Analysis of Bacteriohopanoids from Thermophilic Bacteria by Liquid Chromatography–Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards, Isolation, Molecular Identification, and Cultivation of Four Strains of Thermophilic Bacteria from Hot Springs
2.2. Extraction and Isolation of Hopanoids
2.3. Analysis of Hopanoids by LC–MS
2.4. Quantification
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization and Identification of Bacterial Isolates
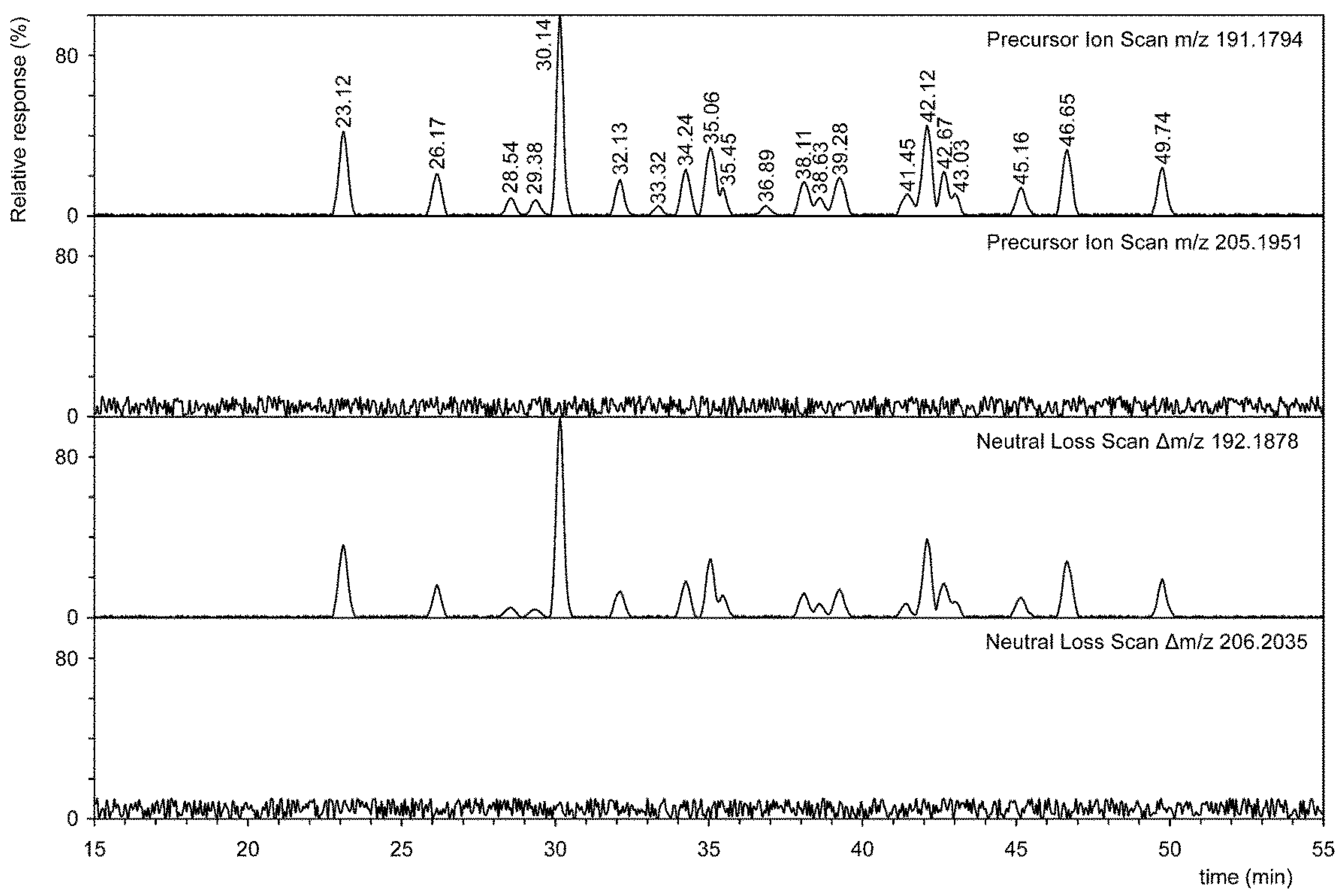
3.2. Analysis of Total Hopanoids by Liquid Chromatography–Mass Spectrometry Using Precursor Ion and Neutral Ion Scans
3.3. The Absence of Methylhopanoids in the Studied Bacteria was Proven by Liquid Chromatography–Mass Spectrometry
3.4. Use of Two Tandem Mass Spectrometry Methods (PIS and/or NLS) for the Identification of Hopanoid Structures
3.5. Influence of Cultivation Temperature on the Structure and Abundance of Hopanoid´s Molecular Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paces, T.; Smejkal, V. Magmatic and Fossil Components of Thermal and Mineral Waters in the Eger River Continental Rift (Bohemian Massif, Central Europe). In Water-Rock Interaction. Proceedings of the Eleventh International Symposium on Water-Rock Interaction; Wanty, R.B., Seal II, R.R., Eds.; Taylor & Francis Group: Abingdon, UK, 2004; pp. 167–172. ISBN 90-5809-641-6. [Google Scholar]
- Peckova, M. Properties of a Hyperthermophilic Bacterium (Thermus sp.) Isolated from a Carlsbad Spring. Folia Microbiol. 1991, 36, 515–521. [Google Scholar] [CrossRef]
- Gharwalova, L.; Palyzova, A.; Maresova, H.; Kolouchova, I.; Kyselova, L.; Rezanka, T. Identification of Homologous Polyprenols from Thermophilic Bacteria. Microorganisms 2021, 9, 1168. [Google Scholar] [CrossRef]
- Mehta, D.; Satyanarayana, T. Diversity of Hot Environments and Thermophilic Microbes. In Thermophilic Microbes in Environmental and Industrial Biotechnology: Biotechnology of Thermophiles; Satyanarayana, T., Littlechild, J., Kawarabayasi, Y., Eds.; Springer: Dordrecht, The Netherland, 2013; pp. 3–60. ISBN 978-94-007-5899-5. [Google Scholar]
- Doree, M.; Terrine, C. Enzymatic Synthesis of Ribonucleoside-5’-Phosphates from Some N6-Substituted Adenosines. Phytochemistry 1973, 12, 1017–1023. [Google Scholar] [CrossRef]
- Liu, W.; Sakr, E.; Schaeffer, P.; Talbot, H.M.; Donisi, J.; Härtner, T.; Kannenberg, E.; Takano, E.; Rohmer, M. Ribosylhopane, a Novel Bacterial Hopanoid, as Precursor of C35 Bacteriohopanepolyols in Streptomyces coelicolor A3(2). Chembiochem 2014, 15, 2156–2161. [Google Scholar] [CrossRef] [Green Version]
- Hippchen, B.; Röll, A.; Poralla, K. Occurrence in Soil of Thermo-Acidophilic Bacilli Possessing ω-Cyclohexane Fatty Acids and Hopanoids. Arch. Microbiol. 1981, 129, 53–55. [Google Scholar] [CrossRef]
