Interaction between Grasses and Epichloë Endophytes and Its Significance to Biotic and Abiotic Stress Tolerance and the Rhizosphere
Abstract
:1. Introduction
2. Evolution of Epichloë Mutualistic Association with Grasses
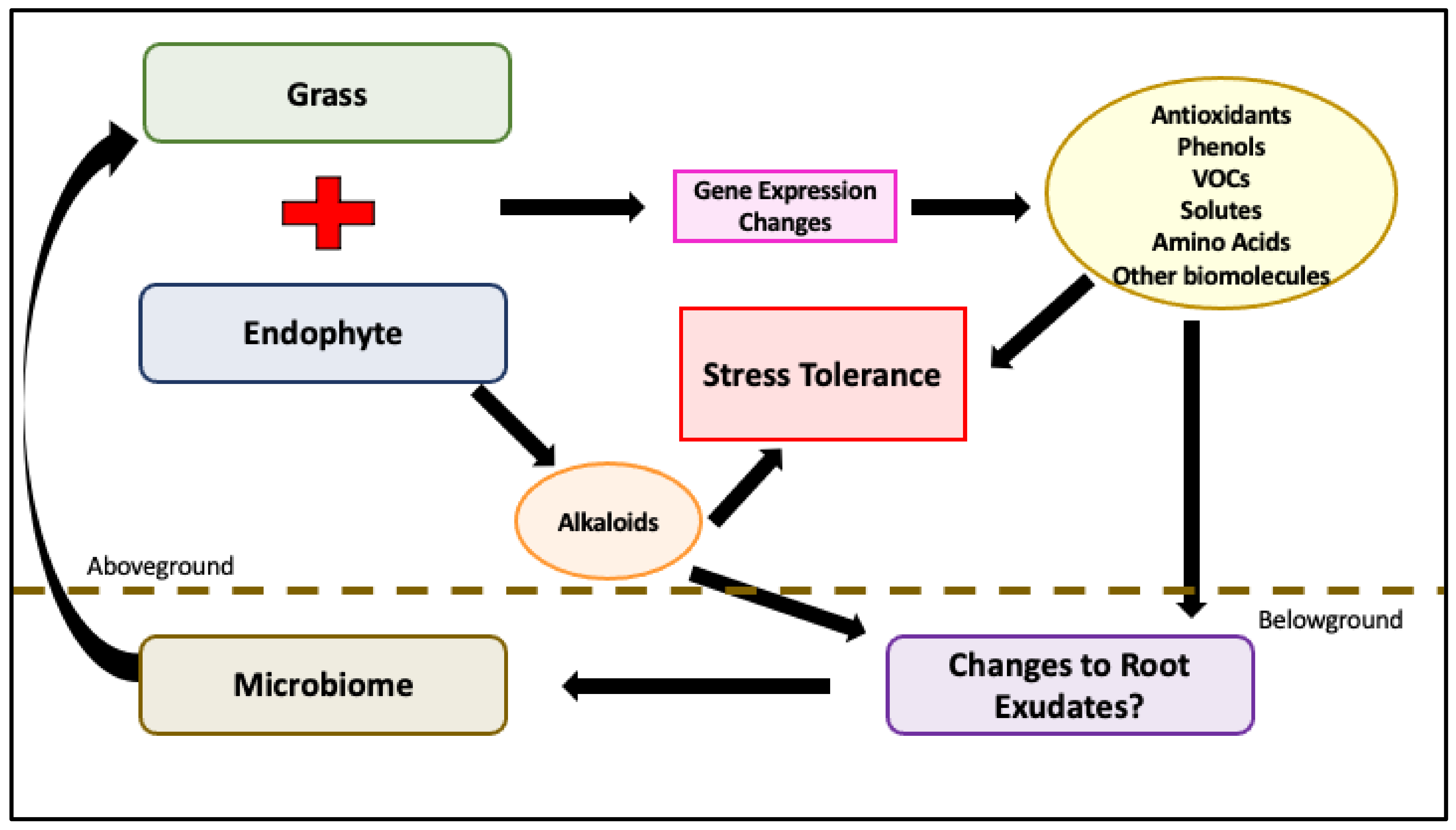
3. Mechanisms of Interaction between Grass and Endophyte
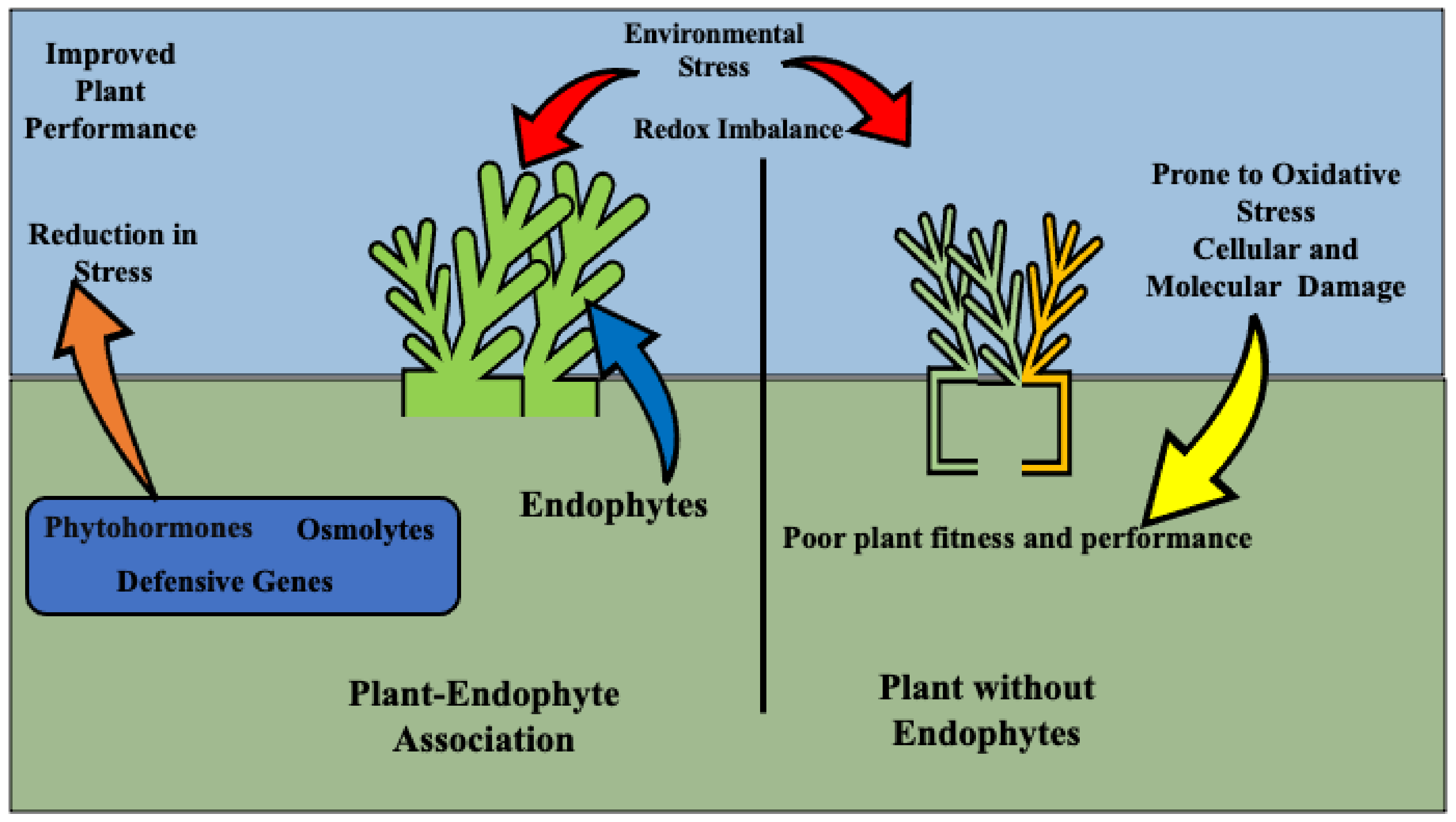
4. Environmental Effect on Endophyte/Grass Associations
5. Stress Tolerance Mechanisms
5.1. Drought
5.2. Salinity
5.3. Heavy Metals
5.4. Low Nutrients
5.5. Cold
5.6. Flood
5.7. Pathogens
5.8. Nematodes
5.9. Insects
5.10. Weed Competition
5.11. Animal Herbivory
6. Epichloë Infected Grass Interaction with Rhizosphere Microbiome
6.1. Epichloë Effect on the Rhizosphere
6.2. Microbiome Changes in Response to Stress
6.3. Microbiome Changes Due to Endophyte Infection
7. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hanson, J.; Cantrell, J.; Proud, J.; Debouck, D.; Cuervo, M.; Schultze-Kraft, R.; Jorge, A. Forage: Grass. 2021. Available online: https://www.genebanks.org/resources/crops/forages-grass/ (accessed on 1 July 2021).
