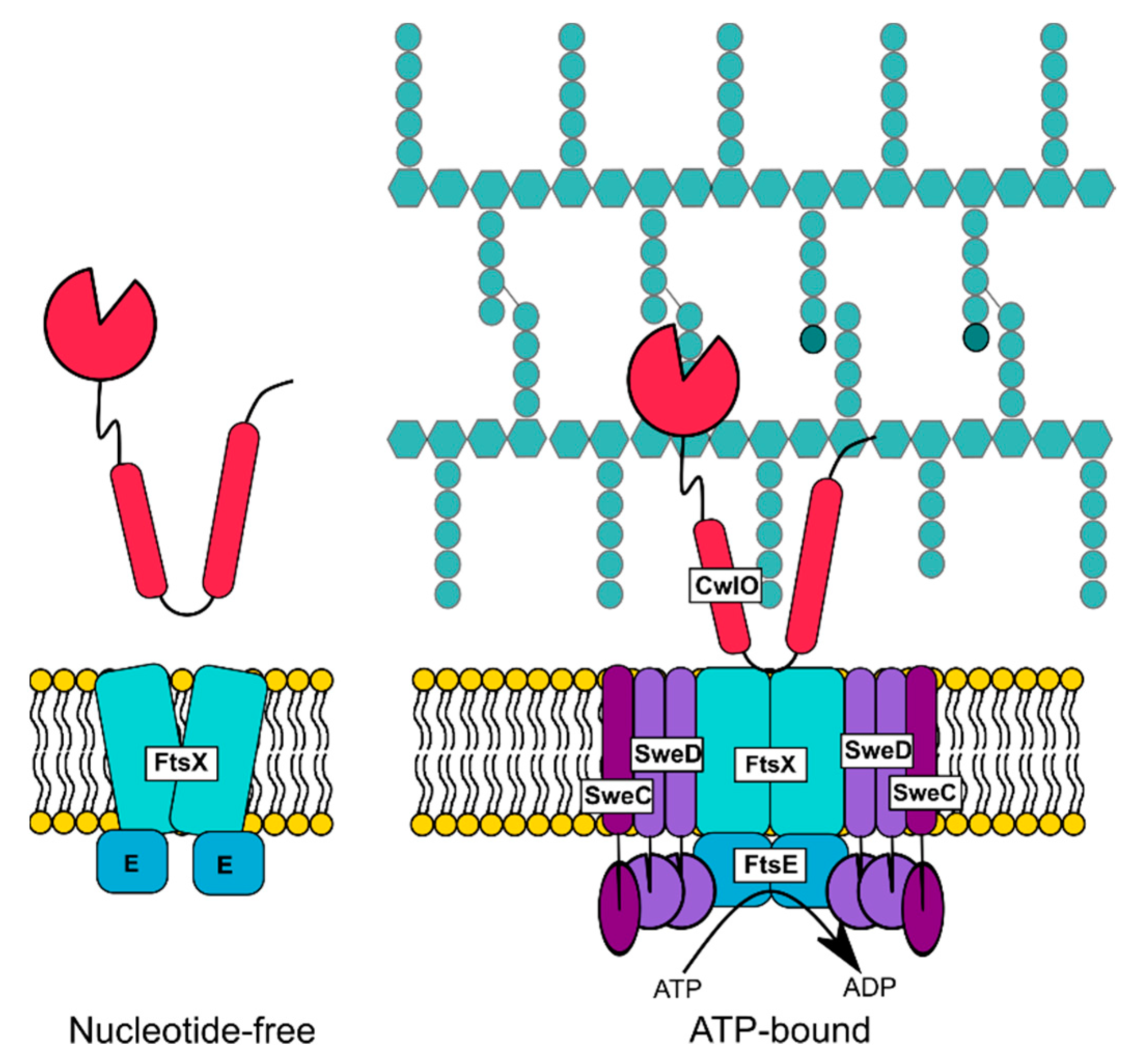
3.3. FtsEX of B. subtilis Regulates the d,l-endopeptidase CwlO
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fath, M.J.; Kolter, R. ABC transporters: Bacterial exporters. Microbiol. Rev. 1993, 57, 995–1017. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.J.; Song, S.; Mason, K.; Pinkett, H.W. Selective substrate uptake: The role of ATP-binding cassette (ABC) importers in pathogenesis. Biochim. Biophys. Acta Biomembr. 2018, 1860, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Gaballa, A.; Helmann, J.D. Identification of a Zinc-Specific Metalloregulatory Protein, Zur, Controlling Zinc Transport Operons in Bacillus subtilis. J. Bacteriol. 1998, 180, 5815–5821. [Google Scholar] [CrossRef] [Green Version]
- Lazarevic, V.; Karamata, D. The tagGH operon of Bacillus subtilis 168 encodes a two-component ABC transporter involved in the metabolism of two wall teichoic acids. Mol. Microbiol. 1995, 16, 345–355. [Google Scholar] [CrossRef]
- Chen, L.; Hou, W.-T.; Fan, T.; Liu, B.; Pan, T.; Li, Y.-H.; Jiang, Y.-L.; Wen, W.; Chen, Z.-P.; Sun, L.; et al. Cryo-electron Microscopy Structure and Transport Mechanism of a Wall Teichoic Acid ABC Transporter. MBio 2020, 11, e02749-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.; Santa Maria, J.P.; Walker, S. Wall Teichoic Acids of Gram-Positive Bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef] [Green Version]
- Dawson, R.J.P.; Locher, K.P. Structure of a bacterial multidrug ABC transporter. Nature 2006, 443, 180–185. [Google Scholar] [CrossRef]
- Locher, K.P. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat. Struct. Mol. Biol. 2016, 23, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Steinfels, E.; Orelle, C.; Fantino, J.-R.; Dalmas, O.; Rigaud, J.-L.; Denizot, F.; Di Pietro, A.; Jault, J.-M. Characterization of YvcC (BmrA), a Multidrug ABC Transporter Constitutively Expressed in Bacillus subtilis. Biochemistry 2004, 43, 7491–7502. [Google Scholar] [CrossRef]
- Davidson, A.L.; Dassa, E.; Orelle, C.; Chen, J. Structure, Function, and Evolution of Bacterial ATP-Binding Cassette Systems. Microbiol. Mol. Biol. Rev. 2008, 72, 317–364. [Google Scholar] [CrossRef] [Green Version]
- Slotboom, D.J. Structural and mechanistic insights into prokaryotic energy-coupling factor transporters. Nat. Rev. Microbiol. 2014, 12, 79–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meisner, J.; Montero Llopis, P.; Sham, L.-T.; Garner, E.; Bernhardt, T.G.; Rudner, D.Z. FtsEX is required for CwlO peptidoglycan hydrolase activity during cell wall elongation in Bacillus subtilis. Mol. Microbiol. 2013, 89, 1069–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalmas, O.; Do Cao, M.-A.; Lugo, M.R.; Sharom, F.J.; Di Pietro, A.; Jault, J.-M. Time-Resolved Fluorescence Resonance Energy Transfer Shows that the Bacterial Multidrug ABC Half-Transporter BmrA Functions as a Homodimer. Biochemistry 2005, 44, 4312–4321. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.; Domene, C.; Forest, E.; Jault, J.-M. Dynamics of a bacterial multidrug ABC transporter in the inward- and outward-facing conformations. Proc. Natl. Acad. Sci. USA 2012, 109, 10832–10836. [Google Scholar] [CrossRef] [Green Version]