- Damsté, J.S.S.; Van Duin, A.C.T.; Hollander, D.; Kohnen, M.E.L.; De Leeuw, J.W. Early Diagenesis of Bacteriohopanepolyol Derivatives: Formation of Fossil Homohopanoids. Geochim. Cosmochim. Acta 1995, 59, 5141–5157. [Google Scholar] [CrossRef]
- Poralla, K.; Härtner, T.; Kannenberg, E. Effect of Temperature and pH on the Hopanoid Content of Bacillus acidocaldarius. FEMS Microbiol. Lett. 1984, 23, 253–256. [Google Scholar] [CrossRef]
- Caron, B.; Mark, A.E.; Poger, D. Some like It Hot: The Effect of Sterols and Hopanoids on Lipid Ordering at High Temperature. J. Phys. Chem. Lett. 2014, 5, 3953–3957. [Google Scholar] [CrossRef]
- Sessions, A.L.; Zhang, L.; Welander, P.V.; Doughty, D.; Summons, R.E.; Newman, D.K. Identification and Quantification of Polyfunctionalized Hopanoids by High Temperature Gas Chromatography-Mass Spectrometry. Org. Geochem. 2013, 56, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Rezanka, T.; Kambourova, M.; Derekova, A.; Kolouchova, I.; Sigler, K. LC-ESI-MS/MS Identification of Polar Lipids of Two Thermophilic Anoxybacillus Bacteria Containing a Unique Lipid Pattern. Lipids 2012, 47, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Talbot, H.; Watson, D.; Murrell, J.; Carter, J.; Farrimond, P. Analysis of Intact Bacteriohopanepolyols from Methanotrophic Bacteria by Reversed-Phase High-Performance Liquid Chromatography-Atmospheric Pressure Chemical Ionisation Mass Spectrometry. J. Chromatogr. A 2001, 921, 175–185. [Google Scholar] [CrossRef]
- Talbot, H.M.; Summons, R.; Jahnke, L.; Farrimond, P. Characteristic Fragmentation of Bacteriohopanepolyols during Atmospheric Pressure Chemical Ionisation Liquid Chromatography/Ion Trap Mass Spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2788–2796. [Google Scholar] [CrossRef]
- Talbot, H.M.; Rohmer, M.; Farrimond, P. Rapid Structural Elucidation of Composite Bacterial Hopanoids by Atmospheric Pressure Chemical Ionisation Liquid Chromatography/Ion Trap Mass Spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Talbot, H.M.; Rohmer, M.; Farrimond, P. Structural Characterisation of Unsaturated Bacterial Hopanoids by Atmospheric Pressure Chemical Ionisation Liquid Chromatography/Ion Trap Mass Spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Talbot, H.M.; Squier, A.H.; Keely, B.J.; Farrimond, P. Atmospheric Pressure Chemical Ionisation Reversed-Phase Liquid Chromatography/Ion Trap Mass Spectrometry of Intact Bacteriohopanepolyols. Rapid Commun. Mass Spectrom. 2003, 17, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Talbot, H.M.; Watson, D.F.; Pearson, E.J.; Farrimond, P. Diverse Biohopanoid Compositions of Non-Marine Sediments. Org. Geochem. 2003, 34, 1353–1371. [Google Scholar] [CrossRef]
