Abstract
Between 2006 and 2019, serological surveys in unvaccinated domestic ducks reared outdoors in Myanmar were performed, using a haemagglutination inhibition (HI) test, to confirm H5 avian influenza virus circulation and assess temporal and spatial distribution. Positive test results occurred every year that samples were collected. The annual proportion of positive farms ranged from 7.1% to 77.2%. The results revealed silent/sub-clinical influenza A (H5) virus circulation, even in years and States/Regions with no highly pathogenic avian influenza (HPAI) outbreaks reported. Further analysis of the 2018/19 results revealed considerable differences in seroconversion rates between four targeted States/Regions and between years, and showed seroconversion before and during the sampling period. By the end of the trial, a high proportion of farms were seronegative, leaving birds vulnerable to infection when sold. Positive results likely indicate infection with Gs/GD/96-lineage H5Nx HPAI viruses rather than other H5 subtype low-pathogenicity avian influenza viruses. The findings suggested persistent, but intermittent, circulation of Gs/GD/96-lineage H5Nx HPAI viruses in domestic ducks, despite the veterinary services’ outbreak detection and control efforts. The role of wild birds in transmission remains unclear but there is potential for spill-over in both directions. The findings of this study assist the national authorities in the design of appropriate, holistic avian influenza control programs.
1. Introduction
Highly pathogenic avian influenza viruses of the H5Nx subtype viruses in the Goose/Guangdong/1/96-lineage (Gs/GD/96-lineage) emerged in China in 1996. Viruses in this lineage are recognised as zoonotic pathogens with pandemic potential and have caused severe outbreaks of disease in poultry, including multi-continent epizootics since 2005 [1,2]. Most countries have eliminated these viruses from poultry following incursions but, in some places, strains of virus within this lineage remain endemic [3].
Gs/GD/96-lineage H5N1 virus was first detected in Myanmar in an outbreak in 2006 [4]. Since then, the Livestock Breeding and Veterinary Department (LBVD) of the Ministry of Agriculture, Livestock and Irrigation (MOALI) has reported multiple outbreaks, predominantly in chickens. Viruses within this lineage have also been detected in live poultry markets in samples from the environment and live birds [5]. In addition to the duck sero-surveillance, the LBVD conducted targeted, active virus surveillance from 2014 to 2019, with technical support from the Food and Agriculture Organization of the United Nations (FAO) to detect potentially zoonotic avian influenza virus (AIV) incursion and its in-country spread. H5Nx virus was detected on multiple occasions (Wong et al., in preparation).
A combination of factors places Myanmar at high risk for HPAI virus introduction, spread and persistence: poultry are commonly sold through live bird markets (LBM); Myanmar has areas with large and growing duck populations; there are shared borders with HPAI-endemic countries, where cross-border-trade control measures for animal diseases can be challenging to enforce [6]; and, during the study period, most poultry production was conducted in household flocks or small farms with low biosecurity.
In Myanmar, several genetically distinct clades within the Gs/Gd/96-lineage have been detected. Some were detected over a number of years (e.g., clade 2.3.4.2), whereas others have disappeared/been eliminated (e.g., clade 7 viruses detected only in 2006), similar to reports from other countries [7].
From 2007 to 2019, Gs/GD/96-lineage H5N1 HPAI was reported from Myanmar in nine States/Regions. (In Myanmar, States and Regions are similar administrative areas. States have a predominant ethnic group). Outbreaks occurred more than once in several States/Regions. The affected domestic poultry species included chickens, ducks, quails, and geese [8,9,10].
From 2014 onwards, the LBVD detected Gs/GD/96-lineage H5N6 subtype HPAI viruses in samples both from border townships and from Mandalay and Yangon LBM [9] but not from farms. A number of these H5N6 positive samples were from ducks but the virus has not been associated with any reported outbreaks.
The role of domestic ducks in Myanmar in the epidemiology of H5 avian influenza remains unclear. In other countries, such as Cambodia, China, and Vietnam, domestic ducks are considered important for Gs/GD/96-lineage H5Nx avian influenza virus transmission and likely play a role in virus persistence. HPAI virus infection in ducks may be subclinical: clinical signs in ducks depend on both the age of the birds and the strain of virus [11,12,13]. In experimental studies, most ducks do not shed viable virus for more than seven to 10 days post-infection. Long-term carriage by individual ducks is rarely detected, with viral RNA shedding reported for up to 28 days in one experimental study [14]. Limited information is available on virus persistence at the flock level.
Compared to several other countries in the region, Myanmar does not have a large duck population, but certain parts of the country have relatively high concentrations of ducks, especially some lower and southern States/Regions. The Myanmar duck population grew steadily between 2007 and 2017 [15]. More than 80% of the duck population was in four of 14 States/Regions that have particularly high duck densities, namely, Ayeyarwady Region, Bago Region, Mon State and Yangon Region. In contrast, the national chicken population was more evenly distributed throughout the country. The 2018 MOALI National Livestock Survey estimated national poultry populations at 7.3 million ducks and 73.2 million chickens, of which 55% and 60%, respectively, were recorded as ‘native type’ [16].
Duck production in Myanmar is tied to seasonal factors associated with rice farming. In the wet season months, generally May to October, duck farmers sell mature ducks and introduce the young ones for rearing. Around July, they sell older, ‘once-moulted’ (one year old) and ‘twice-moulted’ (two-year old) laying ducks. From July to September/October, farmers acquire day-old ducks from traditional hatcheries and then, after the seasonal rice harvest, keep the duck flocks in the fields to feed on spilt grains and produce eggs.
Since 2006, with technical support from the FAO, the LBVD carried out influenza A (H5) sero-surveillance in the Myanmar duck population as described in this article. Serological studies in Myanmar are not complicated by vaccination against H5 avian influenza. Vaccination is prohibited and, given that most infections with H5 viruses in ducks are subclinical, there is no incentive for farmers to vaccinate ducks illegally.
Following Gs/GD/96-lineage H5N1 HPAI detection in poultry outbreaks in the Mandalay Region in 2006, in the same year, the first serological surveillance for subtype H5Nx AIV was carried out in Myanmar duck populations. Subsequently, serological surveillance in duck populations was conducted in all years except 2013. For convenience, the LBVD collected the sera in February, March and April as these months are in the dry season, fit with the duck production cycle, and precede the spring festival.
Some work on serology in Myanmar ducks [6,17] as well as in ducks in other Asian countries [3,18,19,20] is reported elsewhere. These studies have demonstrated that domestic ducks are exposed to H5 avian influenza viruses and have the potential to play an important role as bridge species for avian influenza viruses between wild birds and terrestrial poultry. Serological evidence of Gs/GD/96-lineage H5Nx circulation in Ayeyarwady in 2009 was previously published [21]. A longitudinal study, conducted in 2009–2010, assessed the extent of transmission of H5 avian influenza viruses in duck flocks in Bago Region near a wetland/wild bird sanctuary [6]. Seroconversion occurred in ducks over a 7-month period. No virus was isolated from specimens collected from these birds, but the results strongly suggested that infection occurred throughout the drier months from November to March. By the end of the study, 61 of 64 previously negative flocks had evidence of at least one seropositive bird.
The FAO supported the LBVD to conduct the duck sero-surveillance described here to enhance the veterinary authorities’ knowledge of influenza A (H5) epidemiology in the country and to provide information for control policy options. As a result, disease contingency plans were revised, even though the results of this work have not yet been fully consolidated. The results were used to prepare a policy brief, for senior veterinary officials, outlining measures that could be used to contain avian influenza. The first part of this article collates and describes broad findings from duck surveillance since 2006. In 2018 and 2019, additional studies and data analysis were undertaken on sentinel farms over a 3-month period during the dry season in four States/Regions with high duck numbers. The article includes the findings from the broad sero-surveillance program, the sentinel surveillance, and an assessment of ways that the results since 2006 can be used to shape control and surveillance policies.
2. Materials and Methods
2.1. Sampling Locations
Each year, other than 2013, the FAO and the LBVD selected high-risk States/Regions as surveillance sites to acquire serological evidence of Gs/GD/96-lineage H5Nx virus circulation in duck populations. The States/Regions targeted for duck serological surveillance varied from year to year. State/Region selection was risk-based according to the following factors: high duck population density, locations with previous outbreak reports, proximity to wetlands that are over-wintering sites for migratory wildfowl, presence of populations of other poultry species, and places where poultry value chain studies identified virus incursion risks. Table 1 shows selection criteria that applied to specific States/Regions surveyed.