- Sanderson, M.; Jolley, L.W.; Dobrowolski, J. Pasture and hayland in the USA: Land resources, conservation practices, and ecosystem services. In Conservation Outcomes from Pastureland and Hayland Practices: Assessment, Recommendations, and Knowledge Gaps; Allen Press: Lawrence, KS, USA, 2012; pp. 25–40. [Google Scholar]
- Wilkins, P.W.; Humphreys, M.O. Progress in breeding perennial forage grasses for temperate agriculture. J. Agric. Sci. 2003, 140, 129–150. [Google Scholar] [CrossRef]
- Moser, L.E.; Hoveland, C.S. Cool-Season Grass Overview; Moser, L.E., Buxton, D.R., Casler, M.D., Eds.; ASA, CSSA, SSSA: Madison, WI, USA, 1996; Volume 34. [Google Scholar]
- Leuchtmann, A. Systematics, distribution, and host specificity of grass endophytes. Nat. Toxins 1993, 1, 150–162. [Google Scholar] [CrossRef]
- Young, C.A.; Charlton, N.D.; Takach, J.E.; Swoboda, G.A.; Trammell, M.A.; Huhman, D.V.; Hopkins, A.A. Characterization of Epichloe coenophiala within the US: Are all tall fescue endophytes created equal? Front. Chem. 2014, 2, 95. [Google Scholar] [CrossRef] [Green Version]
- Faeth, S.H.; Hamilton, C.E. Does An asexual endophyte symbiont alter life stage and long-term survival in a perennial host grass? Microb. Ecol. 2006, 52, 748–755. [Google Scholar] [CrossRef]
- Easton, H.S. Grasses and neotyphodium endophytes: Co-adaptation and adaptive breeding. Euphytica 2007, 154, 295–306. [Google Scholar] [CrossRef]
- Schardl, C.L.; Craven, K.D.; Speakman, S.; Stromberg, A.; Lindstrom, A.; Yoshida, R. A novel test for host-symbiont codivergence indicates ancient origin of fungal endophytes in grasses. Syst. Biol. 2008, 57, 483–498. [Google Scholar] [CrossRef] [Green Version]
- Frank, S.A. Genetics of mutualism: The evolution of altruism between species. J. Theor. Biol. 1994, 170, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.F.; Liu, J.S.; Staben, C.; Christensen, M.J.; Latch, G.C.; Siegel, M.R.; Schardl, C.L. Evolutionary diversification of fungal endophytes of tall fescue grass by hybridization with Epichloë species. Proc. Natl. Acad. Sci. USA 1994, 91, 2542–2546. [Google Scholar] [CrossRef] [Green Version]
- Moon, C.D.; Craven, K.D.; Leuchtmann, A.; Clement, S.L.; Schardl, C.L. Prevalence of interspecific hybrids amongst asexual fungal endophytes of grasses. Mol. Ecol. 2004, 13, 1455–1467. [Google Scholar] [CrossRef]
- Saikkonen, K.; Young, C.A.; Helander, M.; Schardl, C.L. Endophytic Epichloë species and their grass hosts: From evolution to applications. Plant Mol. Biol. 2016, 90, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Schardl, C.L.; Leuchtmann, A.; Chung, K.R.; Penny, D.; Siegel, M.R. Coevolution by common descent of fungal symbionts (Epichloe spp.) and grass hosts. Mol. Biol. Evol. 1997, 14, 133. [Google Scholar] [CrossRef] [Green Version]
- Bacon, C.W.; Siegel, M.R. Endophyte parasitism of tall fescue. J. Prod. Agric. 1988, 1, 45–55. [Google Scholar] [CrossRef]
- Young, C.A.; Felitti, S.; Shields, K.; Spangenberg, G.; Johnson, R.D.; Bryan, G.T.; Saikia, S.; Scott, B. A complex gene cluster for indole-diterpene biosynthesis in the grass endophyte Neotyphodium lolii. Fungal Genet. Biol. 2006, 43, 679–693. [Google Scholar] [CrossRef]
- Hettiarachchige, I.K.; Elkins, A.C.; Reddy, P.; Mann, R.C.; Guthridge, K.M.; Sawbridge, T.I.; Forster, J.W.; Spangenberg, G.C. Genetic modification of asexual Epichloë endophytes with the perA gene for peramine biosynthesis. Mol. Genet. Genom. 2019, 294, 315–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, C.A.; Schardl, C.L.; Panaccione, D.G.; Florea, S.; Takach, J.E.; Charlton, N.D.; Moore, N.; Webb, J.S.; Jaromczyk, J. Genetics, genomics and evolution of ergot alkaloid diversity. Toxins 2015, 7, 1273–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, C.; Li, N.; Zhang, Y.; Li, C.; Zhang, X.; Nan, Z. Role of Epichloë endophytes in defense responses of cool-season grasses to pathogens: A Review. Plant Dis. 2018, 102, 2061–2073. [Google Scholar] [CrossRef] [Green Version]
- Freitas, P.P.; Hampton, J.G.; Rolston, M.P.; Glare, T.R.; Miller, P.P.; Card, S.D. A tale of two grass species: Temperature affects the symbiosis of a mutualistic Epichloë endophyte in both tall fescue and perennial ryegrass. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Bouton, J.H.; Gates, R.N.; Belesky, D.P.; Owsley, M. Yield and persistence of tall fescue in the southeastern coastal plain after removal of its endophyte. Agron. J. 1993, 85, 52–55. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Belesky, D.P. Adaptations of endophyte-infected cool-season grasses to environmental stresses: Mechanisms of drought and mineral stress tolerance. Crop. Sci. 2000, 40, 923–940. [Google Scholar] [CrossRef]
- West, C.P.; Izekor, E.; Turner, K.E.; Elmi, A.A. Endophyte effects on growth and persistence of tall fescue along a water-supply gradient. Agron. J. 1993, 85, 264–270. [Google Scholar] [CrossRef]
- Hume, D.E.; Cooper, B.; Panckhurst, K. The role of endophyte in determining the persistence and productivity of ryegrass, tall fescue and meadow fescue in Northland. In Proceedings of the New Zealand Grassland Association, Waitangi, New Zealand, 1 January 2009. [Google Scholar]
- Eaton, C.J.; Cox, M.P.; Scott, B. What triggers grass endophytes to switch from mutualism to pathogenism? Plant Sci. 2011, 180, 190–195. [Google Scholar] [CrossRef]
- Passarge, A.; Demir, F.; Green, K.; Depotter, J.R.L.; Scott, B.; Huesgen, P.F.; Doehlemann, G.; Misas Villamil, J.C. Host apoplastic cysteine protease activity is suppressed during the mutualistic association of Lolium perenne and Epichloë festucae. J. Exp. Bot. 2021, 72, 3410–3426. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, D.; Belesky, D. Epichloë (formerly neotyphodium) fungal endophytes increase adaptation of cool-season perennial grasses to environmental stresses. Acta Agrobot. 2019, 2, 72. [Google Scholar] [CrossRef]
- Tanaka, A.; Christensen, M.J.; Takemoto, D.; Park, P.; Scott, B. Reactive oxygen species play a role in regulating a fungus–perennial ryegrass mutualistic interaction. Plant Cell 2006, 18, 1052–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimentel, M.R.; Molina, G.; Dionísio, A.P.; Maróstica Junior, M.R.; Pastore, G.M. The use of endophytes to obtain bioactive compounds and their application in biotransformation process. Biotechnol. Res. Int. 2011, 2011, 576286. [Google Scholar] [CrossRef] [Green Version]
- Pan, F.; Hou, K.; Gao, F.; Hu, B.; Chen, Q.; Wu, W. Peimisine and peiminine production by endophytic fungus Fusarium sp. isolated from Fritillaria unibracteata var. wabensis. Phytomedicine 2014, 21, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Nagabhyru, P.; Dinkins, R.D.; Schardl, C.L. Transcriptomics of Epichloë-Grass symbioses in host vegetative and reproductive stages. Mol. Plant-Microbe Interact. 2018, 32, 194–207. [Google Scholar] [CrossRef] [Green Version]