- Talbot, H.M.; Sidgwick, F.R.; Bischoff, J.; Osborne, K.A.; Rush, D.; Sherry, A.; Spencer-Jones, C.L. Analysis of Non-Derivatised Bacteriohopanepolyols by Ultrahigh-Performance Liquid Chromatography/Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 2087–2098. [Google Scholar] [CrossRef] [Green Version]
- Zarzycki, P.K.; Portka, J.K. Recent Advances in Hopanoids Analysis: Quantification Protocols Overview, Main Research Targets and Selected Problems of Complex Data Exploration. J. Steroid Biochem. Mol. Biol. 2015, 153, 3–26. [Google Scholar] [CrossRef]
- Rezanka, T.; Siristova, L.; Melzoch, K.; Sigler, K. N-Acylated Bacteriohopanehexol-Mannosamides from the Thermophilic Bacterium Alicyclobacillus acidoterrestris. Lipids 2011, 46, 249–261. [Google Scholar] [CrossRef]
- Bligh, E.; Dyer, W. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Vitova, M.; Stranska, M.; Palyzova, A.; Rezanka, T. Detailed Structural Characterization of Cardiolipins from Various Biological Sources Using a Complex Analytical Strategy Comprising Fractionation, Hydrolysis and Chiral Chromatography. J. Chromatogr. A 2021, 1648, 462185. [Google Scholar] [CrossRef] [PubMed]
- Talbot, H.M.; Farrimond, P.; Schaeffer, P.; Pancost, R.D. Bacteriohopanepolyols in Hydrothermal Vent Biogenic Silicates. Org. Geochem. 2005, 36, 663–672. [Google Scholar] [CrossRef]
- Welander, P.V.; Coleman, M.L.; Sessions, A.L.; Summons, R.E.; Newman, D.K. Identification of a Methylase Required for 2-Methylhopanoid Production and Implications for the Interpretation of Sedimentary Hopanes. Proc. Natl. Acad. Sci. USA 2010, 107, 8537–8542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohmer, M.; Bouvier-Nave, P.; Ourisson, G. Distribution of Hopanoid Triterpenes in Prokaryotes. Microbiology 1984, 130, 1137–1150. [Google Scholar] [CrossRef] [Green Version]
- Simonin, P.; Jürgens, U.J.; Rohmer, M. Bacterial Triterpenoids of the Hopane Series from the Prochlorophyte Prochlorothrix hollandica and Their Intracellular Localization. Eur. J. Biochem. 1996, 241, 865–871. [Google Scholar] [CrossRef]
- Summons, R.E.; Jahnke, L.L.; Hope, J.M.; Logan, G.A. 2-Methylhopanoids as Biomarkers for Cyanobacterial Oxygenic Photosynthesis. Nature 1999, 400, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Rashby, S.E.; Sessions, A.L.; Summons, R.E.; Newman, D.K. Biosynthesis of 2-Methylbacteriohopanepolyols by an Anoxygenic Phototroph. Proc. Natl. Acad. Sci. USA 2007, 104, 15099–15104. [Google Scholar] [CrossRef] [Green Version]
- Zundel, M.; Rohmer, M. Prokaryotic Triterpenoids. Eur. J. Biochem. 1985, 150, 23–27. [Google Scholar] [CrossRef]