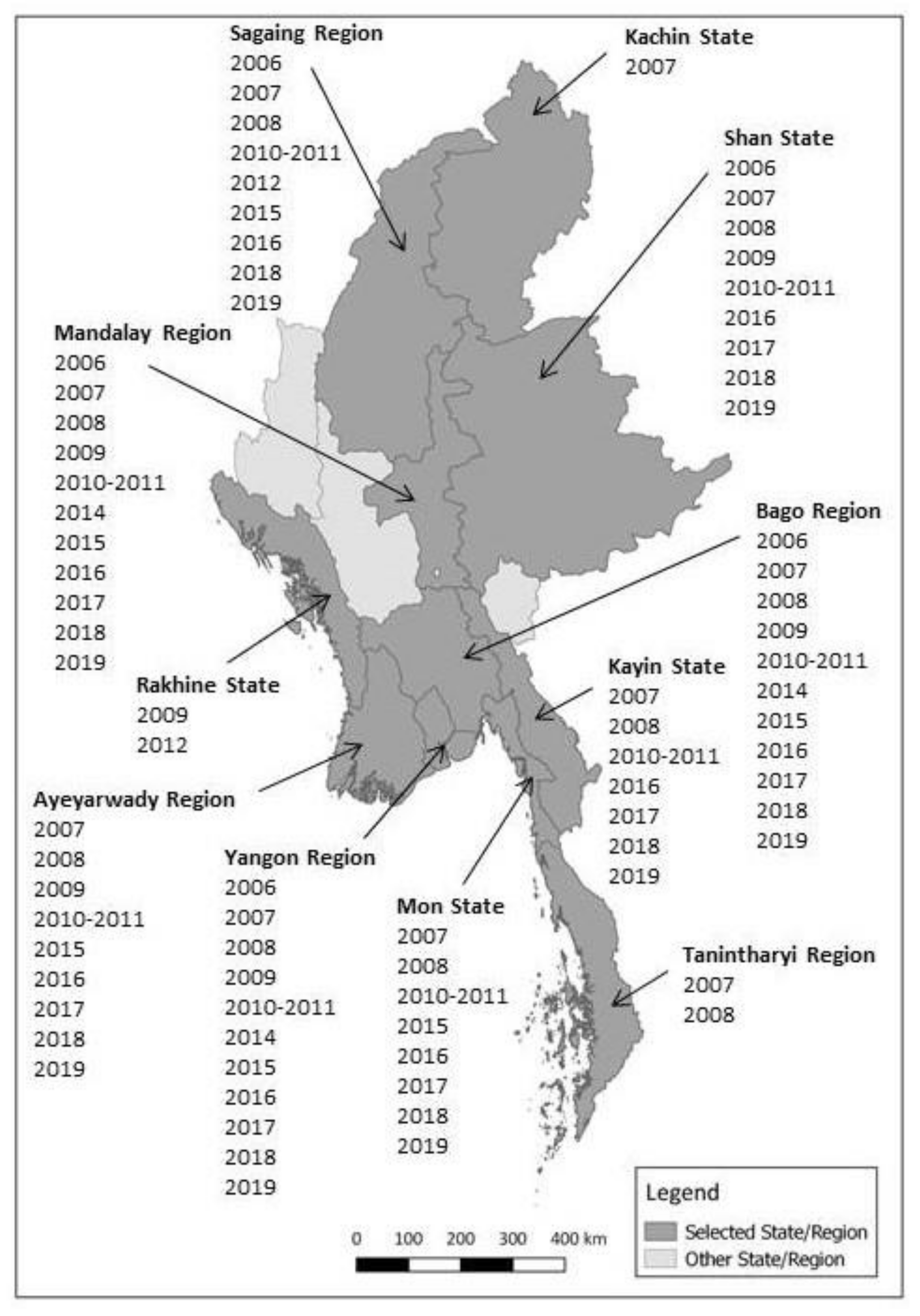
Figure 1 shows the locations of selected States and Regions, and also indicates the years when samples were collected. Townships were selected within each State/Region and then farms/flocks were selected in each township.
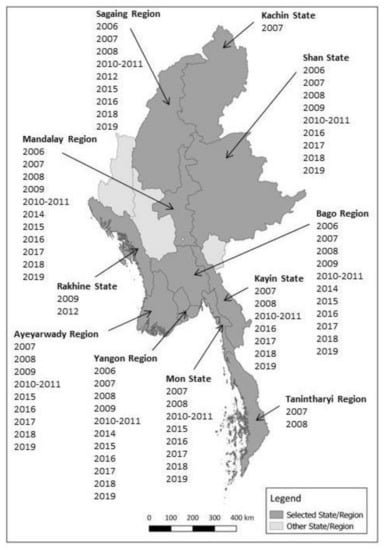
Figure 1.
Map of Myanmar States and Regions selected for duck sero-surveillance and years of sampling.

Table 1.
Criteria used for selecting states/regions for sero-surveillance in Myanmar.
Table 1.
Criteria used for selecting states/regions for sero-surveillance in Myanmar.
| State/Region | Selection Criteria |
|---|---|
| Ayeyarwady Region |
|
| Bago Region |
|
| Yangon Region |
|
| Mandalay Region |
|
| Mon State |
|
| Kayin State |
|
| Shan State |
|
| Sagaing Region |
|
| Thanintharyi Region |
|
2.2. Sampling Procedure
Throughout the study, sampling was purposive, not randomized. Within States or Regions, logistic practicality influenced township and farm/flock selection. Generally, specimens (blood drawn from the ulnar vein) were collected from 30 birds per flock at each sampling throughout the study period, though sometimes fewer than 30 could be tested at each sampling due to poor sample quality or other practical considerations in a particular year. As described in Table 2, the study included only one round of sampling in its first years. Later, multiple collection rounds were conducted, that is, repeated visits to the same flocks, with sampling once a month over three to five consecutive months, to detect sero-conversion in selected flocks in a particular year.
The number of farms and samples, and the locations where sampling was carried out, are summarised in Table 2. In most years, the sampling focused on targeting high-risk duck populations, but in some years, a national cross-sectional study or cohort study was carried out as well. According to design parameters, the sampling surveillance can be considered in four time periods during the whole study period from 2006 to 2019. The four periods were: 2006–2009 risk-based surveillance; 2009 cross-sectional study; 2010–2011 cohort study; and 2012–2019 risk-based surveillance.

Table 2.
Overview of duck sero-surveillance from 2006 to 2019.
Table 2.
Overview of duck sero-surveillance from 2006 to 2019.
| Time Period and Type of Surveillance | Year | No. of Farms/Flocks Sampled | No. of Sample Rounds | No. of Samples Collected/Tested | Samples Tested Per Farm Per Round | No. of Townships Sampled | No of States/Regions | State/Region Sampled |
|---|---|---|---|---|---|---|---|---|
| 2006–2009: Risk-based surveillance | 2006 | 84 | 1 | 2,331 | 28 | 17 | 5 | Bago, Mandalay, Sagaing, Shan, Yangon |
| 2007 | 418 | 1 | 8,898 | 21 | 54 | 10 | Ayeyarwady, Bago, Kachin, Kayin, Mandalay, Mon, Sagaing, Shan, Tanintharyi, Yangon | |
| 2008 | 337 | 1 | 7,378 | 22 | 39 | 9 | Ayeyarwady, Bago, Kayin, Mandalay, Mon, Sagaing, Shan, Tanintharyi, Yangon | |
| 2009 | 94 | 1 | 1,378 | 15 | 7 | 3 | Ayeyarwady, Rakhine, Yangon | |
| 2009: Cross-sectional study | 2009 | 281 | 1 | 8,237 | 30 | 28 | 5 | Ayeyarwady, Bago, Mandalay, Shan, Yangon |
| 2010–2011: Cohort study | 2010–2011 | 101 | 5 | 14,467 | 30 | 50 | 8 | Ayeyarwady, Bago, Kayin, Mandalay, Mon, Sagaing, Shan, Yangon |
| 2012–2019: Risk-based surveillance | 2012 | 101 | 1 | 612 | 6 | 2 | 2 | Rakhine, Sagaing |
| 2014 | 9 | 1 | 270 | 30 | 3 | 3 | Bago, Mandalay, Yangon | |
| 2015 | 50 | 3 | 4,516 | 30 | 10 | 6 | Ayeyarwady, Bago, Mandalay, Mon, Sagaing, Yangon | |
| 2016 | 100 | 3 | 8,997 | 30 | 20 | 8 | Ayeyarwady, Bago, Kayin, Mandalay, Mon, Sagaing, Shan, Yangon | |
| 42 | 3 | 3,780 | 30 | 14 | 7 | Ayeyarwady, Bago, Kayin, Mandalay, Mon, Shan, Yangon | ||
| 2018 | 100 | 3 | 9,000 | 30 | 20 | 8 | Ayeyarwady, Bago, Kayin, Mandalay, Mon, Sagaing, Shan, Yangon | |
| 2019 | 100 | 3 | 8,940 | 30 | 20 | 8 | Ayeyarwady, Bago, Kayin, Mandalay, Mon, Sagaing, Shan, Yangon | |
| Total | 1817 | 78,804 | 87 | 11 |
2.3. Laboratory Test Procedure
Although the sampling strategy varied from year to year, the test procedure was consistent and, despite imperfections, the analysis considers the entire study period together. The Veterinary Diagnostic Laboratories in Yangon and Mandalay tested serum samples using a haemagglutination inhibition (HI) assay to measure antibody titres against circulating H5 subtypes. Note that samples were not tested using a type A-specific ELISA test, which was in line with the aim of this study to detect evidence of infection with H5 viruses in ducks. Other sub-national laboratories tested samples from 2009 to 2011. HI tests were conducted according to the OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals [22]. A farm was considered positive if at least one of the serum samples collected from its duck flock was positive. Serum samples were considered positive if there was inhibition at a serum dilution of 1/16 or more against four hemagglutination units of antigen.