- Kayano, Y.; Tanaka, A.; Takemoto, D. Two closely related Rho GTPases, Cdc42 and RacA, of the en-dophytic fungus Epichloë festucae have contrasting roles for ROS production and symbiotic infection synchronized with the host plant. PLoS Pathog. 2018, 14, e1006840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, K.A.; Becker, Y.; Tanaka, A.; Takemoto, D.; Fitzsimons, H.L.; Seiler, S.; Lalucque, H.; Silar, P.; Scott, B. SymB and SymC, two membrane associated proteins, are required for Epichloë festucae hyphal cell-cell fusion and maintenance of a mutualistic interaction with Lolium perenne. Mol Microbiol 2017, 103, 657–677. [Google Scholar] [CrossRef]
- Green, K.A.; Becker, Y.; Fitzsimons, H.L.; Scott, B. An Epichloë festucae homologue of MOB3, a component of the STRIPAK complex, is required for the establishment of a mutualistic symbiotic interaction with Lolium perenne. Mol. Plant Pathol. 2016, 17, 1480–1492. [Google Scholar] [CrossRef]
- Eaton, C.J.; Dupont, P.-Y.; Solomon, P.; Clayton, W.; Scott, B.; Cox, M.P. A core gene set describes the molecular basis of mutualism and antagonism in Epichloë spp. Mol. Plant-Microbe Interact. 2014, 28, 218–231. [Google Scholar] [CrossRef] [Green Version]
- Schmid, J.; Day, R.; Zhang, N.; Dupont, P.-Y.; Cox, M.P.; Schardl, C.L.; Minards, N.; Truglio, M.; Moore, N.; Harris, D.R.; et al. Host tissue environment directs activities of an Epichloë endophyte, while it induces systemic hormone and defense responses in its native perennial ryegrass host. Mol. Plant-Microbe Interact. 2016, 30, 138–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helander, M.; Phillips, T.; Faeth, S.H.; Bush, L.P.; McCulley, R.; Saloniemi, I.; Saikkonen, K. Alkaloid quantities in endophyte-infected tall fescue are affected by the plant-fungus combination and environment. J. Chem. Ecol. 2016, 42, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennessy, L.M.; Popay, A.J.; Finch, S.C.; Clearwater, M.J.; Cave, V.M. Temperature and plant genotype alter alkaloid concentrations in ryegrass infected with an Epichloë endophyte and this affects an insect herbivore. Front. Plant Sci. 2016, 7, 1097. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Parsons, A.J.; Bassett, S.; Christensen, M.J.; Hume, D.E.; Johnson, L.J.; Johnson, R.D.; Simpson, W.R.; Stacke, C.; Voisey, C.R.; et al. High nitrogen supply and carbohydrate content reduce fungal endophyte and alkaloid concentration in Lolium perenne. New Phytol. 2007, 173, 787–797. [Google Scholar] [CrossRef]
- Hill, N.S. Ecological relationships of balansiae-infected graminoids. In Biotechnology of Endophytic Fungi of Grasses; Bacon, C.W., White, J., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 59–73. [Google Scholar]
- Faeth, S.; Helander, M.; Saikkonen, K. Asexual Neotyphodium endophytes in a native grass reduce competitive abilities. Ecol. Lett. 2004, 7, 304–313. [Google Scholar] [CrossRef]
- Cheplick, G.P. Costs of fungal endophyte infection in Lolium perenne genotypes from Eurasia and North Africa under extreme resource limitation. Environ. Exp. Bot. 2007, 60, 202–210. [Google Scholar] [CrossRef]
- Bacon, C.W. Abiotic stress tolerances (moisture, nutrients) and photosynthesis in endophyte-infected tall fescue. Agric. Ecosyst. Environ. 1993, 44, 123–141. [Google Scholar] [CrossRef]
- Latch, G.C.M.; Christensen, M.J. Artificial infection of grasses with endophytes. Ann. Appl. Biol. 1985, 107, 17–24. [Google Scholar] [CrossRef]
- De Battista, J.; Altier, N.; Galdames, D.R.; Dall’Agnol, M. Significance of endophyte toxicosis and current practices in dealing with the problem in South America. In Neotyphodium/Grass Interactions; Bacon, C.W., Hill, N.S., Eds.; Springer US: Boston, MA, USA, 1997; pp. 383–388. [Google Scholar]
- De Battista, J.P.; Bouton, J.H.; Bacon, C.W.; Siegel, M.R. Rhizome and herbage production of endophyte-removed tall fescue clones and populations. Agron. J. 1990, 82, 651–654. [Google Scholar] [CrossRef]
- Elmi, A.A.; West, C.P. Endophyte infection effects on stomatal conductance, osmotic adjustment and drought recovery of tall fescue. New Phytol. 1995, 131, 61–67. [Google Scholar] [CrossRef]
- Xu, W.; Li, M.; Lin, W.; Nan, Z.; Tian, P. Effects of Epichloë sinensis endophyte and host ecotype on physiology of festuca sinensis under different soil moisture conditions. Plants 2021, 10, 1649. [Google Scholar] [CrossRef]
- Zhao, Z.; Kou, M.; Zhong, R.; Xia, C.; Christensen, M.J.; Zhang, X. Transcriptome analysis revealed plant hormone biosynthesis and response pathway modification by Epichloëgansuensis in achnatheruminebrians under different soil moisture availability. J. Fungi 2021, 7, 640. [Google Scholar] [CrossRef]
- Nagabhyru, P.; Dinkins, R.D.; Wood, C.L.; Bacon, C.W.; Schardl, C.L. Tall fescue endophyte effects on tolerance to water-deficit stress. BMC Plant Biol. 2013, 13, 127. [Google Scholar] [CrossRef] [Green Version]
- Dupont, P.-Y.; Eaton, C.J.; Wargent, J.J.; Fechtner, S.; Solomon, P.; Schmid, J.; Day, R.C.; Scott, B.; Cox, M.P. Fungal endophyte infection of ryegrass reprograms host metabolism and alters development. New Phytol. 2015, 208, 1227–1240. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [Green Version]
- Reza Sabzalian, M.; Mirlohi, A. Neotyphodium endophytes trigger salt resistance in tall and meadow fescues. J. Plant Nutr. Soil Sci. 2010, 173, 952–957. [Google Scholar] [CrossRef]
- Wang, J.; Tian, P.; Christensen, M.J.; Zhang, X.; Li, C.; Nan, Z. Effect of Epichloë gansuensis endophyte on the activity of enzymes of nitrogen metabolism, nitrogen use efficiency and photosynthetic ability of achnatherum inebrians under various NaCl concentrations. Plant Soil 2019, 435, 57–68. [Google Scholar] [CrossRef]
- Song, M.; Chai, Q.; Li, X.; Yao, X.; Li, C.; Christensen, M.J.; Nan, Z. An asexual Epichloë endophyte modifies the nutrient stoichiometry of wild barley (Hordeum brevisubulatum) under salt stress. Plant Soil 2015, 387, 153–165. [Google Scholar] [CrossRef]
- Chen, T.; Johnson, R.; Chen, S.; Lv, H.; Zhou, J.; Li, C. Infection by the fungal endophyte Epichloë bromicola enhances the tolerance of wild barley (Hordeum brevisubulatum) to salt and alkali stresses. Plant Soil 2018, 428, 353–370. [Google Scholar] [CrossRef]
- Yang, W.-J.; Rich, P.J.; Axtell, J.D.; Wood, K.V.; Bonham, C.C.; Ejeta, G.; Mickelbart, M.V.; Rhodes, D. Genotypic Variation for Glycinebetaine in Sorghum. Crop. Sci. 2003, 43, 162–169. [Google Scholar] [CrossRef]
- Wang, J.; Hou, W.; Christensen, M.J.; Xia, C.; Chen, T.; Zhang, Z.; Nan, Z. The fungal endophyte Epichloë gansuensis increases NaCl-tolerance in Achnatherum inebrians through enhancing the activity of plasma membrane H+-ATPase and glucose-6-phosphate dehydrogenase. Sci. China Life Sci. 2021, 64, 452–465. [Google Scholar] [CrossRef]
- Chen, T.; White, J.F.; Li, C. Fungal endophyte Epichloë bromicola infection regulates anatomical changes to account for salt stress tolerance in wild barley (Hordeum brevisubulatum). Plant Soil 2021, 461, 533–546. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Nan, Z. Effects of cadmium stress on growth and anti-oxidative systems in Achnatherum inebrians symbiotic with neotyphodium gansuense. J. Hazard. Mater. 2010, 175, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Gao, Y.; Zhang, L.; Xie, F. Effects of cadmium on growth parameters of endophyte-infected endophyte-free ryegrass. J. Plant Nutr. Soil Sci. 2006, 169, 857–860. [Google Scholar] [CrossRef]