- Welander, P.V.; Summons, R.E. Discovery, Taxonomic Distribution, and Phenotypic Characterization of a Gene Required for 3-Methylhopanoid Production. Proc. Natl. Acad. Sci. USA 2012, 109, 12905–12910. [Google Scholar] [CrossRef] [Green Version]
- Elvert, M.; Niemann, H. Occurrence of Unusual Steroids and Hopanoids Derived from Aerobic Methanotrophs at an Active Marine Mud Volcano. Org. Geochem. 2008, 39, 167–177. [Google Scholar] [CrossRef]
- Damsté, J.S.S.; Rijpstra, W.I.C.; Dedysh, S.N.; Foesel, B.U.; Villanueva, L. Pheno- and Genotyping of Hopanoid Production in Acidobacteria. Front. Microbiol. 2017, 8, 968. [Google Scholar] [CrossRef]
- Seemann, M.; Bisseret, P.; Tritz, J.-P.; Hooper, A.B.; Rohmer, M. Novel Bacterial Triterpenoids of the Hopane Series from Nitrosomonas europaea and Their Significance for the Formation of the C35 Bacteriohopane Skeleton. Tetrahedron Lett. 1999, 40, 1681–1684. [Google Scholar] [CrossRef]
- Neunlist, S.; Holst, O.; Rohmer, M. Prokaryotic Triterpenoids—the Hopanoids of the Purple Non-Sulfur Bacterium Rhodomicrobium vannielii—an Aminotriol and Its Aminoacyl Derivatives, N-Tryptophanyl and N-Ornithinyl Aminotriol. Eur. J. Biochem. 1985, 147, 561–568. [Google Scholar] [CrossRef]
- Bradley, A.S.; Pearson, A.; Saenz, J.P.; Marx, C.J. Adenosylhopane: The First Intermediate in Hopanoid Side Chain Biosynthesis. Org. Geochem. 2010, 41, 1075–1081. [Google Scholar] [CrossRef]
- Kannenberg, E.L.; Poralla, K. Hopanoid Biosynthesis and Function in Bacteria. Naturwissenschaften 1999, 86, 168–176. [Google Scholar] [CrossRef]
- Welander, P.V.; Hunter, R.C.; Zhang, L.; Sessions, A.L.; Summons, R.E.; Newman, D.K. Hopanoids Play a Role in Membrane Integrity and pH Homeostasis in Rhodopseudomonas palustris TIE-1. J. Bacteriol. 2009, 191, 6145–6156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Winden, J.F.; Reichart, G.-J.; McNamara, N.P.; Benthien, A.; Damste, J.S.S. Temperature-Induced Increase in Methane Release from Peat Bogs: A Mesocosm Experiment. PLoS ONE 2012, 7, e39614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Winden, J.F.; Talbot, H.M.; Kip, N.; Reichart, G.-J.; Pol, A.; McNamara, N.P.; Jetten, M.S.M.; Op den Camp, H.J.M.; Sinninghe Damsté, J.S. Bacteriohopanepolyol Signatures as Markers for Methanotrophic Bacteria in Peat Moss. Geochim. Cosmochim. Acta 2012, 77, 52–61. [Google Scholar] [CrossRef]