- Lacabanne, D.; Orelle, C.; Lecoq, L.; Kunert, B.; Chuilon, C.; Wiegand, T.; Ravaud, S.; Jault, J.-M.; Meier, B.H.; Böckmann, A. Flexible-to-rigid transition is central for substrate transport in the ABC transporter BmrA from Bacillus subtilis. Commun. Biol. 2019, 2, 149. [Google Scholar] [CrossRef]
- Orelle, C.; Gubellini, F.; Durand, A.; Marco, S.; Lévy, D.; Gros, P.; Di Pietro, A.; Jault, J.-M. Conformational Change Induced by ATP Binding in the Multidrug ATP-Binding Cassette Transporter BmrA. Biochemistry 2008, 47, 2404–2412. [Google Scholar] [CrossRef]
- Wiegand, T.; Lacabanne, D.; Keller, K.; Cadalbert, R.; Lecoq, L.; Yulikov, M.; Terradot, L.; Jeschke, G.; Meier, B.H.; Böckmann, A. Solid-state NMR and EPR Spectroscopy of Mn 2+ -Substituted ATP-Fueled Protein Engines. Angew. Chem. Int. Ed. 2017, 56, 3369–3373. [Google Scholar] [CrossRef] [Green Version]
- Fribourg, P.F.; Chami, M.; Sorzano, C.O.S.; Gubellini, F.; Marabini, R.; Marco, S.; Jault, J.-M.; Lévy, D. 3D Cryo-Electron Reconstruction of BmrA, a Bacterial Multidrug ABC Transporter in an Inward-Facing Conformation and in a Lipidic Environment. J. Mol. Biol. 2014, 426, 2059–2069. [Google Scholar] [CrossRef]
- Krügel, H.; Licht, A.; Biedermann, G.; Petzold, A.; Lassak, J.; Hupfer, Y.; Schlott, B.; Hertweck, C.; Platzer, M.; Brantl, S.; et al. Cervimycin C resistance in Bacillus subtilis is due to a promoter up-mutation and increased mRNA stability of the constitutive ABC-transporter gene bmrA. FEMS Microbiol. Lett. 2010, 313, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Torres, C.; Galián, C.; Freiberg, C.; Fantino, J.-R.; Jault, J.-M. The YheI/YheH heterodimer from Bacillus subtilis is a multidrug ABC transporter. Biochim. Biophys. Acta Biomembr. 2009, 1788, 615–622. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Verhalen, B.; Stein, R.A.; Wen, P.-C.; Tajkhorshid, E.; Mchaourab, H.S. Conformational dynamics of the nucleotide binding domains and the power stroke of a heterodimeric ABC transporter. Elife 2014, 3, e02740. [Google Scholar] [CrossRef] [PubMed]
- Dezi, M.; Di Cicco, A.; Bassereau, P.; Levy, D. Detergent-mediated incorporation of transmembrane proteins in giant unilamellar vesicles with controlled physiological contents. Proc. Natl. Acad. Sci. USA 2013, 110, 7276–7281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutter, B.; Schaab, C.; Albrecht, S.; Borgmann, M.; Brunner, N.A.; Freiberg, C.; Ziegelbauer, K.; Rock, C.O.; Ivanov, I.; Loferer, H. Prediction of Mechanisms of Action of Antibacterial Compounds by Gene Expression Profiling. Antimicrob. Agents Chemother. 2004, 48, 2838–2844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.T.; Connelly, M.B.; Amolo, C.; Otani, S.; Yaver, D.S. Global Transcriptional Response of Bacillus subtilis to Treatment with Subinhibitory Concentrations of Antibiotics That Inhibit Protein Synthesis. Antimicrob. Agents Chemother. 2005, 49, 1915–1926. [Google Scholar] [CrossRef] [Green Version]
- Reilman, E.; Mars, R.A.T.; van Dijl, J.M.; Denham, E.L. The multidrug ABC transporter BmrC/BmrD of Bacillus subtilis is regulated via a ribosome-mediated transcriptional attenuation mechanism. Nucleic Acids Res. 2014, 42, 11393–11407. [Google Scholar] [CrossRef] [Green Version]
- Chumsakul, O.; Takahashi, H.; Oshima, T.; Hishimoto, T.; Kanaya, S.; Ogasawara, N.; Ishikawa, S. Genome-wide binding profiles of the Bacillus subtilis transition state regulator AbrB and its homolog Abh reveals their interactive role in transcriptional regulation. Nucleic Acids Res. 2011, 39, 414–428. [Google Scholar] [CrossRef]