- Żurek, G.; Wiewióra, B.; Rybka, K.; Prokopiuk, K. Different response of perennial ryegrass—Epichloë endophyte symbiota to the elevated concentration of heavy metals in soil. J. Appl. Genet. 2021, 1–13. [Google Scholar] [CrossRef]
- Monnet, F.; Vaillant, N.; Hitmi, A.; Coudret, A.; Sallanon, H. Endophytic neotyphodium lolii induced tolerance to Zn stress in Lolium perenne. Physiol. Plant. 2001, 113, 557–563. [Google Scholar] [CrossRef]
- Salehi, M.; Shabani, L.; Sabzalian, M.R. Epichloe endophyte modifies antioxidative defense and aquaporin genes expression in response to Ni contamination in lolium perenne maryam salehi 1, Leil. J. Plant Process. Funct. 2021, 10, 56. [Google Scholar]
- Wang, J.; Nan, Z.; Christensen, M.J.; Zhang, X.; Tian, P.; Zhang, Z.; Niu, X.; Gao, P.; Chen, T.; Ma, L. Effect of Epichloë gansuensis endophyte on the nitrogen metabolism, nitrogen use efficiency, and stoichiometry of achnatherum inebrians under nitrogen limitation. J. Agric. Food Chem. 2018, 66, 4022–4031. [Google Scholar] [CrossRef]
- Wang, J.; Nan, Z.; Christensen, M.J.; Li, C. Glucose-6-phosphate dehydrogenase plays a vital role in achnatherum inebrians plants host to Epichloë gansuensis by improving growth under nitrogen deficiency. Plant Soil 2018, 430, 37–48. [Google Scholar] [CrossRef]
- Li, X.; Ren, A.; Han, R.; Yin, L.; Wei, M.; Gao, Y. Endophyte-mediated effects on the growth and physiology of achnatherum sibiricum are conditional on both N and P availability. PLoS ONE 2012, 7, e48010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Hou, W.; Jin, J.; Christensen, M.J.; Gu, L.; Cheng, C.; Wang, J. Epichloë gansuensis increases the tolerance of achnatherum inebrians to low-P stress by modulating amino acids metabolism and phosphorus utilization efficiency. J. Fungi 2021, 7, 390. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Wang, J.; Christensen, M.J.; Liu, J.; Zhang, Y.; Liu, Y.; Cheng, C. Metabolomics insights into the mechanism by which Epichloë gansuensis endophyte increased achnatherum inebrians tolerance to low nitrogen stress. Plant Soil 2021, 463, 487–508. [Google Scholar] [CrossRef]
- Heineck, G.C.; Watkins, E.; Ehlke, N.J. The fungal endophyte Epichloë festucae var. lolii does not improve the freezing tolerance of perennial ryegrass. Crop. Sci. 2018, 58, 1788–1800. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; He, R.; Chai, Q.; Li, C.; Nan, Z. Transcriptomic analyses giving insights into molecular regulation mechanisms involved in cold tolerance by Epichloë endophyte in seed germination of Achnatherum inebrians. Plant Growth Regul. 2016, 80, 367–375. [Google Scholar] [CrossRef]
- De Santis, A.; Landi, P.; Genchi, G. Changes of mitochondrial properties in maize seedlings associated with selection for germination at low temperature. Fatty acid composition, cytochrome c oxidase, and adenine nucleotide translocase activities. Plant Physiol. 1999, 119, 743–754. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Li, C.; Zhang, X.; Johnson, R.; Bao, G.; Yao, X.; Chai, Q. Effects of cold shocked Epichloë infected festuca sinensis on ergot alkaloid accumulation. Fungal Ecol. 2015, 14, 99–104. [Google Scholar] [CrossRef]
- Arachevaleta, M.; Bacon, C.W.; Hoveland, C.S.; Radcliffe, D.E. Effect of the tall fescue endophyte on plant response to environmental stress. Agron. J. 1989, 81, 83–90. [Google Scholar] [CrossRef]
- Adams, A.E.; Kazenel, M.R.; Rudgers, J.A. Does a foliar endophyte improve plant fitness under flooding? Plant Ecol. 2017, 218, 711–723. [Google Scholar] [CrossRef]
- Song, M.; Li, X.; Saikkonen, K.; Li, C.; Nan, Z. An asexual Epichloë endophyte enhances waterlogging tolerance of hordeum brevisubulatum. Fungal Ecol. 2015, 13, 44–52. [Google Scholar] [CrossRef]
- Saedi, T.; Mosaddeghi, M.R.; Sabzalian, M.R.; Zarebanadkouki, M. Effect of Epichloë fungal endophyte symbiosis on tall fescue to cope with flooding-derived oxygen-limited conditions depends on the host genotype. Plant Soil 2021, 1–21. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Lin, W.; Li, M.; Wang, M.; Wang, Z.; Kuang, Y.; Tian, P. Effect of an Epichloë endophyte on adaptability to water stress in festuca sinensis. Fungal Ecol. 2017, 30, 39–47. [Google Scholar] [CrossRef]
- Chu-Chou, M.; Guo, B.; An, Z.Q.; Hendrix, J.W.; Ferriss, R.S.; Siegel, M.R.; Dougherty, C.T.; Burrus, P.B. Suppression of mycorrhizal fungi in fescue by the acremonium coenophialum endophyte. Soil Biol. Biochem. 1992, 24, 633–637. [Google Scholar] [CrossRef]
- Clarke, B.B.; White, J.F.; Hurley, R.H.; Torres, M.S.; Sun, S.; Huff, D.R. Endophyte-mediated suppression of dollar spot disease in fine fescues. Plant Dis. 2006, 90, 994–998. [Google Scholar] [CrossRef] [Green Version]
- Ambrose, K.V.; Belanger, F.C. SOLiD-SAGE of endophyte-infected red fescue reveals numerous effects on host transcriptome and an abundance of highly expressed fungal secreted proteins. PLoS ONE 2012, 7, e53214. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Christensen, M.J.; Nan, Z. Effects of the endophyte Epichloë festucae var. lolii of perennial ryegrass (Lolium perenne) on indicators of oxidative stress from pathogenic fungi during seed germination and seedling growth. Eur. J. Plant Pathol. 2015, 141, 571–583. [Google Scholar] [CrossRef]
- Schmidt, D.; Scehovic, J. Results of one trial examining the influence of Acremonium uncinatum and leaf spot diseases on different chemical components and nutritive value of Festuca pratensis. Bull. OILB SROP 1994, 17, 187–192. [Google Scholar]
- Li, F.; Duan, T.; Li, Y. Effect of fungal endophytes against rust disease of perennial ryegrass (Lolium perenne) on growth and physiological indices. Acta Prataculturae Sin. 2011, 20, 150–156. [Google Scholar] [CrossRef]
- Perez, L.I.; Gundel, P.E.; Marrero, H.J.; Arzac, A.G.; Omacini, M. Symbiosis with systemic fungal endophytes promotes host escape from vector-borne disease. Oecologia 2017, 184, 237–245. [Google Scholar] [CrossRef]
- Iannone, L.J.; Vignale, M.V.; Pinget, A.D.; Re, A.; Mc Cargo, P.D.; Novas, M.V. Seed-transmitted Epichloë sp. endophyte alleviates the negative effects of head smut of grasses (Ustilago bullata) on Bromus auleticus. Fungal Ecol. 2017, 29, 45–51. [Google Scholar] [CrossRef]
- Górzyńska, K.; Ryszka, P.; Anielska, T.; Turnau, K.; Lembicz, M. Effect of Epichloë typhina fungal endophyte on the diversity and incidence of other fungi in Puccinellia distans wild grass seeds. Flora 2017, 228, 60–64. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; White, J.F.; Wei, X.; He, Y.; Li, C. Epichloë endophyte improves ergot disease resistance of host (achnatherum inebrians) by regulating leaf senescence and photosynthetic capacity. J. Plant Growth Regul. 2021, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Qin, J.; Chen, W.; Zhou, Y.; Ren, A.; Gao, Y. Pathogen resistant advantage of endophyte-infected over endophyte-free Leymus chinensis is strengthened by pre-drought treatment. Eur. J. Plant Pathol. 2016, 144, 477–486. [Google Scholar] [CrossRef]