HI test antigens were prepared from influenza A (H5N1) viruses isolated from Myanmar chickens that died from infection in field outbreaks during 2006–2017. A clade 7 H5N1 virus isolated by Yangon Veterinary Diagnostic Laboratory (YVDL) was used as the test antigen in 2006. A clade 2.3.4 H5N1 virus isolated in YVDL in 2007 was used as the test antigen from 2007 to 2015. H5N1 virus (clade 2.3.4.2) from a 2015 Monywa outbreak was isolated by YVDL and used in 2016 and 2017. A clade 2.3.2.1c H5N1 virus isolated in 2017 was used as the antigen in 2018 and 2019. The Australian Animal Health Laboratory, now the Australian Centre for Disease Preparedness (ACDP), produced this antigen and the positive and negative sera. It was supplemented by locally produced antigen from the same virus, tested in parallel with the ACDP antigen.
2.4. Case Definition and Interpretation of Results
In the first part of this analysis, for data from 2010 to 2011 and from 2015 to 2019, the number of farms visited in each sampling round was counted. The number of “infected farms” included any farm that had at least one seropositive bird in any round of the study. For example, if samples were collected from 30 farms per month over a three-month period, this would be counted as 90 farms. If the same farm tested positive in two months, it was recorded as two positive farms.
For the period from 2018 to 2019, additional analyses were undertaken on sentinel flocks in four States/Regions known to have a high concentration of ducks, namely, Ayeyarwady, Bago, Mon and Yangon. In each of these States/Regions, up to five locations and five sentinel farms in each location were selected. Specimens were collected from 30 ducks each month for three months between February and April (with a few samples extending into May). In 2018, the set of 30 tested farms was different from those tested in 2019.
Results from individual flocks were examined for evidence of seroconversion. For this component of the study, individual flocks in each month were categorised, based on the percentage of birds with evidence of a titre at or above log24, as follows: zero seropositive (no samples with a titre ≥log24); low seropositive (≤10% of individual birds seropositive with a titre ≥log24); medium seropositive (>10% but ≤ 50% of individual birds in a flock with a titre ≥log24); and high seropositive (>50% in flock with a titre ≥ log24).
Additional details of the methods used are provided in Appendix A.
3. Results
3.1. Overall Results
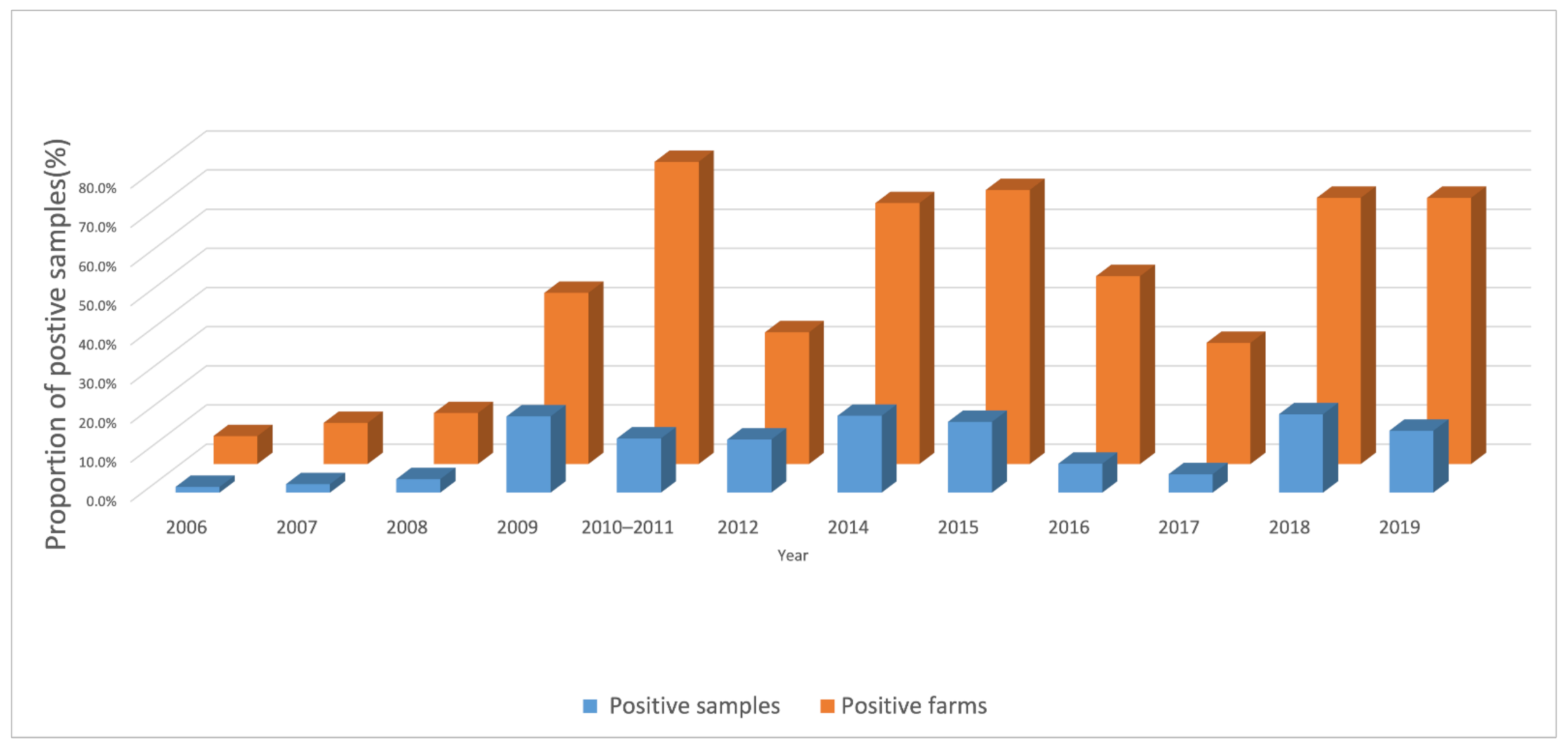
From 2006 to 2019, 78,804 duck serum samples were tested (Table 2, above). During that period, the annual proportion of positive duck farms ranged from 7.1% to 77.2% and the proportion of positive samples ranged from 1.5% to 20.0% (Figure 2). Across all years during the study, 33.5% of 1817 farms and 11.9% of 78,804 samples were H5 antibody positive.
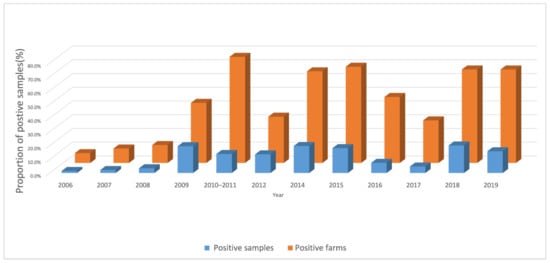
Figure 2.
Proportion H5-positive farms and samples, from 2006 to 2019, by year.
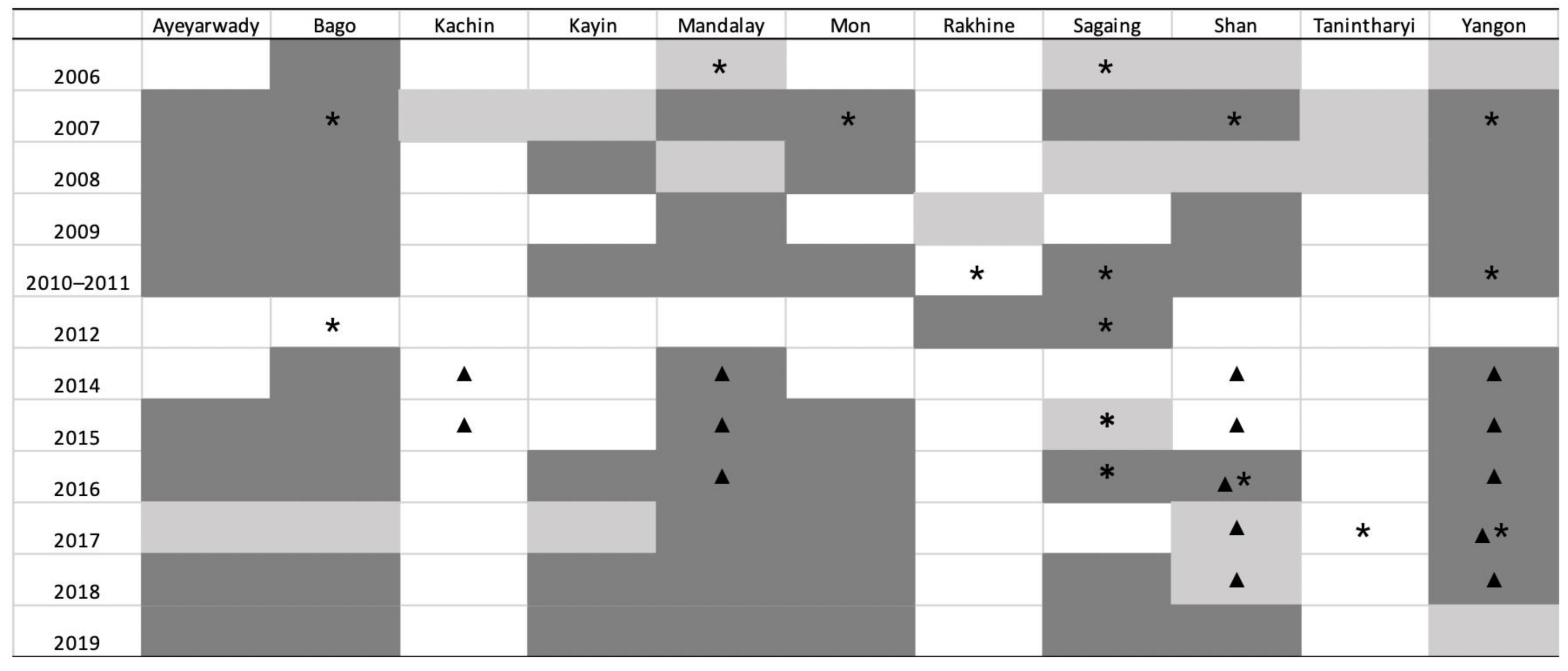
The geographical implementation of the sero-surveillance in Myanmar over time is summarised in Figure 3, which also presents the States/Regions where sero-positive farms were found. Additional information about HPAI outbreaks reported to the OIE, and about AIV detection through active surveillance [9,10], are superimposed on Figure 3.