- Nicolas, P.; Mader, U.; Dervyn, E.; Rochat, T.; Leduc, A.; Pigeonneau, N.; Bidnenko, E.; Marchadier, E.; Hoebeke, M.; Aymerich, S.; et al. Condition-Dependent Transcriptome Reveals High-Level Regulatory Architecture in Bacillus subtilis. Science 2012, 335, 1103–1106. [Google Scholar] [CrossRef]
- Trach, K.A.; Hoch, J.A. Multisensory activation of the phosphorelay initiating sporulation in Bacillus subtilis: Identification and sequence of the protein kinase of the alternate pathway. Mol. Microbiol. 1993, 8, 69–79. [Google Scholar] [CrossRef]
- Jiang, M.; Shao, W.; Perego, M.; Hoch, J.A. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol. Microbiol. 2000, 38, 535–542. [Google Scholar] [CrossRef]
- Fukushima, S.; Yoshimura, M.; Chibazakura, T.; Sato, T.; Yoshikawa, H. The putative ABC transporter YheH/YheI is involved in the signalling pathway that activates KinA during sporulation initiation. FEMS Microbiol. Lett. 2006, 256, 90–97. [Google Scholar] [CrossRef]
- Ohki, R.; Tateno, K.; Masuyama, W.; Moriya, S.; Kobayashi, K.; Ogasawara, N. The BceRS two-component regulatory system induces expression of the bacitracin transporter, BceAB, in Bacillus subtilis. Mol. Microbiol. 2003, 49, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Staroń, A.; Finkeisen, D.E.; Mascher, T. Peptide Antibiotic Sensing and Detoxification Modules of Bacillus subtilis. Antimicrob. Agents Chemother. 2011, 55, 515–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dintner, S.; Heermann, R.; Fang, C.; Jung, K.; Gebhard, S. A Sensory Complex Consisting of an ATP-binding Cassette Transporter and a Two-component Regulatory System Controls Bacitracin Resistance in Bacillus subtilis. J. Biol. Chem. 2014, 289, 27899–27910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemens, R.; Zaschke-Kriesche, J.; Khosa, S.; Smits, S.H.J. Insight into Two ABC Transporter Families Involved in Lantibiotic Resistance. Front. Mol. Biosci. 2018, 4, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dintner, S.; Staron, A.; Berchtold, E.; Petri, T.; Mascher, T.; Gebhard, S. Coevolution of ABC Transporters and Two-Component Regulatory Systems as Resistance Modules against Antimicrobial Peptides in Firmicutes Bacteria. J. Bacteriol. 2011, 193, 3851–3862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascher, T. Intramembrane-sensing histidine kinases: A new family of cell envelope stress sensors in Firmicutes bacteria. FEMS Microbiol. Lett. 2006, 264, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Joseph, P.; Fichant, G.; Quentin, Y.; Denizot, F. Regulatory relationship of two-component and ABC transport systems and clustering of their genes in the Bacillus/Clostridium group, suggest a functional link between them. J. Mol. Microbiol. Biotechnol. 2002, 4, 503–513. [Google Scholar] [PubMed]
- Bernard, R.; Guiseppi, A.; Chippaux, M.; Foglino, M.; Denizot, F. Resistance to Bacitracin in Bacillus subtilis: Unexpected Requirement of the BceAB ABC Transporter in the Control of Expression of Its Own Structural Genes. J. Bacteriol. 2007, 189, 8636–8642. [Google Scholar] [CrossRef] [Green Version]