- Funnell-Harris, D.L.; Pedersen, J.F.; Sattler, S.E. Alteration in lignin biosynthesis restricts growth of fusarium spp. in brown midrib sorghum. Phytopathology 2010, 100, 671–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, L.J.; Johnson, R.D.; Schardl, C.L.; Panaccione, D.G. Identification of differentially expressed genes in the mutualistic association of tall fescue with Neotyphodium coenophialum. Physiol. Mol. Plant Pathol. 2003, 63, 305–317. [Google Scholar] [CrossRef]
- Bastias, D.A.; Martínez-Ghersa, M.A.; Ballaré, C.L.; Gundel, P.E. Epichloë fungal endophytes and plant defenses: Not just alkaloids. Trends Plant Sci. 2017, 22, 939–948. [Google Scholar] [CrossRef]
- Kou, M.-Z.; Bastías, D.A.; Christensen, M.J.; Zhong, R.; Nan, Z.-B.; Zhang, X.-X. The plant salicylic acid signalling pathway regulates the infection of a biotrophic pathogen in grasses associated with an Epichloë endophyte. J. Fungi 2021, 7, 633. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Parsons, A.J.; Fraser, K.; Xue, H.; Newman, J.A. Metabolic profiles of lolium perenne are differentially affected by nitrogen supply, carbohydrate content, and fungal endophyte infection. Plant Physiol. 2008, 146, 1440–1453. [Google Scholar] [CrossRef] [Green Version]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef]
- Arras, G.; Agabbio, M.; Piga, A.; D’hallewin, G. Fungicide effect of volatile compounds of thymus capitatus essential oil. Acta Hortic 1995, 379, 593–600. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, C.; Li, C.; Nan, Z. Chemical composition and antifungal activity of the volatile oil from Epichloë gansuensis, endophyte-infected and non-infected achnatherum inebrians. Sci. China Life Sci. 2015, 58, 512–514. [Google Scholar] [CrossRef] [Green Version]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Guy, P.L.; Davis, L.T. Variation in the incidence of Barley yellow dwarf virus and in the ability of neotyphodium endophytes to deter feeding by aphids (rhopalosiphum padi) on Australasian tall fescue. Australas. Plant Pathol. 2002, 31, 307–308. [Google Scholar] [CrossRef]
- Bastías, D.A.; Gianoli, E.; Gundel, P.E. Fungal endophytes can eliminate the plant growth–defence trade-off. New Phytol. 2021, 230, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Kimmons, C.A.; Gwinn, K.D.; Bernard, E.C. Nematode reproduction on endophyte-infected and endophyte-free tall fescue. Plant Dis. 1990, 74, 757–761. [Google Scholar] [CrossRef]
- Pedersen, J.F.; Rodriguez-Kabana, R.; Shelby, R.A. Ryegrass cultivars and endophyte in tall fescue affect nematodes in grass and succeeding soybean. Agron. J. 1988, 80, 811–814. [Google Scholar] [CrossRef]
- Stewart, T.M.; Mercep, C.F.; Grante, J.L. Development of meloidogyne naasi on endophyte-infected and endophyte-free perennial ryegrass. Australas. Plant Pathol. 1993, 22, 40–41. [Google Scholar] [CrossRef]
- Elmi, A.A.; West, C.P.; Robbins, R.T.; Kirkpatrick, T.L. Endophyte effects on reproduction of a root-knot nematode (Meloidogyne marylandi) and osmotic adjustment in tall fescue. J. Br. Grassl. Soc. 2000, 55, 166–172. [Google Scholar] [CrossRef]
- Guo, C.-H.; Li, X.-Z.; Liu, L.; Cao, J.-X.; Li, C.-J. Effect of the epichloe endophyte on the soil nematode community in the rhizosphere of achnatherum inebrians. Acta Prataculturae Sin. 2016, 25, 140–148. [Google Scholar] [CrossRef]
- Timper, P.; Gates, R.N.; Bouton, J.H. Response of pratylenchus spp. in tall fescue infected with different strains of the fungal endophyte neotyphodium coenophialum. Nematology 2005, 7, 105–110. [Google Scholar] [CrossRef]
- Panaccione, D.G. Origins and significance of ergot alkaloid diversity in fungi. FEMS Microbiol. Lett. 2005, 251, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panaccione, D.; Kotcon, J.; Schardl, C.; Johnson, R.; Morton, J. Ergot alkaloids are not essential for endophytic fungus-associated population suppression of the lesion nematode, Pratylenchus scribneri, on perennial ryegrass. Nematology 2006, 8, 583–590. [Google Scholar] [CrossRef]
- Bacon, C.W.; Hinton, D.M.; Glenn, A.E. The Endophytic Niche and Grass Defense; Taylor & Francis Group, LLC: Abingdon, UK, 2009. Available online: https://www.ars.usda.gov/research/publications/publication/?seqNo115=218387 (accessed on 7 July 2021).
- Ball, O.; Christensen, M.; Prestidge, R. Effect of isolates of acremonium endophyte on adult black beetle (heteronychus arator) feeding. In Proceedings of the New Zealand Plant Protection Society Conference, Waitangi, New Zealand, 1 August 1994. [Google Scholar]
- Popay, A.; Tapper, B.; Podmore, C. Endophyte infected meadow fescue and loline alkaloids affect Argentine stem weevil larvae. In Proceedings of the New Zealand Plant Protection, Waitangi, New Zealand, 1 August 2009; pp. 19–27. [Google Scholar]
- Bastias, D.A.; Ueno, A.C.; Machado Assefh, C.R.; Alvarez, A.E.; Young, C.A.; Gundel, P.E. Metabolism or behavior: Explaining the performance of aphids on alkaloid-producing fungal endophytes in annual ryegrass (Lolium multiflorum). Oecologia 2017, 185, 245–256. [Google Scholar] [CrossRef]
- Bills, G.F.; González-Menéndez, V.; Martín, J.; Platas, G.; Fournier, J.; Peršoh, D.; Stadler, M. Hypoxylon pulicicidum sp. nov. (Ascomycota, Xylariales), a pantropical insecticide-producing endophyte. PLoS ONE 2012, 7, e46687. [Google Scholar] [CrossRef] [PubMed]
- Craven, K.; Blankenship, J.; Leuchtmann, A.; Hignight, K.; Schardl, C. Hybrid fungal endophytes symbiotic with the grass Lolium pratense. Sydowia -Horn 2001, 53, 44–73. [Google Scholar]
- He, Y.; Chen, T.; Zhang, H.; White, J.F.; Li, C. Fungal endophytes help grasses to tolerate sap-sucking herbivores through a hormone-signaling system. J. Plant Growth Regul. 2021, 1–16. [Google Scholar] [CrossRef]
- Jiang, Y.; Qiu, Y.; Hu, Y.; Yu, D. Heterologous expression of AtWRKY57 confers drought tolerance in oryza sativa. Front. Plant Sci. 2016, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, B.; Krauss, J. Can Epichloë endophytes enhance direct and indirect plant defence? Fungal Ecol. 2019, 38, 98–103. [Google Scholar] [CrossRef]
- Hennessy, L.M.; Popay, A.J.; Glare, T.R.; Finch, S.C.; Cave, V.M.; Rostás, M. Olfactory responses of Argentine stem weevil to herbivory and endophyte-colonisation in perennial ryegrass. J. Pest Sci. 2021, 1–15. [Google Scholar] [CrossRef]
- Rostás, M.; Cripps, M.G.; Silcock, P. Aboveground endophyte affects root volatile emission and host plant selection of a belowground insect. Oecologia 2015, 177, 487–497. [Google Scholar] [CrossRef]
- Qawasmeh, A.; Raman, A.; Wheatley, W. Volatiles in perennial ryegrass infected with strains of endophytic fungus: Impact on African black beetle host selection. J. Appl. Entomol. 2015, 139, 94–104. [Google Scholar] [CrossRef]