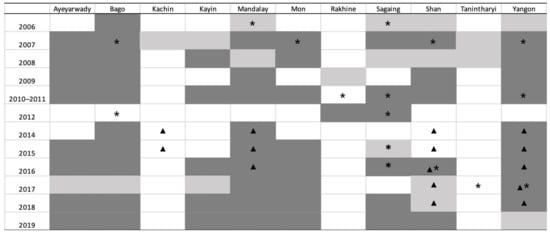
Figure 3.
Study implementation from 2006 to 2019 by State/Region and year. Light shading: State/Region where serological surveillance was carried out, but no positives were found. Dark shading: State/Region where serological surveillance was carried out and positive farms were detected. Asterisk (*): State/Region where HPAI outbreak was reported in the OIE WAHIS. Triangle (▲): State/Region where HPAI was confirmed by PCR in active surveillance.
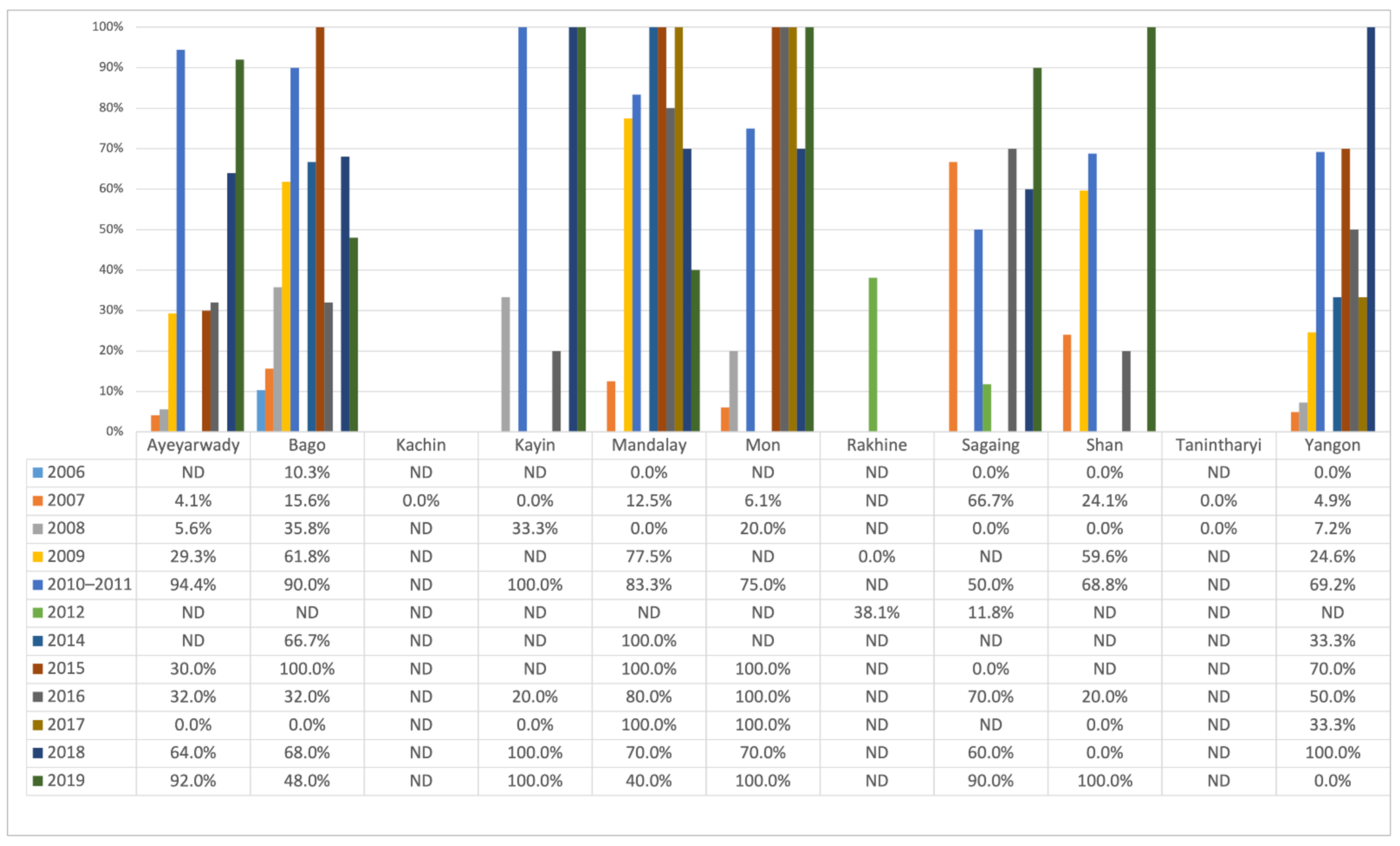
Figure 4 shows that the proportion of positive farms varied considerably at the State/Region level over the years. The variation observed differed between the States/Regions, and no common trend was observed within and between the different States/Regions.
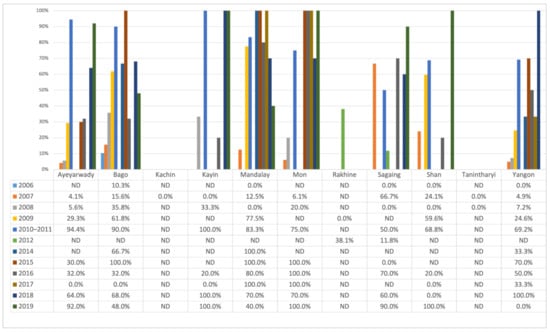
Figure 4.
Annual farm sero-positivity percentage by State/Region from 2006 to 2019.
3.2. Additional Data Analysis from 2018 to 2019
Results from 70 farms in four states (Ayeyarwady (25), Bago (25), Mon (10) and Yangon (10)) from 2018 to 2019 were reviewed in greater detail than those in previous years. In these States/Regions, in each year, five farms were sampled from either two or five townships. Samples were collected each month in February, March and April. With few exceptions, different farms were used in 2018 and 2019, but in each year, the same farms were tested three times. Two farms sold their flock in April 2019 and, therefore, the results for those farms during that month were not available.
At each collection, farms were classified as belonging to one of four categories: zero seropositive ducks, low seropositive, medium seropositive and high seropositive (see Methods). Results are summarised in Table 3.

Table 3.
Serological status of 70 sentinel duck flocks, 2018 and 2019.
At the first collection in February 2018 and 2019, 42/70 and 38/70 farms had no seropositive birds. Of these initially seronegative farms, 25/42 (2018) and 12/38 (2019) had evidence of seropositive birds in subsequent rounds. Eleven of the 42 seronegative farms in 2018 had at least 50% of birds tested as seropositive in at least one subsequent month, providing evidence of recent infection and likely transmission in the flock. In 2019, only three flocks moved from zero positive to >50% positive.
