Metabolic Engineering of Extremophilic Bacterium Deinococcus radiodurans for the Production of the Novel Carotenoid Deinoxanthin
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids, and Culture Medium
2.2. DNA Manipulation and Plasmid Construction
2.3. Shake Flask Cultivation
2.4. Carotenoid Extraction and Analytical Methods
2.5. Real-Time Quantitative PCR (RT-qPCR)
2.6. DPPH Assays
3. Results
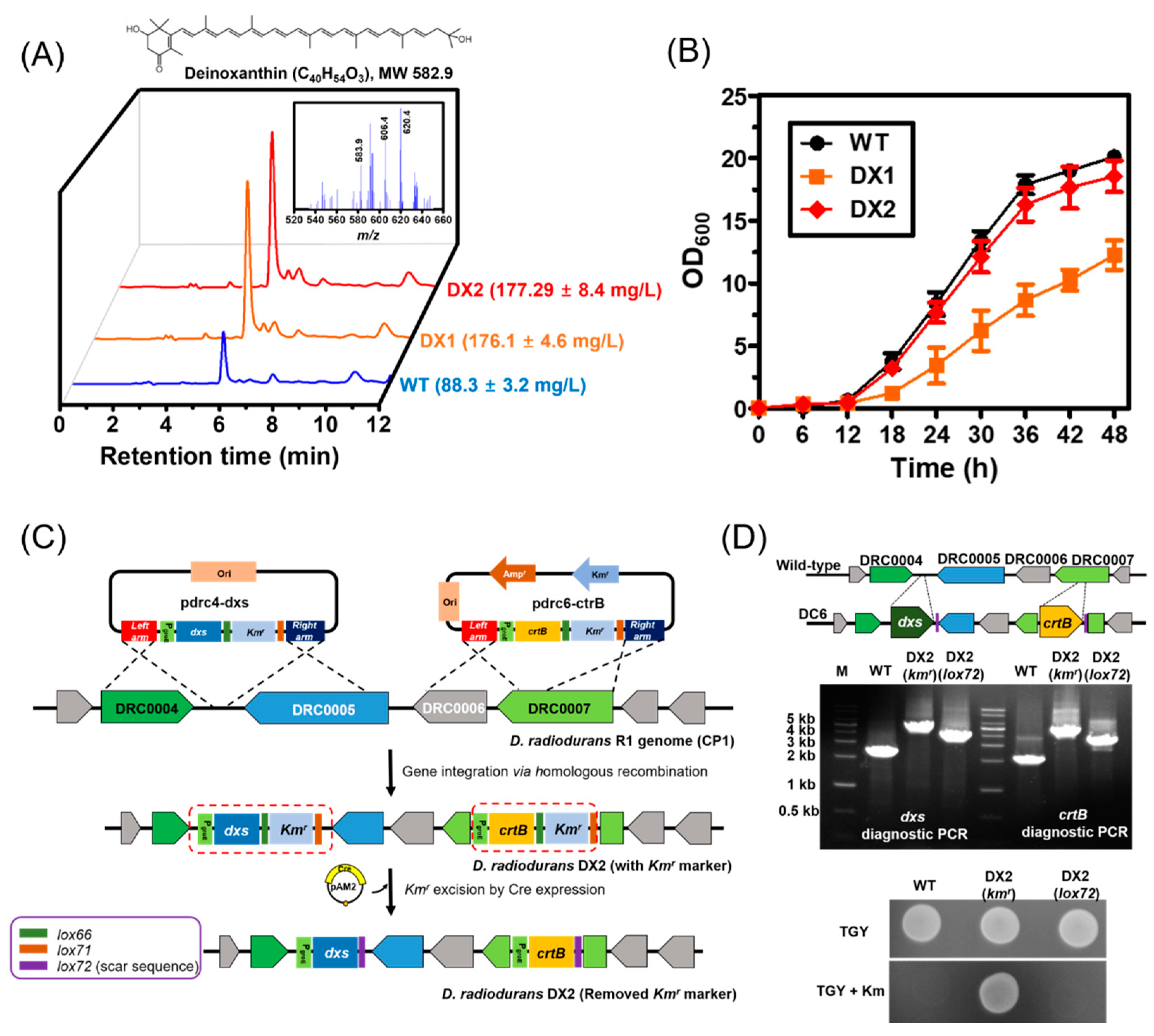
3.1. Construction of a Base Strain via the Genome-Based Overexpression of Rate-Limiting Steps
3.2. Optimization of Culture Conditions to Enhance the Production of Deinoxanthin
3.3. Evaluation of the Antioxidant Activity of Deinoxanthin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Young, A.J.; Lowe, G.L. Carotenoids-Antioxidant properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Mordi, R.C.; Ademosun, O.T.; Ajanaku, C.O.; Olanrewanju, I.O.; Walton, J.C. Free radical mediated oxidative degradation of carotenes and xanthophylls. Molecules 2020, 25, 1038. [Google Scholar] [CrossRef] [PubMed]
- Zaripheh, S.; Erdman, J.W. Factors that influence the bioavailability of xanthophylls. J. Nutr. 2020, 132, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Bocanegra, A.R.; Ponce-Noyola, T.; Torres-Muñoz, J.A. Astaxanthin production by Phaffia rhodozyma and Haematococcus pluvialis: A comparative study. Appl. Microbiol. Biotechnol. 2007, 75, 783–791. [Google Scholar] [CrossRef]
- Asker, D.; Beppu, T.; Ueda, K. Zeaxanthinibacter enoshimensis gen. nov., sp. nov., a novel zeaxanthin-producing marine bacterium of the family Flavobacteriaceae, isolated from sea water off Enoshima island, Japan. Int. J. Sys. Evol. Microbiol. 2007, 57, 837–843. [Google Scholar] [CrossRef]
- Saha, S.K.; Ermis, H.; Murray, P. Marine microalgae for potential lutein production. Appl. Sci. 2020, 10, 6457. [Google Scholar] [CrossRef]
- Shindo, K.; Misawa, N. New and rare carotenoids isolated from marine bacteria and their antioxidant activities. Mar. Drugs 2014, 12, 1690–1698. [Google Scholar] [CrossRef]
- Sugawara, T.; Ganesan, P.; Li, Z.; Manabe, Y.; Hirata, T. Siphoxanthin, a green algal carotenoid, as a novel functional compound. Mar. Drugs 2014, 12, 3660–3668. [Google Scholar] [CrossRef]
- Takatani, N.; Nishida, K.; Sawabe, T.; Maoka, T.; Miyashita, K.; Hosokawa, M. Identification of a novel carotenoid, 2′-isopentenylsaproxanthin, by Jejuia palldilutea strain 11shimoA1 and its increased production under alkaline condition. Appl. Microbiol. Biotechnol. 2014, 98, 6633–6640. [Google Scholar] [CrossRef]
- Mandelli, F.; Couger, M.B.; Paixão, D.A.A.; Machado, C.B.; Carnielli, C.M.; Aricetii, J.A.; Polikarpov, I.; Prade, R.; Caldana, C.; Paes Leme, A.F.; et al. Thermal adaptation strategies of the extremophile bacterium Thermus filiformis based on multi-omics analysis. Extremophiles 2017, 20, 775–788. [Google Scholar] [CrossRef]
- Lemee, L.; Peuchant, E.; Clerc, M.; Brunner, M.; Pfander, H. Deinoxanthin: A new carotenoid isolated from Deinococcus radiodurans. Tetrahedron 1997, 53, 919–926. [Google Scholar] [CrossRef]