- Sutherland, B.; Hoglund, J. Effect of ryegrass containing the endophyte (Acremonium lolii), on the performance of associated white clover and subsequent crops. In Proceedings of the New Zealand Grassland Association, Balclutha, New Zealand, 1 January 1989; Volume 50, pp. 265–269. [Google Scholar]
- Stevens, D.R.; Hickey, M.J. Effects of endophytic ryegrass on white clover. Proc. Int. Symp. Acremonium/Grass Interact. 1990, 58–61. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: New York, NY, USA, 1984. [Google Scholar]
- Vázquez-de-Aldana, B.R.; Romo, M.; García-Ciudad, A.; Petisco, C.; García-Criado, B. Infection with the fungal endophyte Epichloë festucae may alter the allelopathic potential of red fescue. Ann. Appl. Biol. 2011, 159, 281–290. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Belesky, D.P. Ecological importance of neotyphodium spp. grass endophytes in agroecosystems. Grassl. Sci. 2006, 52, 1–14. [Google Scholar] [CrossRef]
- Guo, J.; McCulley, R.L.; McNear, D.H. Tall fescue cultivar and fungal endophyte combinations influence plant growth and root exudate composition. Front. Plant Sci. 2015, 6, 183. [Google Scholar] [CrossRef] [Green Version]
- Sherif, E.; Hegazy, A.; Gomaa, N.; Hassan, N.H.; Hassan, M. Allelopathic effect of black mustard tissues and root exudates on some crops and weeds. Planta Daninha 2013, 31, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.Q.; Matsui, Y. Phytotoxic substances in root exudates of cucumber (Cucumis sativus L.). J. Chem. Ecol. 1994, 20, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Huangpu, J.J.; Chen, T.; Zhang, Z.Y.; Lin, W.X. Allelopathic potential and identification of allelochemicals in Pseudostellariae heterophylla rhizosphere soil in different crop rotations. Allelopath. J. 2014, 33, 151–161. [Google Scholar]
- Roberts, C.; Andrae, J. Tall Fescue Toxicosis and Management. Crop Manag. 2004, 3, 1–18. [Google Scholar] [CrossRef]
- Strickland, J.R.; Oliver, J.W.; Cross, D.L. Fescue toxicosis and its impact on animal agriculture. Vet. Hum. Toxicol. 1993, 35, 454–464. [Google Scholar] [PubMed]
- Cross, D.L.; Redmond, L.M.; Strickland, J.R. Equine fescue toxicosis-signs and and solutions. J. Anim. Sci. 1995, 73, 899–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klotz, J.L.; Brown, K.R.; Bush, L.P.; Matthews, J.C.; Boling, J.A.; Strickland, J.R. Grazing high versus low endophyte-infected tall fescue reduces contractility of bovine lateral saphenous veins. J. Anim. Sci. Suppl. 2007, 85, 12–13. [Google Scholar]
- Trotta, R.J.; Harmon, D.L.; Klotz, J.L. Interaction of ergovaline with serotonin receptor 5-HT2A in bovine ruminal and mesenteric vasculature1. J. Anim. Sci. 2018, 96, 4912–4922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Nan, Z.; Li, C.; Gao, K. Cytotoxic effect of ergot alkaloids in achnatherum inebrians infected by the neotyphodium gansuense endophyte. J. Agric. Food Chem. 2014, 62, 7419–7422. [Google Scholar] [CrossRef] [PubMed]
- Mote, R.S.; Hill, N.S.; Skarlupka, J.H.; Tran, V.T.; Walker, D.I.; Turner, Z.B.; Sanders, Z.P.; Jones, D.P.; Suen, G.; Filipov, N.M. Toxic tall fescue grazing increases susceptibility of the Angus steer fecal microbiota and plasma/urine metabolome to environmental effects. Sci. Rep. 2020, 10, 2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mote, R.S.; Hill, N.S.; Skarlupka, J.H.; Turner, Z.B.; Sanders, Z.P.; Jones, D.P.; Suen, G.; Filipov, N.M. Response of beef cattle fecal microbiota to grazing on toxic tall fescue. Appl. Environ. Microbiol. 2019, 85, e00032-19. [Google Scholar] [CrossRef] [Green Version]
- Mote, R.S.; Hill, N.S.; Uppal, K.; Tran, V.T.; Jones, D.P.; Filipov, N.M. Metabolomics of fescue toxicosis in grazing beef steers. Food Chem. Toxicol. 2017, 105, 285–299. [Google Scholar] [CrossRef] [Green Version]
- Mote, R.S.; Filipov, N.M. Use of Integrative interactomics for improvement of farm animal health and welfare: An example with fescue toxicosis. Toxins 2020, 12, 633. [Google Scholar] [CrossRef]
- Rudgers, J.A.; Clay, K. Endophyte symbiosis with tall fescue: How strong are the impacts on communities and ecosystems? Fungal Biol. Rev. 2007, 21, 107–124. [Google Scholar] [CrossRef]
- Conover, M.R.; Messmer, T.A. Feeding preferences and changes in mass of Canada geese grazing endophyte-infected tall fescue. Condor 1996, 98, 859–862. [Google Scholar] [CrossRef]
- Mueller, C.W.; Carminati, A.; Kaiser, C.; Subke, J.-A.; Gutjahr, C. Rhizosphere functioning and structural development as complex interplay between plants, microorganisms and soil minerals. Front. Environ. Sci. 2019, 7, 130. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, K.; Missaoui, A.; Lee, K.C.; Ghimire, B.; Presley, H.W.; Makaju, S. Rhizosphere microbiome manipulation for sustainable crop production. Curr. Plant Biol. 2021, 27, 100210. [Google Scholar] [CrossRef]
- Jones, D.L.; Nguyen, C.; Finlay, R.D. Carbon flow in the rhizosphere: Carbon trading at the soil–root interface. Plant Soil 2009, 321, 5–33. [Google Scholar] [CrossRef]
- Chauhan, A.; Saini, R.; Sharma, J.C. Plant growth promoting rhizobacteria and their biological properties for soil enrichment and growth promotion. J. Plant Nutr. 2021, 12, 1–27. [Google Scholar] [CrossRef]
- Johansson, J.F.; Paul, L.R.; Finlay, R.D. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiol. Ecol. 2004, 48, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schlaeppi, K.; Bulgarelli, D. The plant microbiome at work. Mol. Plant-Microbe Interact. 2015, 28, 212–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alori, E.; Glick, B.; Babalola, O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.A.; Rudgers, J.A. Plant–soil feedbacks promote negative frequency dependence in the coexistence of two aridland grasses. Proc. R. Soc. B: Biol. Sci. 2016, 283, 20160608. [Google Scholar] [CrossRef] [Green Version]
- Tilman, D. Resource Competition and Community Structure.(MPB-17); Princeton University Press: Princeton, NJ, USA, 2020; Volume 17. [Google Scholar]
- MacArthur, R.H. Population ecology of some warblers of northeastern coniferous forests. Ecology 1958, 39, 599–619. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Bergmann, G.T.; Bates, S.T.; Eilers, K.G.; Lauber, C.L.; Caporaso, J.G.; Walters, W.A.; Knight, R.; Fierer, N. The under-recognized dominance of Verrucomicrobia in soil bacterial communities. Soil Biol. Biochem. 2011, 43, 1450–1455. [Google Scholar] [CrossRef] [Green Version]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Vorholt, J.A.; Vogel, C.; Carlström, C.I.; Müller, D.B. Establishing causality: Opportunities of synthetic communities for plant microbiome research. Cell Host Microbe 2017, 22, 142–155. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; Strauss, S.L. Insights into the taxonomic and functional characterization of agricultural crop core rhizobiomes and their potential microbial drivers. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Tisdall, J.M. Possible role of soil microorganisms in aggregation in soils. Plant Soil 1994, 159, 115–121. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, K.; Almohimeed, I.; Moslem, M.; Bahkali, A. M13-microsatellite PCR and rDNA sequence markers for identification of Trichoderma (Hypocreaceae) species in Saudi Arabian soil. Genetics 2010, 9, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Marks, S.; Clay, K. Physiological responses of Festuca arundinacea to fungal endophyte infection. New Phytol. 1996, 133, 727–733. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Hill, N.S. Soil carbon, nitrogen and ergot alkaloids with short- and long-term exposure to endophyte-infected and endophyte-free tall fescue. Soil Sci. Soc. Am. J. 2005, 69, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Omacini, M.; Chaneton, E.J.; Ghersa, C.M.; Otero, P. Do foliar endophytes affect grass litter decomposition? A microcosm approach using Lolium multiflorum. Oikos 2004, 104, 581–590. [Google Scholar] [CrossRef]