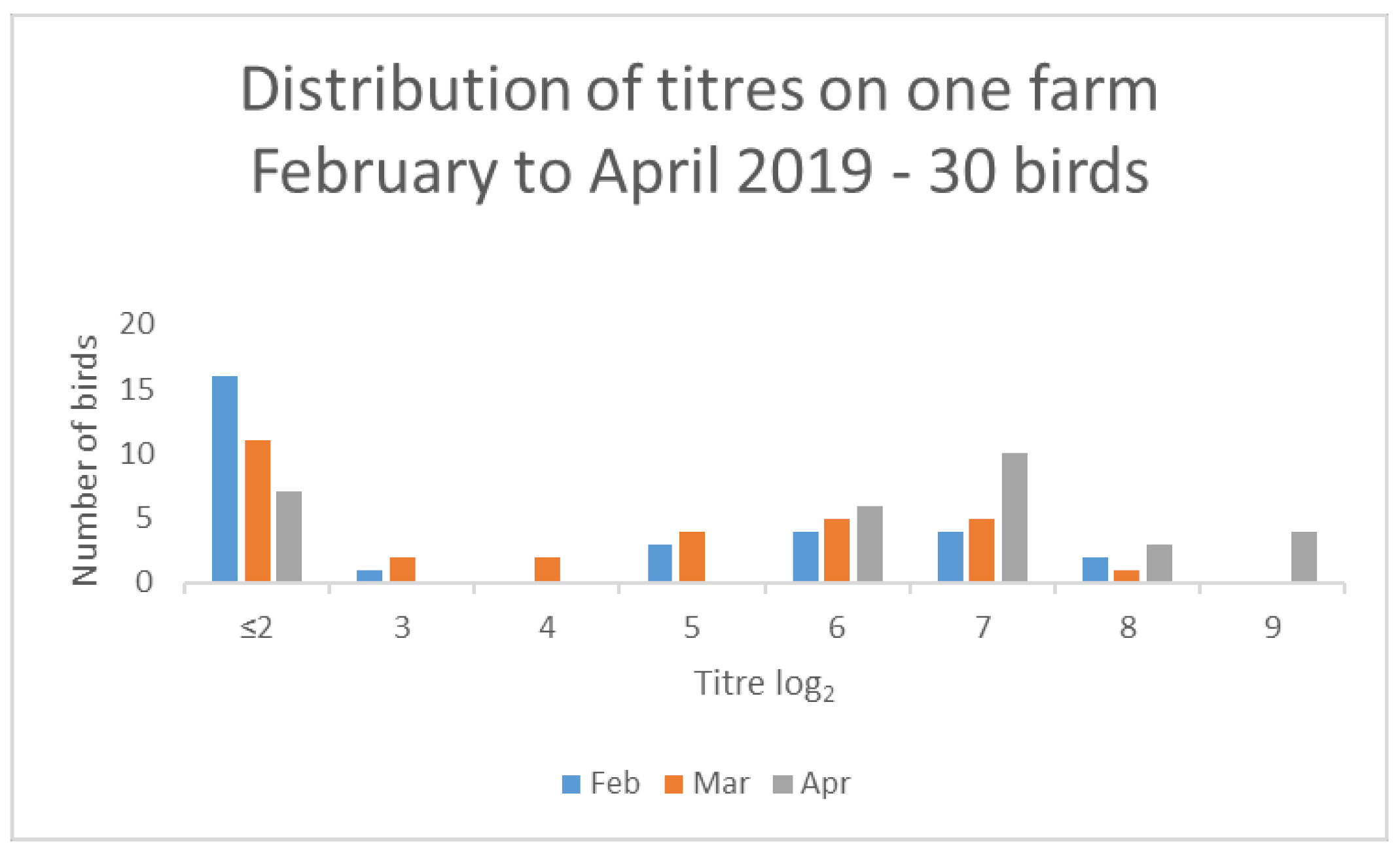
Several farms classified as medium seropositive (with titres > 10% but ≤ 50%) in February also had evidence of increased titres. For example, one farm had 13/30 seropositive birds with a median titre of log22 in February 2019 but, by April 2019, had 24/30 seropositive birds with a median titre of log27 (Figure 5).
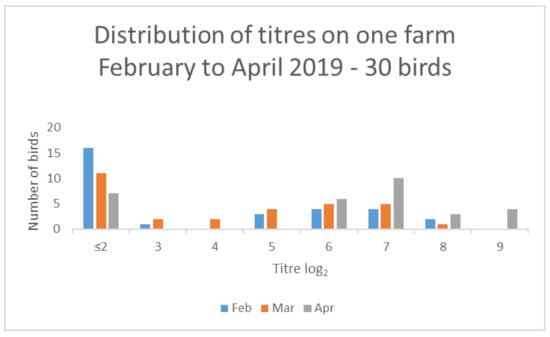
Figure 5.
Results from one farm demonstrating changes in titre distribution for 30 birds over a 3-month period.
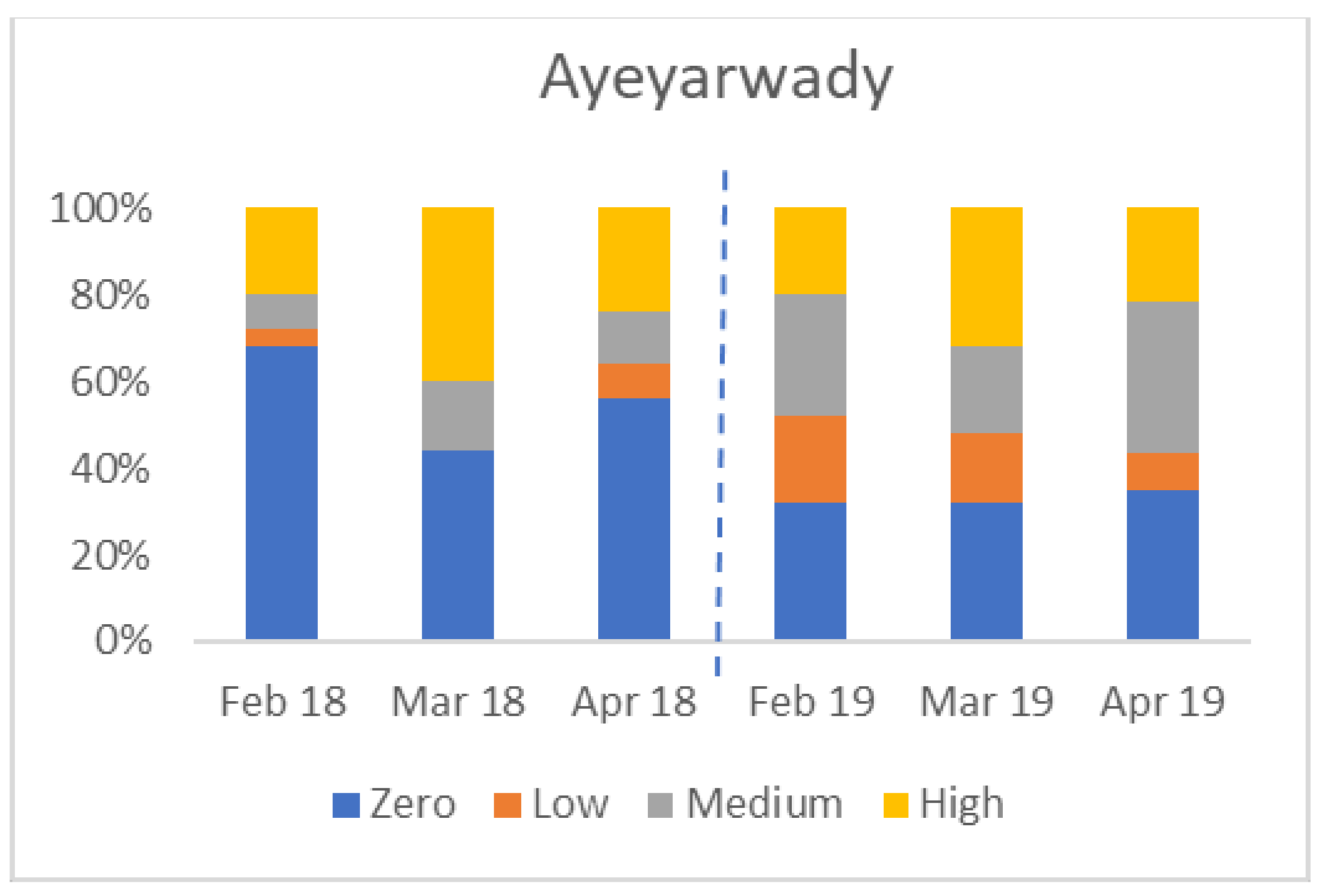
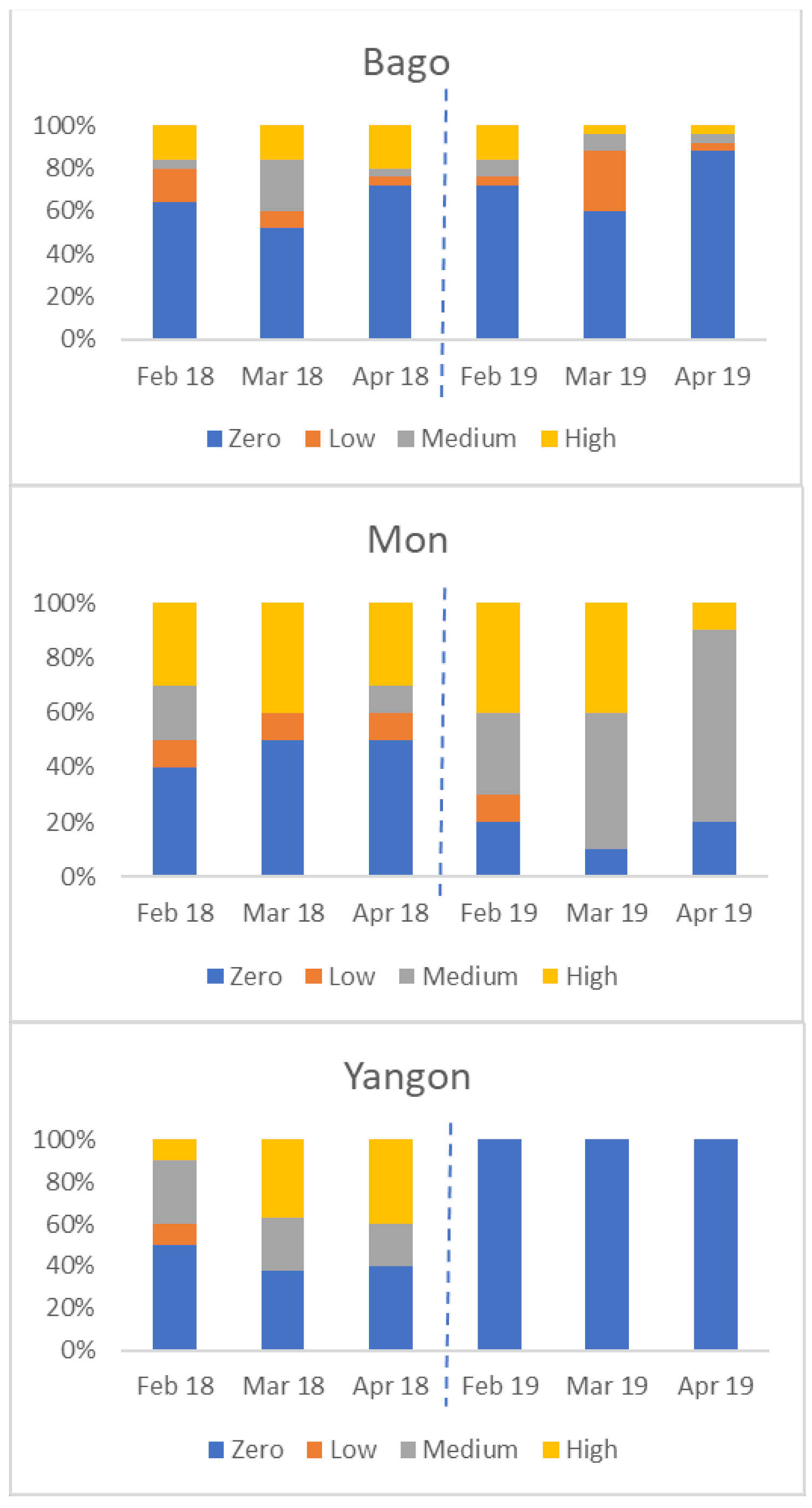
There was considerable variation between States/Regions. The four charts in Figure 6 show the percentage of farms in each of four States/Regions in each category (zero, low, medium, and high seropositive) by month. Not all areas were affected in both years; for example, Yangon had no seropositive birds in 2019.