- Rietkötter, E.; Hoyer, D.; Mascher, T. Bacitracin sensing in Bacillus subtilis. Mol. Microbiol. 2008, 68, 768–785. [Google Scholar] [CrossRef]
- Mascher, T.; Margulis, N.G.; Wang, T.; Ye, R.W.; Helmann, J.D. Cell wall stress responses in Bacillus subtilis: The regulatory network of the bacitracin stimulon. Mol. Microbiol. 2003, 50, 1591–1604. [Google Scholar] [CrossRef]
- Koh, A.; Gibbon, M.J.; Van der Kamp, M.W.; Pudney, C.R.; Gebhard, S. Conformation control of the histidine kinase BceS of Bacillus subtilis by its cognate ABC-transporter facilitates need-based activation of antibiotic resistance. Mol. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Radeck, J.; Gebhard, S.; Orchard, P.S.; Kirchner, M.; Bauer, S.; Mascher, T.; Fritz, G. Anatomy of the bacitracin resistance network in B acillus subtilis. Mol. Microbiol. 2016, 100, 607–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, G.; Dintner, S.; Treichel, N.S.; Radeck, J.; Gerland, U.; Mascher, T.; Gebhard, S. A New Way of Sensing: Need-Based Activation of Antibiotic Resistance by a Flux-Sensing Mechanism. MBio 2015, 6, e00975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingston, A.W.; Zhao, H.; Cook, G.M.; Helmann, J.D. Accumulation of heptaprenyl diphosphate sensitizes B acillus subtilis to bacitracin: Implications for the mechanism of resistance mediated by the BceAB transporter. Mol. Microbiol. 2014, 93, 37–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobras, C.M.; Piepenbreier, H.; Emenegger, J.; Sim, A.; Fritz, G.; Gebhard, S. BceAB-Type Antibiotic Resistance Transporters Appear To Act by Target Protection of Cell Wall Synthesis. Antimicrob. Agents Chemother. 2019, 64, e02241-19. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Geng, Y.; Ren, S.; Yu, T.; Li, Y.; Liu, G.; Wang, H.; Meng, H.; Shi, L. The VirAB-VirSR-AnrAB Multicomponent System Is Involved in Resistance of Listeria monocytogenes EGD-e to Cephalosporins, Bacitracin, Nisin, Benzalkonium Chloride, and Ethidium Bromide. Appl. Environ. Microbiol. 2019, 85, e01470-19. [Google Scholar] [CrossRef]
- Grubaugh, D.; Regeimbal, J.M.; Ghosh, P.; Zhou, Y.; Lauer, P.; Dubensky, T.W.; Higgins, D.E. The VirAB ABC Transporter Is Required for VirR Regulation of Listeria monocytogenes Virulence and Resistance to Nisin. Infect. Immun. 2017, 86, e00901-17. [Google Scholar] [CrossRef] [Green Version]
- Collins, B.; Curtis, N.; Cotter, P.D.; Hill, C.; Ross, R.P. The ABC Transporter AnrAB Contributes to the Innate Resistance of Listeria monocytogenes to Nisin, Bacitracin, and Various β-Lactam Antibiotics. Antimicrob. Agents Chemother. 2010, 54, 4416–4423. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Wiedmann, M.; Boor, K.J.; Bergholz, T.M. VirR-Mediated Resistance of Listeria monocytogenes against Food Antimicrobials and Cross-Protection Induced by Exposure to Organic Acid Salts. Appl. Environ. Microbiol. 2015, 81, 4553–4562. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Long, F.; Chen, Y.; Knøchel, S.; She, Q.; Shi, X. A Putative ABC Transporter Is Involved in Negative Regulation of Biofilm Formation by Listeria monocytogenes. Appl. Environ. Microbiol. 2008, 74, 7675–7683. [Google Scholar] [CrossRef] [Green Version]