- Osborne, K.A.; Gray, N.D.; Sherry, A.; Leary, P.; Mejeha, O.; Bischoff, J.; Rush, D.; Sidgwick, F.R.; Birgel, D.; Kalyuzhnaya, M.G.; et al. Methanotroph-Derived Bacteriohopanepolyol Signatures as a Function of Temperature Related Growth, Survival, Cell Death and Preservation in the Geological Record. Environ. Microbiol. Rep. 2017, 9, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, L.L.; Summons, R.E.; Hope, J.M.; Marais, D.J.D. Carbon Isotopic Fractionation in Lipids from Methanotrophic Bacteria II: The Effects of Physiology and Environmental Parameters on the Biosynthesis and Isotopic Signatures of Biomarkers. Geochim. Cosmochim. Acta 1999, 63, 79–93. [Google Scholar] [CrossRef]
- Bale, N.J.; Rijpstra, W.I.C.; Sahonero-Canavesi, D.X.; Oshkin, I.Y.; Belova, S.E.; Dedysh, S.N.; Sinninghe Damsté, J.S. Fatty Acid and Hopanoid Adaption to Cold in the Methanotroph Methylovulum psychrotolerans. Front. Microbiol. 2019, 10, 589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, A.; Bringer-Meyer, S.; Poralla, K.; Sahm, H. Effect of Alcohols and Temperature on the Hopanoid Content of Zymomonas mobilis. Appl. Microbiol. Biotechnol. 1986, 25, 32–36. [Google Scholar] [CrossRef]
- Joyeux, C.; Fouchard, S.; Llopiz, P.; Neunlist, S. Influence of the Temperature and the Growth Phase on the Hopanoids and Fatty Acids Content of Frateuria aurantia (DSMZ 6220). FEMS Microbiol. Ecol. 2004, 47, 371–379. [Google Scholar] [CrossRef]
- Cvejic, J.H.; Bodrossy, L.; Kovács, K.L.; Rohmer, M. Bacterial Triterpenoids of the Hopane Series from the Methanotrophic Bacteria Methylocaldum spp.: Phylogenetic Implications and First Evidence for an Unsaturated Aminobacteriohopanepolyol. FEMS Microbiol. Lett. 2000, 182, 361–365. [Google Scholar] [CrossRef]
- Flesch, G.; Rohmer, M. Prokaryotic Hopanoids—the Biosynthesis of the Bacteriohopane Skeleton—Formation of Isoprenic Units from 2 Distinct Acetate Pools and a Novel Type of Carbon Carbon Linkage between a Triterpene and D-Ribose. Eur. J. Biochem. 1988, 175, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.; Leavitt, W.D.; Saenz, J.P.; Summons, R.E.; Tam, M.C.-M.; Close, H.G. Diversity of Hopanoids and Squalene-Hopene Cyclases across a Tropical Land-Sea Gradient. Environ. Microbiol. 2009, 11, 1208–1223. [Google Scholar] [CrossRef]
- Pearson, A.; Page, S.R.F.; Jorgenson, T.L.; Fischer, W.W.; Higgins, M.B. Novel Hopanoid Cyclases from the Environment. Environ. Microbiol. 2007, 9, 2175–2188. [Google Scholar] [CrossRef] [PubMed]

| 1b | 1c | 1d | 1e | 2e | 3e | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ion Description | m/z | Abund a | m/z | Abund | m/z | Abund | m/z | Abund | m/z | Abund | m/z | Abund |
| [M + H]+ | 998.7294 | 15 | 1012.7450 | 19 | 1026.7609 | 23 | 1040.7764 | 26 | 982.7702 | 22 | 924.7648 | 19 |
| [M + H-COCH2]+ | 956.7188 | 9 | 970.7345 | 10 | 984.7502 | 10 | 998.7659 | 11 | 940.7597 | 10 | 882.7541 | 12 |
| [M + H-CH3COOH]+ | 938.7083 | 100 | 952.7240 | 100 | 966.7397 | 100 | 980.7553 | 100 | 922.7493 | 100 | 864.7432 | 100 |