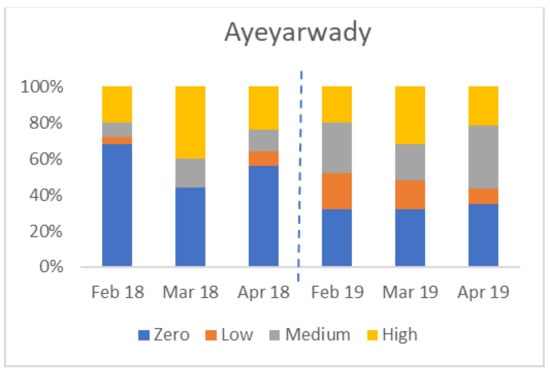
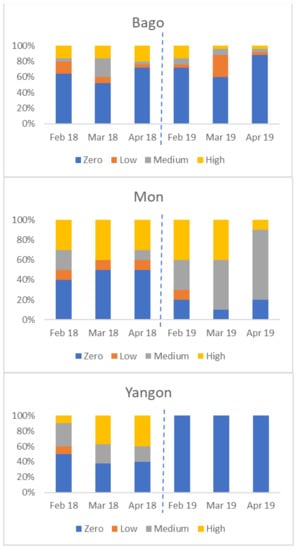
Figure 6.
Variation in percentage of flocks by serological category (Y axis) in four States/Regions, from February to April 2018 and 2019 (zero seroconversion, low seroconversion, medium seroconversion, and high seroconversion—see Materials and Methods for definitions).
4. Discussion
This study reviewed and consolidated the available information on sero-surveillance for H5 avian influenza infection in ducks, between 2006 and 2019, in Myanmar. The authors undertook these studies and the consolidation of data to gain a greater understanding of the behaviour of H5 viruses in ducks and to guide/improve future surveillance programmes and avian influenza management in Myanmar. The results indicate that most individual States and Regions had years with high numbers of positive duck farms and ducks, but there were some variations between years and between States/Regions in terms of the extent of infection.
Serological status in ducks did not always correlate with the known extent of disease. Duck populations in Ayeyarwady Region and Kayin State sero-converted to H5 virus even though no Gs/GD/96-lineage H5Nx HPAI outbreaks were ever officially reported from these two States/Regions. It is probable that farmers did not report all outbreaks of disease in small-scale poultry flocks, as has been found to occur elsewhere in South and Southeast Asia [23,24], or that infection was confined to free-ranging ducks that did not develop severe disease. In addition, the study detected seroconversion in multiple States/Regions with no recorded outbreaks in the same year, suggesting that spill-over to species in which disease is more evident did not occur or that infection was not identified/reported.
The States/Regions with the highest proportions of positive farms were Bago, Mandalay, Mon, Rakhine, and Shan, but they showed considerable variation between years. Throughout the period from 2007 to 2015, Bago Region was the most densely populated duck-raising area in Myanmar [15]; Ayeyarwady, Mon, and Yangon were the States/Regions with the second, third and fourth-largest duck populations, respectively. Results from sentinel flocks examined in these four States/Regions over two 3-month periods (February to April in 2018–2019) confirmed the variability between years and variation in infection status from month-to-month. Somewhat unexpectedly, high proportions of seropositive birds and farms were also found in Mandalay and Shan, where duck numbers are comparatively low.
These results from the past 15 years demonstrate that most areas with high concentrations of ducks in Myanmar had multiple flocks that were or became seropositive to H5 avian influenza. In the work by Cristalli et al., 61/64 of negative flocks studied in Bago State seroconverted across a 7-month period [6]. In 2018–2019, using sentinel farms, seroconversion was detected in some farms between February and April, with fewer farms being infected in 2019 than 2018.
Other studies in the region have demonstrated the presence of infected ducks in live bird markets [3]. Given the (usually) short duration of virus shedding by ducks, these findings suggest that infection can occur in the period close to sale and may result from holding ducks in traders’ yards prior to transport to markets, or prolonged holding of birds in markets, allowing them to become infected and shed virus once in the market. Results from the current study demonstrate that some flocks in Myanmar that were seropositive in February were negative by April, suggesting that immunity in these flocks is not always long-lived. The high proportion of seronegative ducks means that when these ducks were sold, they may not have had sufficient humoral immunity to resist infection. Stresses associated with transport might also play a role in increasing susceptibility to infection.
This study had some constraints and inconsistencies. Over the study period, sample areas, sample populations, sample months, and test antigen varied. In most years, the sample design used purposive, rather than random sampling, and the same farms were sampled monthly, but the number of months varied. The proportions reported in the results should be considered as indicative and may be an over-estimation or under-estimation of population prevalence, given that samples were collected using a risk-based approach rather than a random approach. Variations in the antigens employed in HI tests also need to be considered. Some gaps in background data, especially for early rounds of testing, were also noted. Although these study design features complicated and prevented statistical analysis and interpretation of combined data, the positive HI test results provided evidence that influenza A (H5) viruses were present each year that sera were tested. Despite imperfections in study design, the analysis gave indications on the spatial and temporal distribution of infection.
As only 30 samples were collected from each flock, a negative result did not rule out the possibility of the presence of low numbers of seropositive birds in the flock that were not detected in the sample of birds tested. Some positive results may also have been “false positives”, although this was unlikely given the cut-off point selected for a positive bird (log24). This was one rationale for the more detailed assessment of results in 2018 and 2019, that is, to demonstrate that seroconversion had occurred, based on a marked increase in the number of birds testing positive and increases in the median titre, rather than using a single positive sample in a flock as the indicator of seroconversion.
It is likely that an H5 HI positive test result in this study, using HA antigen derived from Gs/GD/96-lineage H5N1 virus isolated in Myanmar, indicates past infection with viruses from this lineage. Low pathogenicity H5 viruses are likely to be present in Myanmar based on the detection of a single low pathogenicity H5N2 virus in 2017 in a consignment of virus samples sent to an international reference laboratory for characterisation (Wong et al., in preparation). Future serological testing will include tests using this virus as well as significant reference strains of H5 LPAIVs as antigens to compare results with samples tested with the Gs/Gd/96 H5 antigen. Low pathogenicity H5Nx viruses are not thought to have contributed significantly to positive results. Research in wild ducks in Australia demonstrated that serum from birds that had tested positive using an HI test with Australian (Eurasian) LPAI H5 virus antigens did not test positive against any of the seven different antigens prepared from Gs/GD/96-lineage viruses [25]. Multiple Gs/GD/96-lineage H5N1 viruses have been isolated from samples from ducks in Myanmar, confirming their presence in ducks, although not from any of the farms in the 2018–2019 study. Regardless, infection with any H5 subtype virus in domestic ducks is a notifiable event and, therefore, any seroconversion of ducks to an H5 virus warrants investigation. Apart from testing for virus and viral RNA in affected flocks, this investigation could include HI assays using two H5 antigens with heterologous neuraminidase (e.g., H5N1 and H5N2) on samples to ensure that positive results are not due to steric interference mediated by anti-N antibodies [22], arising from infection with an HxN1 strain other than an H5N1 virus.