- Mandin, P.; Fsihi, H.; Dussurget, O.; Vergassola, M.; Milohanic, E.; Toledo-Arana, A.; Lasa, I.; Johansson, J.; Cossart, P. VirR, a response regulator critical for Listeria monocytogenes virulence. Mol. Microbiol. 2005, 57, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Thedieck, K.; Hain, T.; Mohamed, W.; Tindall, B.J.; Nimtz, M.; Chakraborty, T.; Wehland, J.; Jänsch, L. The MprF protein is required for lysinylation of phospholipids in listerial membranes and confers resistance to cationic antimicrobial peptides (CAMPs) on Listeria monocytogenes. Mol. Microbiol. 2006, 62, 1325–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staubitz, P.; Peschel, A. MprF-mediated lysinylation of phospholipids in Bacillus subtilis—Protection against bacteriocins in terrestrial habitats? Microbiology 2002, 148, 3331–3332. [Google Scholar] [CrossRef] [PubMed]
- Heaton, M.P.; Neuhaus, F.C. Biosynthesis of D-alanyl-lipoteichoic acid: Cloning, nucleotide sequence, and expression of the Lactobacillus casei gene for the D-alanine-activating enzyme. J. Bacteriol. 1992, 174, 4707–4717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristian, S.A.; Dürr, M.; Van Strijp, J.A.G.; Neumeister, B.; Peschel, A. MprF-Mediated Lysinylation of Phospholipids in Staphylococcus aureus Leads to Protection against Oxygen-Independent Neutrophil Killing. Infect. Immun. 2003, 71, 546–549. [Google Scholar] [CrossRef] [Green Version]
- Abachin, E.; Poyart, C.; Pellegrini, E.; Milohanic, E.; Fiedler, F.; Berche, P.; Trieu-Cuot, P. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 2002, 43, 1–14. [Google Scholar] [CrossRef]
- Peschel, A.; Otto, M.; Jack, R.W.; Kalbacher, H.; Jung, G.; Götz, F. Inactivation of the dlt Operon in Staphylococcus aureus Confers Sensitivity to Defensins, Protegrins, and Other Antimicrobial Peptides. J. Biol. Chem. 1999, 274, 8405–8410. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.S.; Hossain, H.; Otten, S.; Kuenne, C.; Kuchmina, K.; Machata, S.; Domann, E.; Chakraborty, T.; Hain, T. Intracellular Gene Expression Profile of Listeria monocytogenes. Infect. Immun. 2006, 74, 1323–1338. [Google Scholar] [CrossRef] [Green Version]
- Camejo, A.; Buchrieser, C.; Couvé, E.; Carvalho, F.; Reis, O.; Ferreira, P.; Sousa, S.; Cossart, P.; Cabanes, D. In Vivo Transcriptional Profiling of Listeria monocytogenes and Mutagenesis Identify New Virulence Factors Involved in Infection. PLoS Pathog. 2009, 5, e1000449. [Google Scholar] [CrossRef]
- Pazos, M.; Peters, K. Peptidoglycan. In Subcellular Biochemistry; Springer: Berlin/Heidelberg, Germany, 2019; pp. 127–168. [Google Scholar]
- Egan, A.J.F.; Errington, J.; Vollmer, W. Regulation of peptidoglycan synthesis and remodelling. Nat. Rev. Microbiol. 2020, 18, 446–460. [Google Scholar] [CrossRef]
- Nakagawa, J.; Tamaki, S.; Tomioka, S.; Matsuhashi, M. Functional biosynthesis of cell wall peptidoglycan by polymorphic bifunctional polypeptides. Penicillin-binding protein 1Bs of Escherichia coli with activities of transglycosylase and transpeptidase. J. Biol. Chem. 1984, 259, 13937–13946. [Google Scholar] [CrossRef]
- Mayer, C. Bacterial Cell Wall Recycling. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- Mayer, C.; Kluj, R.M.; Mühleck, M.; Walter, A.; Unsleber, S.; Hottmann, I.; Borisova, M. Bacteria’s different ways to recycle their own cell wall. Int. J. Med. Microbiol. 2019, 309, 151326. [Google Scholar] [CrossRef] [PubMed]