- Jeong, S.-W.; Kang, C.K.; Choi, Y.J. Metabolic engineering of Deinococcus radiodurans for the production of phytoene. J. Microbiol. Biotechnol. 2018, 28, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.K.; Jeong, S.-W.; Yang, J.E.; Choi, Y.J. High-yield production of lycopene from corn steep liquor and glycerol using the metabolically engineered Deinococcus radiodurans R1 strain. J. Agric. Food Chem. 2020, 68, 5147–5153. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.K.; Yang, J.E.; Park, H.W.; Choi, Y.J. Enhanced lycopene production by UV-C irradiation in radiation-resistant Deinococcus radiodurans R1. J. Microbiol. Biotechnol. 2020, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Xu, Z.; Sun, Z.; Lin, J.; Hua, Y. Evaluation of the antioxidant effects of carotenoids from Deinococcus radiodurans through targeted mutagenesis, chemiluminescence, and DNA damage analyses. Biochim. Biophys. Acta Gen. Subj. 2007, 1770, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Hua, Y. Carotenoid biosynthesis in extremophilic Deinococcus-Thermus bacteria. Trends Microbiol. 2010, 18, 512–520. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, S.; Shao, X.; Park, J.-B.; Jeong, S.-H.; Park, H.-J.; Kwak, W.-J.; Wei, G.; Kim, S.-W. Challenges and tackles in metabolic engineering for microbial production of carotenoids. Microb. Cell Fact. 2019, 18, 55. [Google Scholar] [CrossRef]
- Airo, A.; Chan, S.L.; Martinez, Z.; Platt, M.O.; Trent, J.D. Heat shock and cold shock in Deinococcus radiodurans. Cell Biochem. Biophys. 2004, 40, 277–288. [Google Scholar] [CrossRef]
- Meima, R.; Lidstrom, M.E. Characterization of the minimal replicon of a cryptic Deinococcus radiodurans SARK plasmid and development of versatile Escherchia coli-D. radiodurans shuttle vectors. Appl. Environ. Microbiol. 2000, 66, 3856–3867. [Google Scholar] [CrossRef]
- Xu, Z.; Tian, B.; Sun, Z.; Lin, J.; Hua, Y. Identification and functional analysis of a phytoene desaturase gene from the extremely radioresistant bacterium Deinococcus radiodurans. Microbiology 2007, 153, 1642–1652. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Q.; Luo, X.; Fang, C.; Zhang, Q.; Tang, Y. Knockout of crtB or crtI gene blocks the carotenoid biosynthesis pathway in Deinococcus radiodurans R1 and influences its resistance to oxidative DNA-damaging agents due to change of free radicals scavenging activity. Arch. Microbiol. 2007, 188, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, W.; Su, S.; Chen, M.; Lu, W.; Lin, M.; Molnár, I.; Xu, Y. CYP287A1 is a carotenoid 2-β-hydroxylase required for deinoxanthin biosysnthesis in Deinococcus radiodurans R1. Appl. Microbiol. Biotechnol. 2015, 99, 10539–10546. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Sun, Z.; Xu, Z.; Shen, S.; Wang, H.; Hua, Y. Carotenoid 3′, 4′-desaturase is involved in carotenoid biosynthesis in the radioresistant bacterium Deinococcus radiodurans. Microbiology 2008, 154, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lee, K.J.; Lee, P.C. Characterization of carotenoid biosynthesis in newly isolated Deinococcus sp. AJ005 and investigation of the effects of environmental conditions on cell growth and carotenoid biosynthesis. Mar. Drugs 2019, 17, 705. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-W.; Yang, J.E.; Im, S.H.; Choi, Y.J. Development of Cre-lox based multiple knockout system in Deinococcus radiodurans R1. Korean J. Chem. Eng. 2017, 34, 1728–1733. [Google Scholar] [CrossRef]