| [M + H-CH3COOH-COCH2]+ | 896.6977 | 8 | 910.7134 | 8 | 924.7281 | 10 | 938.7448 | 12 | 880.7386 | 9 | 822.7330 | 8 |
| [M + H-2 × CH3COOH]+ | 878.6872 | 35 | 892.7029 | 37 | 906.7185 | 41 | 920.7341 | 42 | 862.7281 | 41 | 804.7222 | 40 |
| [M + H-2 × CH3COOH-COCH2]+ | 836.6766 | 18 | 850.6923 | 23 | 864.7080 | 22 | 878.7235 | 29 | 820.7175 | 23 | 762.7120 | 21 |
| [M + H-3 × CH3COOH]+ | 818.6662 | 26 | 832.6819 | 29 | 846.6974 | 34 | 860.7130 | 37 | 802.7070 | 35 | 744.7014 | 33 |
| [M + H-3 × CH3COOH-COCH2]+ | 776.6555 | 14 | 790.6711 | 16 | 804.6869 | 21 | 818.7024 | 25 | 760.6964 | 24 | - | 0 |
| [M + H-4 × CH3COOH]+ | 758.6450 | 25 | 772.6606 | 27 | 786.6763 | 32 | 800.6920 | 35 | 742.6859 | 34 | - | 0 |
| [M + H-4 × CH3COOH-COCH2]+ | 716.6344 | 12 | 730.6501 | 13 | 744.6657 | 16 | 758.6814 | 17 | - | 0 | - | 0 |
| [M + H-5 × CH3COOH]+ | 698.6240 | 20 | 712.6395 | 22 | 726.6552 | 26 | 740.6708 | 29 | - | 0 | - | 0 |
| [M + H-CH3COOH-RC=O]+ | 728.5100 | 60 | 728.5099 | 62 | 728.5101 | 68 | 728.5000 | 70 | 670.5038 | 67 | 612.4984 | 70 |
| [M + H-2 × CH3COOH-RC=O]+ | 668.4888 | 18 | 668.4888 | 18 | 668.4885 | 19 | 668.4888 | 21 | 610.4827 | 23 | 552.4773 | 17 |
| [M + H-3 × CH3COOH-RC=O]+ | 608.4677 | 16 | 608.4677 | 16 | 608.4677 | 17 | 608.4678 | 17 | 550.4616 | 14 | 492.4563 | 9 |
| [M + H-4 × CH3COOH-RC=O]+ | 548.4465 | 12 | 548.4466 | 10 | 548.4467 | 11 | 548.4467 | 9 | 490.4405 | 8 | - | 0 |
| [M + H-5 × CH3COOH-RC=O]+ | 488.4255 | 8 | 488.4256 | 7 | 488.4255 | 6 | 488.4254 | 5 | - | 0 | - | 0 |
| [A+B cycles]+ | 191.1796 | 13 | 191.1795 | 15 | 191.1796 | 16 | 191.1794 | 18 | 191.1796 | 11 | 191.1795 | 9 |
| Structure of Ion | m/z |
|---|---|
| [M + H]+ | 788.5323 |
| [M + H-terminal group + H]+ | 611.4678 |
| [M + H-terminal group-CH3COOH]+ | 551.4466 |
| [M + H-terminal group-CH3COOH-COCH2]+ | 509.4355 |
| [M + H-terminal group-2 × CH3COOH]+ | 491.4249 |
| [M + H-terminal group-A + B rings, i.e., ring C cleavage]+ | 419.2799 |
| [M + H-terminal group-A + B rings-CH3COOH]+ | 359.2583 |
| [M + H-terminal group-A + B rings-2 × CH3COOH]+ | 299.2377 |
| Cultiv. Temp | Bacterium | 1a | 1b | 1c | 1d | 1e | 2a | 2b | 2c | 2d | 2e | 3a | 3b | 3c | 3d | 3e | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 55 | G. stearothermophilus CCM 2062 | 5.5 | 1.3 | 4.5 | 2.3 | 2.9 | 5.2 | 0.3 | 2.9 | 1.6 | 1.9 | 14.7 | 2.6 | 10.7 | 9.1 | 6.8 | 12.8 | 14.0 | 1.0 |
| 58 | G. stearothermophilus ST-YPDa | 7.7 | 1.9 | 5.2 | 3.5 | 4.1 | 5.2 | 1.1 | 3.9 | 1.9 | 2.5 | 9.6 | 1.6 | 9.1 | 5.8 | 5.7 | 6.3 | 23.5 | 1.4 |
| 58 | G. stearothermophilus VR-1a | 7.4 | 1.7 | 5.1 | 3.7 | 3.9 | 5.6 | 0.8 | 3.9 | 2.2 | 3.1 | 9.3 | 1.7 | 9.2 | 5.6 | 5.5 | 8.1 | 21.8 | 1.4 |