- Benda, M.; Schulz, L.; Rismondo, J.; Stülke, J. The YtrBCDEF ABC transporter is involved in the control of social activities in Bacillus subtilis. bioRxiv 2020. [Google Scholar] [CrossRef]
- Yoshida, K.-I.; Fujita, Y.; Ehrlich, S.D. An Operon for a Putative ATP-Binding Cassette Transport System Involved in Acetoin Utilization ofBacillus subtilis. J. Bacteriol. 2000, 182, 5454–5461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salzberg, L.I.; Luo, Y.; Hachmann, A.-B.; Mascher, T.; Helmann, J.D. The Bacillus subtilis GntR Family Repressor YtrA Responds to Cell Wall Antibiotics. J. Bacteriol. 2011, 193, 5793–5801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, M.; Kohl, B.; Münch, D.; Raatschen, N.; Albada, H.B.; Hamoen, L.; Metzler-Nolte, N.; Sahl, H.-G.; Bandow, J.E. Proteomic Response of Bacillus subtilis to Lantibiotics Reflects Differences in Interaction with the Cytoplasmic Membrane. Antimicrob. Agents Chemother. 2012, 56, 5749–5757. [Google Scholar] [CrossRef] [Green Version]
- Beckering, C.L.; Steil, L.; Weber, M.H.W.; Völker, U.; Marahiel, M.A. Genomewide Transcriptional Analysis of the Cold Shock Response in Bacillus subtilis. J. Bacteriol. 2002, 184, 6395–6402. [Google Scholar] [CrossRef] [Green Version]
- Quentin, Y.; Fichant, G.; Denizot, F. Inventory, assembly and analysis of Bacillus subtilis ABC transport systems. J. Mol. Biol. 1999, 287, 467–484. [Google Scholar] [CrossRef]
- Lopez, J.M.; Thoms, B. Beziehungen zwischen katabolischer Repression und Sporulation bei Bacillus subtilis. Arch. Microbiol. 1976, 109, 181–186. [Google Scholar] [CrossRef]
- Huang, M.; Oppermann-Sanio, F.B.; Steinbüchel, A. Biochemical and Molecular Characterization of theBacillus subtilis Acetoin Catabolic Pathway. J. Bacteriol. 1999, 181, 3837–3841. [Google Scholar] [CrossRef] [Green Version]
- Gardner, J.G.; Grundy, F.J.; Henkin, T.M.; Escalante-Semerena, J.C. Control of Acetyl-Coenzyme A Synthetase (AcsA) Activity by Acetylation/Deacetylation without NAD+ Involvement in Bacillus subtilis. J. Bacteriol. 2006, 188, 5460–5468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, J.G.; Escalante-Semerena, J.C. In Bacillus subtilis, the Sirtuin Protein Deacetylase, Encoded by the srtN Gene (Formerly yhdZ), and Functions Encoded by the acuABC Genes Control the Activity of Acetyl Coenzyme A Synthetase. J. Bacteriol. 2009, 191, 1749–1755. [Google Scholar] [CrossRef] [Green Version]
- Grundy, F.J.; Waters, D.A.; Takova, T.Y.; Henkin, T.M. Identification of genes involved in utilization of acetate and acetoin in Bacillus subtilis. Mol. Microbiol. 1993, 10, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Thanh, T.N.; Jürgen, B.; Bauch, M.; Liebeke, M.; Lalk, M.; Ehrenreich, A.; Evers, S.; Maurer, K.-H.; Antelmann, H.; Ernst, F.; et al. Regulation of acetoin and 2,3-butanediol utilization in Bacillus licheniformis. Appl. Microbiol. Biotechnol. 2010, 87, 2227–2235. [Google Scholar] [CrossRef]
- Grundy, F.J.; Turinsky, A.J.; Henkin, T.M. Catabolite regulation of Bacillus subtilis acetate and acetoin utilization genes by CcpA. J. Bacteriol. 1994, 176, 4527–4533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, B.-M.; Kritikos, G.; Farelli, J.D.; Todor, H.; Tong, K.; Kimsey, H.; Wapinski, I.; Galardini, M.; Cabal, A.; Peters, J.M.; et al. Construction and Analysis of Two Genome-Scale Deletion Libraries for Bacillus subtilis. Cell Syst. 2017, 4, 291–305.e7. [Google Scholar] [CrossRef]