- Chintong, S.; Phatvej, W.; Rerk-Am, U.; Waiprib, Y.; Klaypradit, W. In vitro antioxidant, antityrosinase, and cytotoxic activities of astaxanthin from shrimp waste. Antioxidant 2019, 8, 128. [Google Scholar] [CrossRef]
- Schmid, A.K.; Lidstrom, M.E. Involvement of two putative alternative sigma factors in stress response of the radioresistant bacterium Deinococcus radiodurans. J. Bacteriol. 2002, 184, 6182–6189. [Google Scholar] [CrossRef][Green Version]
- Télef, N.; Stammitti-Bert, L.; Mortain-Bertrand, A.; Maucourt, M.; Carde, J.P.; Rolin, D.; Gallusci, P. Sucrose deficiency delays lycopene accumulation in tomato fruit pericarp disc. Plant Mol. Biol. 2006, 62, 453–469. [Google Scholar] [CrossRef]


| Description | Reference | |
|---|---|---|
| Strains | ||
| D. radiodurans R1 | Wild type (ATCC13939) | ATCC |
| D. radiodurans DX1 | Wild-type harboring pAM104 plasmid | This study |
| D. radiodurans DX2 | Integration of dxs and crtB genes with the loxP scar sequence into the D. radiodurans R1 chromosome (CP1) | This study |
| E. coli DH5α | Host for recombinant plasmid construction | Lab stock |
| Plasmids | ||
| pTOP Blunt V2 | TA cloning vector | Enzynomics |
| pRADZ3 | E. coli–D. radiodurans shuttle vector carrying the groE promoter | [25] |
| pAM1 | Derivative of pKatAPH3 containing a lox66-kmr-lox71 cassette | [25] |
| pAM2 | Derivative of p13840 containing PgroE-cre-PgroE-cmr | [25] |
| pAM41 | Derivative of pRADZ3 containing crtB | [12] |
| pAM73 | Derivative of pRADZ3 containing dxs | [12] |
| pAM104 | Derivative of pRADZ3 containing dxs and crtB | [12] |
| Pdrc6-crtB | pTOP Blunt V2 containing two homology arms (the partial sequences of drC0006 and drc0007) and PgroE-crtB- lox66-kmr-lox71 | This study |
| Pdrc4-dxs | pAM1 containing two homology arms (the partial sequences of drC0004 and drc0005) and PgroE-dxs- lox66-kmr-lox71 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-W.; Kim, J.-H.; Kim, J.-W.; Kim, C.Y.; Kim, S.Y.; Choi, Y.J. Metabolic Engineering of Extremophilic Bacterium Deinococcus radiodurans for the Production of the Novel Carotenoid Deinoxanthin. Microorganisms 2021, 9, 44. https://doi.org/10.3390/microorganisms9010044
Jeong S-W, Kim J-H, Kim J-W, Kim CY, Kim SY, Choi YJ. Metabolic Engineering of Extremophilic Bacterium Deinococcus radiodurans for the Production of the Novel Carotenoid Deinoxanthin. Microorganisms. 2021; 9(1):44. https://doi.org/10.3390/microorganisms9010044
Chicago/Turabian StyleJeong, Sun-Wook, Jun-Ho Kim, Ji-Woong Kim, Chae Yeon Kim, Su Young Kim, and Yong Jun Choi. 2021. "Metabolic Engineering of Extremophilic Bacterium Deinococcus radiodurans for the Production of the Novel Carotenoid Deinoxanthin" Microorganisms 9, no. 1: 44. https://doi.org/10.3390/microorganisms9010044
APA StyleJeong, S.-W., Kim, J.-H., Kim, J.-W., Kim, C. Y., Kim, S. Y., & Choi, Y. J. (2021). Metabolic Engineering of Extremophilic Bacterium Deinococcus radiodurans for the Production of the Novel Carotenoid Deinoxanthin. Microorganisms, 9(1), 44. https://doi.org/10.3390/microorganisms9010044