| 70 | G. stearothermophilus CCM 5965 | 9.1 | 2.0 | 7.3 | 4.4 | 4.7 | 4.9 | 1.3 | 4.2 | 2.7 | 2.9 | 5.3 | 1.2 | 6.9 | 4.2 | 5.5 | 4.2 | 27.4 | 1.8 |
| 70 | G. stearothermophilus CCM 2062 | 9.3 | 2.0 | 7.6 | 4.2 | 4.9 | 4.7 | 1.1 | 3.8 | 2.4 | 3.2 | 5.1 | 1.1 | 6.6 | 4.1 | 5.3 | 3.9 | 28.7 | 2.0 |
| 55 | M. ruber CCM 4212 | 9.0 | 1.9 | 4.6 | 1.7 | 6.0 | 9.7 | 1.8 | 2.6 | 3.1 | 4.2 | 6.7 | 1.6 | 8.7 | 5.0 | 7.9 | 4.9 | 19.9 | 0.8 |
| 65 | M. ruber CCM 4212 | 11.9 | 2.1 | 5.2 | 2.4 | 6.3 | 9.5 | 1.9 | 2.6 | 2.6 | 3.3 | 5.5 | 1.5 | 7.4 | 4.9 | 7.2 | 4.0 | 20.6 | 1.2 |
| 70 | M. ruber CCM 4212 | 12.0 | 2.4 | 6.0 | 3.0 | 6.7 | 9.0 | 1.7 | 3.0 | 3.2 | 4.1 | 4.1 | 1.3 | 6.2 | 4.7 | 6.7 | 3.2 | 21.2 | 1.5 |
| 45 | A. acidoterrestris CCM 4660 | 8.9 | 2.6 | 5.6 | 4.6 | 6.6 | 11.6 | 2.9 | 4.0 | 3.1 | 5.3 | 9.6 | 2.1 | 3.0 | 3.4 | 5.3 | 4.0 | 15.2 | 2.3 |
| 70 | A. acidoterrestris CCM 4660 | 18.8 | 3.3 | 6.3 | 4.6 | 7.9 | 10.1 | 2.4 | 3.5 | 3.0 | 5.2 | 3.8 | 1.3 | 2.4 | 3.0 | 4.9 | 3.8 | 13.3 | 2.4 |
| 65 | T. aquaticus CCM 3488 | 15.8 | 3.4 | 5.1 | 2.8 | 5.7 | 9.4 | 3.0 | 3.0 | 2.3 | 5.1 | 10.4 | 2.1 | 4.0 | 2.7 | 6.4 | 4.7 | 10.6 | 3.4 |
| 70 | T. aquaticus CCM 3488 | 16.3 | 3.7 | 5.8 | 3.1 | 6.4 | 8.8 | 2.7 | 3.1 | 2.0 | 4.7 | 8.8 | 1.7 | 3.7 | 2.7 | 5.8 | 5.1 | 11.9 | 3.7 |
| 58 | G. kaustophilus ML-1a | 11.8 | 3.0 | 7.7 | 2.2 | 4.4 | 13.7 | 3.3 | 5.9 | 3.3 | 6.7 | 6.3 | 1.5 | 5.5 | 3.7 | 4.8 | 3.3 | 10.7 | 2.2 |
| 42 | B. agri SA-1a | 10.1 | 3.6 | 9.3 | 2.8 | 5.6 | 15.7 | 2.4 | 4.4 | 4.2 | 6.1 | 3.6 | 2.8 | 3.6 | 2.8 | 7.7 | 2.8 | 10.9 | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolouchová, I.; Timkina, E.; Maťátková, O.; Kyselová, L.; Řezanka, T. Analysis of Bacteriohopanoids from Thermophilic Bacteria by Liquid Chromatography–Mass Spectrometry. Microorganisms 2021, 9, 2062. https://doi.org/10.3390/microorganisms9102062
Kolouchová I, Timkina E, Maťátková O, Kyselová L, Řezanka T. Analysis of Bacteriohopanoids from Thermophilic Bacteria by Liquid Chromatography–Mass Spectrometry. Microorganisms. 2021; 9(10):2062. https://doi.org/10.3390/microorganisms9102062
Chicago/Turabian StyleKolouchová, Irena, Elizaveta Timkina, Olga Maťátková, Lucie Kyselová, and Tomáš Řezanka. 2021. "Analysis of Bacteriohopanoids from Thermophilic Bacteria by Liquid Chromatography–Mass Spectrometry" Microorganisms 9, no. 10: 2062. https://doi.org/10.3390/microorganisms9102062
APA StyleKolouchová, I., Timkina, E., Maťátková, O., Kyselová, L., & Řezanka, T. (2021). Analysis of Bacteriohopanoids from Thermophilic Bacteria by Liquid Chromatography–Mass Spectrometry. Microorganisms, 9(10), 2062. https://doi.org/10.3390/microorganisms9102062