Vaccination is not considered likely as a cause of positive serology in ducks in Myanmar. Throughout the study period (2006–2019,) the Myanmar authorities used stamping-out and movement control as the primary countermeasures against Gs/GD/96-lineage H5Nx HPAI outbreaks. Avian influenza vaccination, including for Gs/GD/96-lineage H5Nx viruses, was officially prohibited. In any case, even if vaccines were available, farmers were unlikely to vaccinate ducks because mortality from avian influenza is generally lower in ducks than in chickens and because ducks are mainly maintained in a low-input, scavenger system, feeding in post-harvest rice paddy fields. Flock owners were unlikely to invest in vaccines for a disease that generally causes low mortality in ducks. Therefore, H5 antibody-positive duck sera were considered to be evidence of past infection, not vaccination.
It is possible that some negative serological results in some years were due to the use of an antigen from a virus that was no longer the dominant field strain. In 2017, no seropositive samples were detected in several States/Regions. This may have been due to the use of an antigen that was no longer a good antigenic match to circulating strains given that a clade 2.3.4.2 virus was used as an antigen. Outbreaks in 2017 were associated with clade 2.3.2,1c viruses (Wong et al., in preparation) and viruses in this clade were detected in markets at that time.
The source of infection for duck flocks is still not clear. Avian influenza viruses should not have survived for an extended period in the hot conditions occurring during the dry season from November to May in much of Myanmar, yet transmission during this period still occurred. Water contaminated by infected birds, including domestic ducks, may have played a significant role in transmission, especially when there were limited flows of water and a high concentration of ducks on existing waterways. Water source and wooden egg collection trays were found to be associated with infection in a case-control study that utilised 2009 data from Ayeyarwady (Win et al.). The role of wild ducks in the introduction and transmission of Gs/GD/96-lineage H5Nx viruses in Myanmar is yet to be determined.
It is evident that the measures in place to contain outbreaks of avian influenza in Myanmar, while effective in preventing disease, are not able to prevent infection in all duck flocks. This is not surprising given experiences with Gs/GD/96-lineage H5 viruses in most other parts of SE Asia. Options for managing infection in these flocks are being considered. As Gs/GD/96-lineage H5 viruses are expected to continue to circulate in ducks, terrestrial poultry are at risk of infection from viruses shed by infected ducks in places where they co-mingle. Segregation of ducks from terrestrial poultry along the market chain is one option to reduce this risk, as was implemented in Hong Kong in 1998 onwards, including greater use of centralised slaughter for spent layer ducks. The possibility of using vaccination could also be considered, especially if a vector vaccine, based on another important duck pathogen such as duck virus enteritis virus, became available commercially and provided protection against both diseases. Silent infection detected in unvaccinated ducks in this study negates one of the main objections to vaccination—that it would lead to silent infection. Significant changes to production are unlikely given the value that current free-grazing methods provide to duck farmers and rice producers. However, unless production methods change, virus elimination from all duck flocks remains a distant goal.
The active serological surveillance in ducks has provided a complementary tool for evidence of H5 virus circulation in areas where other detection methods, such as virus isolation and farmer reporting, may not have sufficiently informed the authorities of potential Gs/GD/96-lineage H5N1 infection. Indeed, the study results consolidated in this report indicate that H5Nx avian influenza viruses, most likely Gs/GD/96-lineage H5N1 HPAI viruses, circulated in ducks in Myanmar every year from 2006 to 2019 (excluding the year 2013, for which there were no data).
The findings confirmed the value of H5 serological surveillance in Myanmar duck populations. This information informed the authorities of likely Gs/GD/96-lineage H5N1 HPAI virus presence, distribution and persistence. It provided information on the extent to which H5 virus is infecting ducks sub-clinically, as well as options for further actions to prevent or reduce the threat posed by infected domestic ducks. Sufficient historical evidence has been collected to demonstrate that ducks are being infected with H5 viruses.
This study focused specifically on H5 avian influenza because it has been deemed a major zoonotic threat with pandemic potential. The serological tests performed only detect antibody to H5 viruses, not influenza A viruses of other subtypes. Testing for antibodies to type A influenza was not conducted because the focus of this work was on H5 avian influenza. However, other surveillance for infections with other avian influenza viruses has been conducted in various locations across Myanmar, especially in live bird markets, and has confirmed the presence of a low pathogenicity H5 virus and infection with H9N2 avian influenza viruses [5,9].
Future surveillance programmes should focus more on the detection of H5 viruses. Given the low rates of detection of H5 viruses at the farm level via swab samples from ducks, a shift to greater use of surveillance in markets or to alternative, environmental samples at the farm level, such as drinking water or feathers, should be considered [26]. Repeated, intensive duck serology is not recommended unless results can be provided in real time to assist in determining sites where H5 viruses are actively circulating and to serve as a tool for disease control and local virus elimination.
Author Contributions
Conceptualization, C.C.S.M., H.H.M. and M.T.M.; methodology, C.C.S.M., H.H.M., H.H.W., W.Z.T., M.M.K. and M.T.M.; software, n/a.; validation, A.B., L.S. and M.M.K.; formal analysis, A.B., L.S. and W.S.M.; investigation, H.H.M., H.H.W., W.Z.T. and M.M.K.; resources, D.H., H.H.M. and M.M.K.; data curation, A.B., L.S. and W.S.M.; writing—original draft preparation, A.B. and L.S.; writing—review and editing, D.H., L.S., A.B. and M.T.M.; visualization, A.B., L.S. and W.S.M.; supervision, D.H., M.T.M. and Y.T.W.; project administration, C.C.S.M. and M.M.K.; funding acquisition, D.H. and Y.T.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the US Agency for International Development and the Ministry of Agriculture, Food and Rural Affairs, the Republic of Korea, through funds to the Food and Agriculture Organization of the United Nations. The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the US Agency for International Development, Ministry of Agriculture, Food and Rural Affairs, the Republic of Korea, or the Food and Agriculture Organization of the United Nations.
Institutional Review Board Statement
This study was carried out in strict accordance with the Myanmar National Veterinary Diagnostic Laboratories regulation, “Care and Use of Laboratory Animals”. The Institutional Animal Care and Use Committee of the Myanmar National Veterinary Diagnostic Laboratories approved the sampling methods.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy concerns for participating farmers: it was not possible to obtain approval from every farmer to share raw data dating back over 10 years.
Acknowledgments
The authors gratefully acknowledge contributions from LBVD Officers who collected the samples, that is, the Township Veterinary Officers and Deputy TVO in States/Regions where sampling took place; the Mandalay and Yangon Laboratory technicians/Research Officers who performed the testing, including Khine Thwe Latt (former Research Officer, Yangon Lab), Win Win Myint (Assistant Director, Mandalay Lab), and Wai Zin Thein (Research Officer, Yangon Lab); FAO-ECTAD Officers who managed or designed the sampling strategy, including Wantanee Kalpravidh (former ECTAD Regional Manager), David Castellan, Kachen Wongsathapornchai (former ECTAD Regional Epidemiologists), Masao Sasaki, Murray Maclean, and Tony Williams (former Myanmar ECTAD Chief Technical Advisers); LBVD Officers who managed or designed the sampling strategy, including Than Hla (LBVD Director Rtd), Kyaw Sunn (Former LBVD Director), and Tin Aye Kyi (Dy. Director Rtd); and FAO colleagues who reviewed the article, including Filip Claes, Sophie von Dobschuetz, and Emma Gardner.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Map
The boundaries and names shown and the designations used on the map do not imply the expression of any opinion whatsoever on the part of FAO concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers and boundaries.
Appendix A. Additional Notes on Sampling Procedure
The sampling approach in each of the four time periods is described below. From 2006 to 2009, the strategy was high-risk area surveillance in duck populations in the States/Regions shown in Table 2. In this period, the results for 19,985 serum sample tests from 11 States/Regions were recorded. In 2009, a National Duck Cross-Sectional Study was conducted, comprising 8237 samples from 281 farms in five States/Regions (Ayeyarwady, Bago, Mandalay, Shan, and Yangon).
From 2010 to 2011, based on the 2009 National Duck Cross-Sectional Study results, serologically negative duck flocks were enrolled in a cohort study. This comprised 101 farms in eight States/Regions. On each farm, 30 serum samples were collected in each of five monthly sampling rounds over the 12-month period and 14,467 serum samples were tested.
In 2012, a more restricted sampling took place, covering one township in Rakhine State and one in Sagaing Region, where Gs/GD/96-lineage H5N1 clade 2.3.2 was previously detected. A total of 612 samples from 101 farms were analysed. No surveillance took place in 2013. In 2014, restricted surveillance resumed, covering three farms in one township in each of three Regions. A total of 270 sera were collected from the nine farms/flocks that were sampled.
From 2015 to 2019, risk-based sero-surveillance continued in locations selected according to risk factors described above. In 2015, serum sample collection took place from June to August in ten townships in six States/Regions. Five farms were selected in each township and, on average, 30 samples were collected from each farm on a monthly basis during the surveillance period. Overall, 4516 serum samples were tested. In 2016, serum samples were collected from May to July in 20 townships in eight States/Regions. Five farms were selected in each township and, on average, 30 samples were collected from each farm on a monthly basis during the surveillance period. A total of 8997 serum samples were tested.