- Toledo-Arana, A.; Dussurget, O.; Nikitas, G.; Sesto, N.; Guet-Revillet, H.; Balestrino, D.; Loh, E.; Gripenland, J.; Tiensuu, T.; Vaitkevicius, K.; et al. The Listeria transcriptional landscape from saprophytism to virulence. Nature 2009, 459, 950–956. [Google Scholar] [CrossRef]
- Rismondo, J.; Schulz, L.M.; Yacoub, M.; Wadhawan, A.; Hoppert, M.; Dionne, M.S.; Gründling, A. EslB is required for cell wall biosynthesis and modification in Listeria monocytogenes. J. Bacteriol. 2020. [Google Scholar] [CrossRef]
- Burke, T.P.; Loukitcheva, A.; Zemansky, J.; Wheeler, R.; Boneca, I.G.; Portnoy, D.A. Listeria monocytogenes Is Resistant to Lysozyme through the Regulation, Not the Acquisition, of Cell Wall-Modifying Enzymes. J. Bacteriol. 2014, 196, 3756–3767. [Google Scholar] [CrossRef] [Green Version]
- Durack, J.; Burke, T.P.; Portnoy, D.A. A prl Mutation in SecY Suppresses Secretion and Virulence Defects of Listeria monocytogenes secA2 Mutants. J. Bacteriol. 2015, 197, 932–942. [Google Scholar] [CrossRef] [Green Version]
- Boneca, I.G.; Dussurget, O.; Cabanes, D.; Nahori, M.-A.; Sousa, S.; Lecuit, M.; Psylinakis, E.; Bouriotis, V.; Hugot, J.-P.; Giovannini, M.; et al. A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proc. Natl. Acad. Sci. USA 2007, 104, 997–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubry, C.; Goulard, C.; Nahori, M.-A.; Cayet, N.; Decalf, J.; Sachse, M.; Boneca, I.G.; Cossart, P.; Dussurget, O. OatA, a Peptidoglycan O-Acetyltransferase Involved in Listeria monocytogenes Immune Escape, Is Critical for Virulence. J. Infect. Dis. 2011, 204, 731–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gueiros-Filho, F.J. A widely conserved bacterial cell division protein that promotes assembly of the tubulin-like protein FtsZ. Genes Dev. 2002, 16, 2544–2556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, K.I.; Hove-Jensen, B. Ribose catabolism of Escherichia coli: Characterization of the rpiB gene encoding ribose phosphate isomerase B and of the rpiR gene, which is involved in regulation of rpiB expression. J. Bacteriol. 1996, 178, 1003–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeger, T.; Mayer, C. The Transcriptional Factors MurR and Catabolite Activator Protein Regulate N-Acetylmuramic Acid Catabolism in Escherichia coli. J. Bacteriol. 2008, 190, 6598–6608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohler, P.R.A.; Choong, E.-L.; Rossbach, S. The RpiR-Like Repressor IolR Regulates Inositol Catabolism in Sinorhizobium meliloti. J. Bacteriol. 2011, 193, 5155–5163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Nandakumar, R.; Sadykov, M.R.; Madayiputhiya, N.; Luong, T.T.; Gaupp, R.; Lee, C.Y.; Somerville, G.A. RpiR Homologues May Link Staphylococcus aureus RNAIII Synthesis and Pentose Phosphate Pathway Regulation. J. Bacteriol. 2011, 193, 6187–6196. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, K.; Campos, E.; Aguilera, L.; Toloza, L.; Giménez, R.; Aguilar, J.; Baldoma, L.; Badia, J. Characterization of the gene cluster involved in allantoate catabolism and its transcriptional regulation by the RpiR-type repressor HpxU in Klebsiella pneumoniae. Int. Microbiol. 2013, 16, 165–176. [Google Scholar] [CrossRef]