- Van Hecke, M.M.; Treonis, A.M.; Kaufman, J.R. How does the fungal endophyte neotyphodium coenophialum affect tall fescue (festuca arundinacea) rhizodeposition and soil microorganisms? Plant Soil 2005, 275, 101–109. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Belesky, D.P. Neotyphodium coenophialum-endophyte infection affects the ability of tall fescue to use sparingly available phosphorus. J. Plant Nutr. 1999, 22, 835–853. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Zuo, H.; Belesky, D.P.; Alloush, G.A. Evidence for copper binding by extracellular root exudates of tall fescue but not perennial ryegrass infected with neotyphodium spp. endophytes. Plant Soil 2004, 267, 1–12. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Potential C and N mineralization and microbial biomass from intact and increasingly disturbed soils of varying texture. Soil Biol. Biochem. 1999, 31, 1083–1090. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, F.; Mosaddeghi, M.R.; Hajabbasi, M.A.; Sabzalian, M.R. Influence of tall fescue endophyte infection on structural stability as quantified by high energy moisture characteristic in a range of soils. Geoderma 2015, 249–250, 87–99. [Google Scholar] [CrossRef]
- Handayani, I.P.; Coyne, M.S.; Phillips, T.D. Soil organic carbon fractions differ in two contrasting tall fescue systems. Plant Soil 2011, 338, 43–50. [Google Scholar] [CrossRef]
- Buta, J.G.; Spaulding, D.W. Allelochemicals in tall fescue-abscisic and phenolic acids. J. Chem. Ecol. 1989, 15, 1629–1636. [Google Scholar] [CrossRef]
- Xue, S.; Yang, X.; Liu, G.; Gai, L.; Zhang, C.; Ritsema, C.J.; Geissen, V. Effects of elevated CO2 and drought on the microbial biomass and enzymatic activities in the rhizospheres of two grass species in Chinese loess soil. Geoderma 2017, 286, 25–34. [Google Scholar] [CrossRef]
- Naylor, D.; DeGraaf, S.; Purdom, E.; Coleman-Derr, D. Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J. 2017, 11, 2691–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- East, R. Microbiome: Soil science comes to life. Nature 2013, 501, S18–S19. [Google Scholar] [CrossRef] [PubMed]
- Groben, G.; Luo, J.; Walsh, E.; Qu, H.; Meyer, W.; Bonos, S.; Clarke, B.; Zhang, N. The microbiome associated with tall Fescue under drought stress. In Proceedings of the Twenty-Seventh Annual Rutgers Turfgrass Symposium, New Brunswick, NJ, USA, 12 January 2018; p. 15. [Google Scholar]
- Masmoudi, K.; Aziz, M.A.; Shamim, A.; Sabeem, M.; Hazzouri, K.M.; Amiri, K.M. Metagenomics of beneficial microbes in abiotic stress tolerance of date palm. In The Date Palm Genome; Springer: Berlin, Germany, 2021; Volume 2, pp. 203–214. [Google Scholar]
- Glick, B.R.; Gamalero, E. Recent Developments in the Study of Plant Microbiomes. Microorganisms 2021, 9, 1533. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Feng, H.; Zhang, D.; Feng, Z.; Zhao, L.; Zhang, Y.; Deakin, G.; Peng, J.; Zhu, H.; Xu, X. Composition of rhizosphere microbial communities associated with healthy and verticillium wilt diseased cotton plants. Front. Microbiol. 2021, 12, 618169. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Song, G.C.; Ryu, C.M. Root exudation by aphid leaf infestation recruits root-associated paenibacillus spp. to lead plant insect susceptibility. J. Microbiol. Biotechnol. 2016, 26, 549–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniou, A.; Tsolakidou, M.-D.; Stringlis, I.A.; Pantelides, I.S. Rhizosphere microbiome recruited from a suppressive compost improves plant fitness and increases protection against vascular wilt pathogens of tomato. Front. Plant Sci. 2017, 8, 2022. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, K.; Franklin, D.; Ney, L.; Cabrera, M.; Habteselassie, M.; Hancock, D.; Newcomer, Q.; Subedi, A.; Dahal, S. Improving inorganic nitrogen in soil and nutrient density of edamame bean in three consecutive summers by utilizing a locally sourced bio-inocula. Org. Agric. 2021, 11, 133–143. [Google Scholar] [CrossRef]
- Ney, L.; Franklin, D.; Mahmud, K.; Cabrera, M.; Hancock, D.; Habteselassie, M.; Newcomer, Q. Examining trophic-level nematode community structure and nitrogen mineralization to assess local effective microorganisms’ role in nitrogen availability of swine effluent to forage crops. Appl. Soil Ecol. 2018, 130, 209–218. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyngaard, N.; Cabrera, M.L.; Jarosch, K.A.; Bünemann, E.K. Phosphorus in the coarse soil fraction is related to soil organic phosphorus mineralization measured by isotopic dilution. Soil Biol. Biochem. 2016, 96, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Oki, Y.; Adachi, T. Mobilization and acquisition of sparingly soluble P-sources by brassica cultivars under P-starved environment II. Rhizospheric pH changes, redesigned root architecture and Pi-uptake kinetics. J. Integr. Plant Biol. 2009, 51, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, W. Stoichiometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of northern China. Soil Biol. Biochem. 2016, 98, 74–84. [Google Scholar] [CrossRef]
- Ding, N.; Guo, H.; Kupper, J.V.; McNear, D.H., Jr. Phosphorus source and Epichloë coenophiala strain interact over time to modify tall fescue rhizosphere microbial community structure and function. Soil Biol. Biochem. 2021, 154, 108125. [Google Scholar] [CrossRef]
- Roberts, E.; Lindow, S. Loline alkaloid production by fungal endophytes of Fescue species select for particular epiphytic bacterial microflora. ISME J. 2014, 8, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, E.L.; Ferraro, A. Rhizosphere microbiome selection by Epichloë endophytes of Festuca arundinacea. Plant Soil 2015, 396, 229–239. [Google Scholar] [CrossRef]
- Hou, W.; Xia, C.; Christensen, M.J.; Wang, J.; Li, X.; Chen, T.; Nan, Z. Effect of Epichloë gansuensis endophyte on rhizosphere bacterial communities and nutrient concentrations and ratios in the perennial grass species Achnatherum inebrians during three growth seasons. Crop Pasture Sci. 2020, 71, 1050–1066. [Google Scholar] [CrossRef]
- Fließach, A.; Mader, P. Carbon Source Utilization by Microbial Communities in Soils Under Organic and Conven- Tional Farming Practice; Springer: Berlin/Heidelberg, Germany, 1997; pp. 109–120. [Google Scholar]