In 2017, serum samples were collected from February to April in 14 townships in seven States/Regions. Three farms were selected in each township and, on average, 30 serum samples were collected from each farm on a monthly basis during the surveillance period. In total, 3780 serum samples were tested.
In 2018, serum sample collection took place from February to April in 20 townships in eight States/Regions. Five farms were selected in each township and, on average, 30 samples were collected from each farm on a monthly basis during the surveillance period. Overall, 9000 serum samples were tested.
In 2019, serum samples were collected from February to April in 20 townships in eight States/Regions. Five farms were selected in each township and, on average, 30 serum samples were collected from each farm on a monthly basis during the surveillance period. In total, 8940 serum samples were tested.
References
- Mostafa, A.; Abdelwhab, E.M.; Mettenleiter, T.C.; Pleschka, S. Zoonotic Potential of Influenza A Viruses: A Comprehensive Overview. Viruses 2018, 10, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaji, R.; Saad, M.D.; Davis, C.T.; Swayne, D.E.; Wang, D.; Wong, F.Y.K.; McCauley, J.W.; Peiris, J.S.M.; Webby, R.J.; Fouchier, R.A.M.; et al. Pandemic potential of highly pathogenic avian influenza clade 2.3.4.4 A(H5) viruses. Rev. Med. Virol. 2020, 30, e2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suttie, A.; Karlsson, E.A.; Deng, Y.-M.; Hurt, A.C.; Greenhill, A.R.; Barr, I.G.; Dussart, P.; Horwood, P.F. Avian influenza in the Greater Mekong Subregion, 2003–2018. Infect. Genet. Evol. 2019, 74, 103920. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Uchida, Y.; Myint, W.W.; Thein, W.Z.; Watanabe, C.; Takemae, N.; Mase, M.; Okamatsu, M.; Mar, A.; Mon, C.C.S.; et al. Characterisation of highly pathogenic avian influenza viruses in Myanmar. Vet. Rec. 2008, 163, 722–723. [Google Scholar] [CrossRef] [PubMed]
- Thurain, K.; Mon, P.P.; Nasamran, C.; Charoenkul, K.; Boonyapisitsopa, S.; Tun, T.N.; San, Y.Y.; Aye, A.M.; Amonsin, A. Surveillance of influenza A virus subtype H5N1 in a live bird market in Yangon, Myanmar: 2017–2018. Transbound. Emerg. Dis. 2020, 67, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Cristalli, A.; Morini, M.; Comin, A.; Capello, K.; Sunn, K.; Martini, M. Avian influenza epidemiology in semi-intensive free ranging duck flocks of the Moyingyi Wetland in Bago East District, Myanmar. Trop. Anim. Heal. Prod. 2018, 50, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.; Nguyen, N.T.B. Evolutionary dynamics of highly pathogenic avian influenza A/H5N1 HA clades and vaccine implementation in Vietnam. Clin. Exp. Vaccine Res. 2014, 3, 117–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mon, P.P.; Lapkuntod, J.; Maw, M.T.; Nuansrichay, B.; Parchariyanon, S.; Tiensin, T.; Htun, T.; Padungtod, P.; Kalpravidh, W.; Sunn, K.; et al. Highly pathogenic avian influenza (H5N1) in Myanmar, 2006–2010. Arch. Virol. 2012, 157, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Win, Y.T.; Gardner, E.; Hadrill, D.; Mon, C.C.S.; Kyin, M.M.; Maw, M.T.; Claes, F.; Von Dobschuetz, S.; Kalpravidh, W.; Wongsathapornchai, K.; et al. Emerging Zoonotic Influenza A Virus Detection in Myanmar: Surveillance Practices and Findings. Heal. Secur. 2017, 15, 483–493. [Google Scholar] [CrossRef]
- World Organization for Animal Health. WAHIS Interface. 2018. Available online: http://www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/diseasehome (accessed on 2 October 2021).
- Gilbert, M.; Chaitaweesub, P.; Parakamawongsa, T.; Premashthira, S.; Tiensin, T.; Kalpravidh, W.; Wagner, H.; Slingenbergh, J. Free-grazing Ducks and Highly Pathogenic Avian Influenza, Thailand. Emerg. Infect. Dis. 2006, 12, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M.; Xiao, X.; Pfeiffer, D.; Epprecht, M.; Boles, S.; Czarnecki, C.; Chaitaweesub, P.; Kalpravidh, W.; Minh, P.Q.; Otte, M.J.; et al. Mapping H5N1 highly pathogenic avian influenza risk in Southeast Asia. Proc. Natl. Acad. Sci. USA 2008, 105, 4769–4774. [Google Scholar] [CrossRef] [Green Version]
- Songserm, T.; Jam-On, R.; Sae-Heng, N.; Meemak, N.; Hulse-Post, D.J.; Sturm-Ramirez, K.M.; Webster, R.G. Domestic Ducks and H5N1 Influenza Epidemic, Thailand. Emerg. Infect. Dis. 2006, 12, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Horwood, P.F.; Fabrizio, T.; Horm, S.V.; Metlin, A.; Ros, S.; Tok, S.; Jeevan, T.; Seiler, P.; Y, P.; Rith, S.; et al. Transmission experiments support clade-level differences in the transmission and pathogenicity of Cambodian influenza A/H5N1 viruses. Emerg. Microbes Infect. 2020, 9, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Livestock Veterinary and Breeding Department. Myanmar Livestock Statistics 2017; Ministry of Agriculture Livestock and Irrigation: Nay Pyi Taw, Mynamar, 2017. [Google Scholar]
- Livestock Breeding and Veterinary Department. National Livestock Baseline Survey 2018 Report; Livestock Breeding and Veterinary Department: Yangon, Myanmar, 2019. [Google Scholar]
- Lin, T.N.; Bunpapong, N.; Boonyapisitsopa, S.; Chaiyawong, S.; Janetanakit, T.; Rain, K.T.; Mon, P.P.; Oo, S.M.; Thontiravong, A.; Amonsin, A. Serological evidence of avian influenza virus subtype H5 and H9 in live bird market, Myanmar. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101562. [Google Scholar] [CrossRef] [PubMed]
- Yuyun, I.; Wibawa, H.; Setiaji, G.; Kusumastuti, T.A.; Nugroho, W.S. Determining highly pathogenic H5 avian influenza clade 2.3.2.1c seroprevalence in ducks, Purbalingga, Central Java, Indonesia. Vet. World 2020, 13, 1138–1144. [Google Scholar] [CrossRef]
- Sarker, R.D.; Giasuddin, M.; Chowdhury, E.H.; Islam, M.R. Serological and virological surveillance of avian influenza virus in domestic ducks of the north-east region of Bangladesh. BMC Vet. Res. 2017, 13, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karki, S.; Lupiani, B.; Budke, C.M.; Manandhar, S.; Ivanek, R. Cross-Sectional Serosurvey of Avian Influenza Antibodies Presence in Domestic Ducks of Kathmandu, Nepal. Zoonoses Public Heal. 2013, 61, 442–448. [Google Scholar] [CrossRef]
- Win, H.H.; Mon, C.S.; Aung, K.M.; Oo, K.N.; Sunn, K.; Htun, T.; Tiensin, T.; Maclean, M.; Kalpravidh, W.; Amonsin, A. Risk factors of Highly Pathogenic Avian Influenza (HPAI) in Duck Farms in Ayeyarwaddy Delta Region, Myanmar. Myanmar Vet. J. 2013, 15, 1. [Google Scholar]
- World Organization for Animal Health. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2021. Avian Influenza (Including Infection with High Pathogenicity Avian Influenza Viruses). May 2021. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.03.04_AI.pdf (accessed on 2 October 2021).
- Delabouglise, A.; Le Thanh, N.T.; Xuyen, H.T.A.; Nguyen-Van-Yen, B.; Tuyet, P.N.; Lam, H.M.; Boni, M.F. Poultry farmer response to disease outbreaks in smallholder farming systems in southern Vietnam. eLife 2020, 9, 9. [Google Scholar] [CrossRef]
- Sultana, R.; Rimi, N.A.; Azad, S.; Islam, M.S.; Khan, M.S.U.; Gurley, E.S.; Nahar, N.; Luby, S. Bangladeshi backyard poultry raisers’ perceptions and practices related to zoonotic transmission of avian influenza. J. Infect. Dev. Ctries. 2011, 6, 156–165. [Google Scholar] [CrossRef]
- Wille, M.; Lisovski, S.; Risely, A.; Ferenczi, M.; Roshier, D.; Wong, F.; Breed, A.; Klaassen, M.; Hurt, A.C. Serologic Evidence of Exposure to Highly Pathogenic Avian Influenza H5 Viruses in Migratory Shorebirds, Australia. Emerg. Infect. Dis. 2019, 25, 1903–1910. [Google Scholar] [CrossRef]
- Hood, G.; Roche, X.; Brioudes, A.; von Dobschuetz, S.; Fasina, F.O.; Kalpravidh, W.; Makonnen, Y.; Lubroth, J.; Sims, L. A literature review of the use of environmental sampling in the surveillance of avian influenza viruses. Transbound. Emerg. Dis. 2021, 68, 110–126. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© FAO, 2021. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Com-mons Attribution (CC BY NC SA) license (https://creativecommons.org/licenses/by-nc-sa/3.0/igo/).