- Aleksandrzak-Piekarczyk, T.; Stasiak-Różańska, L.; Cieśla, J.; Bardowski, J. ClaR—A novel key regulator of cellobiose and lactose metabolism in Lactococcus lactis IL1403. Appl. Microbiol. Biotechnol. 2015, 99, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Aleksandrzak-Piekarczyk, T.; Szatraj, K.; Kosiorek, K. GlaR (YugA)—A novel RpiR-family transcription activator of the Leloir pathway of galactose utilization in Lactococcus lactis IL 1403. Microbiologyopen 2019, 8, e00714. [Google Scholar] [CrossRef] [Green Version]
- Van De Putte, P.; Van Dillewijn, J.; Rörsch, A. The selection of mutants of escherichia coli with impaired cell division at elevated temperature. Mutat. Res. Mol. Mech. Mutagen. 1964, 1, 121–128. [Google Scholar] [CrossRef]
- Garti-Levi, S.; Hazan, R.; Kain, J.; Fujita, M.; Ben-Yehuda, S. The FtsEX ABC transporter directs cellular differentiation in Bacillus subtilis. Mol. Microbiol. 2008, 69, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Furuhata, K.; Fukushima, T.; Yamamoto, H.; Sekiguchi, J. Characterization of a New Bacillus subtilis Peptidoglycan Hydrolase Gene, yvcE (Named cwlO), and the Enzymatic Properties of Its Encoded Protein. J. Biosci. Bioeng. 2004, 98, 174–181. [Google Scholar] [CrossRef]
- Domínguez-Cuevas, P.; Porcelli, I.; Daniel, R.A.; Errington, J. Differentiated roles for MreB-actin isologues and autolytic enzymes in Bacillus subtilis morphogenesis. Mol. Microbiol. 2013, 89, 1084–1098. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.-Y.; Chu, S.-H.; Shaw, G.-C. Deletion of the cell wall peptidoglycan hydrolase gene cwlO or lytE severely impairs transformation efficiency in Bacillus subtilis. J. Gen. Appl. Microbiol. 2018, 64, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, M.; Ooiwa, S.; Sekiguchi, J. Synthetic Lethality of the lytE cwlO Genotype in Bacillus subtilis Is Caused by Lack of d,l-Endopeptidase Activity at the Lateral Cell Wall. J. Bacteriol. 2012, 194, 796–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunet, Y.R.; Wang, X.; Rudner, D.Z. SweC and SweD are essential co-factors of the FtsEX-CwlO cell wall hydrolase complex in Bacillus subtilis. PLoS Genet. 2019, 15, e1008296. [Google Scholar] [CrossRef] [Green Version]
- Crow, A.; Greene, N.P.; Kaplan, E.; Koronakis, V. Structure and mechanotransmission mechanism of the MacB ABC transporter superfamily. Proc. Natl. Acad. Sci. USA 2017, 114, 12572–12577. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rismondo, J.; Schulz, L.M. Not Just Transporters: Alternative Functions of ABC Transporters in Bacillus subtilis and Listeria monocytogenes. Microorganisms 2021, 9, 163. https://doi.org/10.3390/microorganisms9010163
Rismondo J, Schulz LM. Not Just Transporters: Alternative Functions of ABC Transporters in Bacillus subtilis and Listeria monocytogenes. Microorganisms. 2021; 9(1):163. https://doi.org/10.3390/microorganisms9010163
Chicago/Turabian StyleRismondo, Jeanine, and Lisa Maria Schulz. 2021. "Not Just Transporters: Alternative Functions of ABC Transporters in Bacillus subtilis and Listeria monocytogenes" Microorganisms 9, no. 1: 163. https://doi.org/10.3390/microorganisms9010163
APA StyleRismondo, J., & Schulz, L. M. (2021). Not Just Transporters: Alternative Functions of ABC Transporters in Bacillus subtilis and Listeria monocytogenes. Microorganisms, 9(1), 163. https://doi.org/10.3390/microorganisms9010163