- Jenkins, M.B.; Franzluebbers, A.J.; Humayoun, S.B. Assessing short-term responses of prokaryotic communities in bulk and rhizosphere soils to tall fescue endophyte infection. Plant Soil 2006, 289, 309–320. [Google Scholar] [CrossRef]
- Buyer, J.S.; Zuberer, D.A.; Nichols, K.A.; Franzluebbers, A.J. Soil microbial community function, structure, and glomalin in response to tall fescue endophyte infection. Plant Soil 2011, 339, 401–412. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Bacillus: A biological tool for crop improvement through Bio-molecular changes in adverse environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Mahmud, K.; Lee, K.; Hill, N.S.; Mergoum, A.; Missaoui, A. Influence of tall fescue Epichloë endophytes on rhizosphere soil microbiome. Microorganisms 2021, 9, 1843. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jin, Y.; Hou, F.; Bowatte, S. Soil microbial and chemical responses to foliar Epichloë fungal infection in Lolium perenne, Hordeum brevisubulatum and Achnatherum inebrians. Fungal Ecol. 2021, 53, 101091. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, Y.; Yao, X.; Wei, X.; Li, X.; Li, C.; White, J.F.; Nan, Z. Gene analysis reveals that leaf litter from Epichloë endophyte-infected perennial ryegrass alters diversity and abundance of soil microbes involved in nitrification and denitrification. Soil Biol. Biochem. 2021, 154, 108123. [Google Scholar] [CrossRef]
- Ju, Y.; Zhong, R.; Christensen, M.J.; Zhang, X. Effects of Epichloë gansuensis endophyte on the root and rhizosphere soil bacteria of achnatherum inebrians under different moisture conditions. Front. Microbiol. 2020, 11, 747. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Li, C.; Nan, Z.; Li, F. Effects of feeding drunken horse grass infected with Epichloë gansuensis endophyte on animal performance, clinical symptoms and physiological parameters in sheep. BMC Vet. Res. 2017, 13, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mormile, B.W. Influence of Seed Microbiome on Fitness of Epichloë Infected Tall Fescue Seedlings; Southern Connecticut State University: Ann Arbor, MI, USA, 2016. [Google Scholar]
- Smith, S.E.; Gianinazzi-Pearson, V. Physiological interactions between symbionts in vesicular-arbuscular mycorrhizal plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 221–244. [Google Scholar] [CrossRef]
- Omacini, M.; Eggers, T.; Bonkowski, M.; Gange, A.C.; Jones, T.H. Leaf endophytes affect mycorrhizal status and growth of co-infected and neighbouring plants. Funct. Ecol. 2006, 20, 226–232. [Google Scholar] [CrossRef]
- Müller, J. Artificial infection by endophytes affects growth and mycorrhizal colonisation of Lolium perenne. Funct. Plant Biol. 2003, 30, 419–424. [Google Scholar] [CrossRef]
- Vignale, M.V.; Iannone, L.J.; Scervino, J.M.; Novas, M.V. Epichloë exudates promote in vitro and in vivo arbuscular mycorrhizal fungi development and plant growth. Plant Soil 2018, 422, 267–281. [Google Scholar] [CrossRef]
- Zhong, R.; Xia, C.; Ju, Y.; Zhang, X.; Duan, T.; Nan, Z.; Li, C. A foliar Epichloë endophyte and soil moisture modified belowground arbuscular mycorrhizal fungal biodiversity associated with Achnatherum inebrians. Plant Soil 2021, 458, 105–122. [Google Scholar] [CrossRef]
- Li, F.; Deng, J.; Nzabanita, C.; Li, Y.; Duan, T. Growth and physiological responses of perennial ryegrass to an AMF and an Epichloë endophyte under different soil water contents. Symbiosis 2019, 79, 151–161. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, P.; Li, F.; Duan, T. Effects of AM fungi and grass endophytes on perennial ryegrass Bipolaris sorokiniana leaf spot disease under limited soil nutrients. Eur. J. Plant Pathol. 2019, 154, 659–671. [Google Scholar] [CrossRef]
- Charitha Devi, M.; Reddy, M.N. Phenolic acid metabolism of groundnut (Arachis hypogaea L.) plants inoculated with VAM fungus and Rhizobium. Plant Growth Regul. 2002, 37, 151–156. [Google Scholar] [CrossRef]
- Gunter, S.A.; Beck, P.A. Novel endophyte-infected tall fescue for growing beef cattle. J. Anim. Sci. 2004, 82, 75–82. [Google Scholar] [CrossRef]
- Hume, D.E.; Ryan, D.; Cooper, B.; Popay, A.I. Agronomic performance of AR37-infected ryegrass in northern New Zealand. J. N. Z. Grassl. 2007, 69, 201–205. [Google Scholar] [CrossRef]
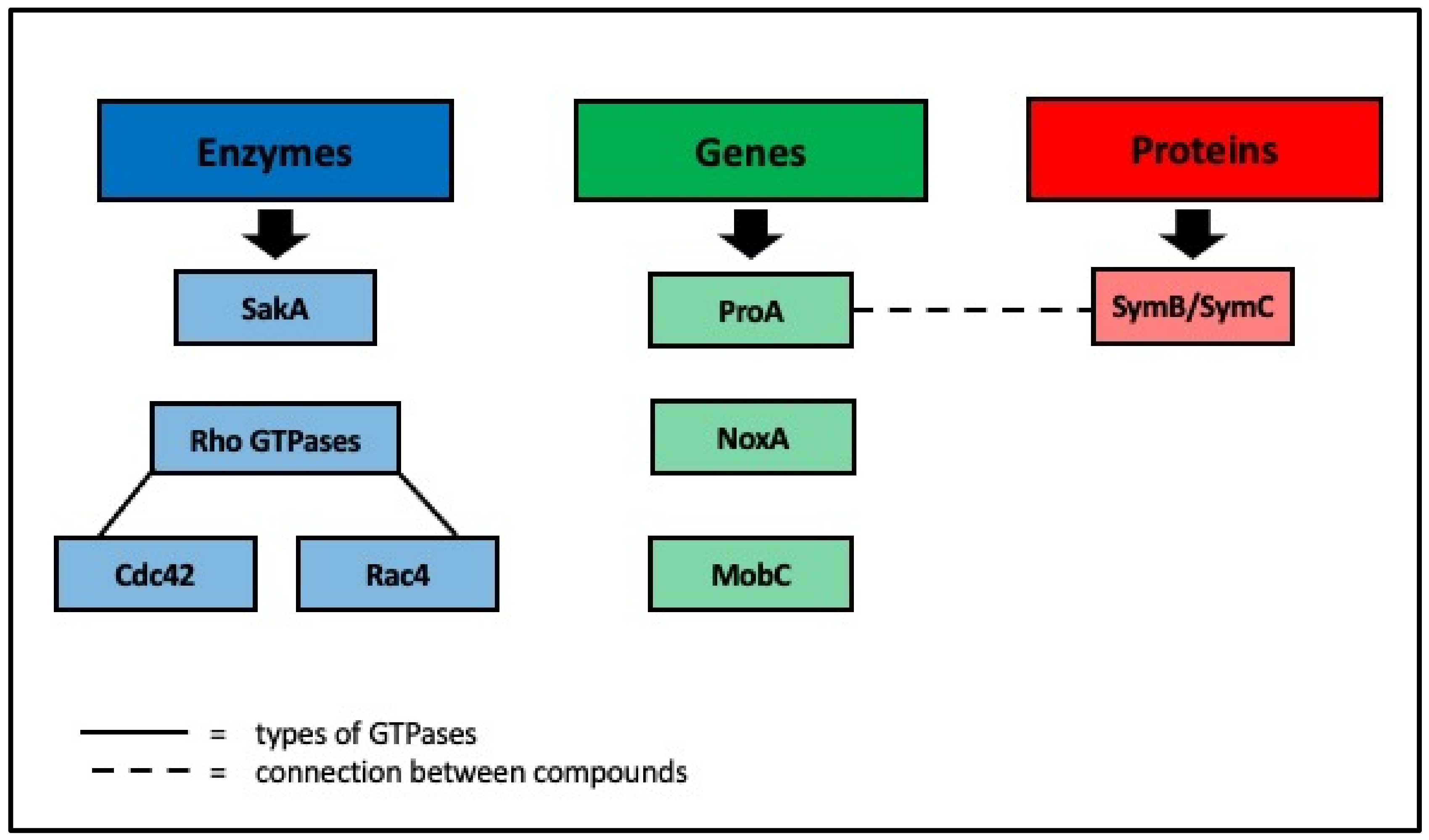
| Stressor | Tolerance/Resistance Potential | Mediating Biomolecules | Mechanisms | References |
|---|---|---|---|---|
| Drought | High | proline, glutamic acid, mannitol, loline alkaloids, oligosaccharides, antioxidant enzymes, ABA |
| [44,45,46,47,48,49,50,51,52] |
| Salinity | High | K+, P, N, glycine betaine, enzymatic and non-enzymatic antioxidants |
| [53,54,55,56,57] |
| Heavy Metals | Medium | Enzymatic antioxidants, H2O, chlorophyll |
| [60,61,62,63] |
| Low Nutrients | High | G6DPH, NADPH, N-related enzymes, glutamine, amino acids, antioxidants, organic acids |
| [65,66,67,68,69] |
| Cold | Low | Fatty acids, proteins |
| [70,71,72,73] |
| Flood | Low | Antioxidants |
| [55,74,75,76,78] |
| Pathogens | High | alkaloids, antioxidants, proteins, salicylic acid, jasmonic acid, phenolic compounds, volatile organic compounds |
| [51,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100] |
| Nematodes | Medium | alkaloids |
| [101,102,103,104,105,106,107,108] |
| Insects | High | Peramine and loline alkaloids, jasmonic acid, volatiles |
| [27,38,92,109,110,111,112,113,114,115,116,117,118,119,120] |
| Weeds | Medium | Phenolic compounds, syringic acid, myristic acid |
| [121,122,123,124,125,126,127,128,129] |
| Animal Herbivory | High | Ergot alkaloids, indole-diterpene alkaloids |
| [130,131,132,133,134,135,136,137,138,139,140,141] |
| Soil Component | E+ Effect | Reference |
|---|---|---|
| Organic C | Increased | [167,171] |
| Carbohydrates | Increased | [167] |
| Phenols | Increased | [168] |
| Organic Matter | Increased | [171] |
| Microbial Biomass C | Mixed Results | [165,172] |
| Mineralizable C | Decreased | [165,172] |
| Microbial Biomass N | Increased | [165] |
| Mineralizable N | Increased | [165] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Missaoui, A.; Mahmud, K.; Presley, H.; Lonnee, M. Interaction between Grasses and Epichloë Endophytes and Its Significance to Biotic and Abiotic Stress Tolerance and the Rhizosphere. Microorganisms 2021, 9, 2186. https://doi.org/10.3390/microorganisms9112186
Lee K, Missaoui A, Mahmud K, Presley H, Lonnee M. Interaction between Grasses and Epichloë Endophytes and Its Significance to Biotic and Abiotic Stress Tolerance and the Rhizosphere. Microorganisms. 2021; 9(11):2186. https://doi.org/10.3390/microorganisms9112186
Chicago/Turabian StyleLee, Kendall, Ali Missaoui, Kishan Mahmud, Holly Presley, and Marin Lonnee. 2021. "Interaction between Grasses and Epichloë Endophytes and Its Significance to Biotic and Abiotic Stress Tolerance and the Rhizosphere" Microorganisms 9, no. 11: 2186. https://doi.org/10.3390/microorganisms9112186
APA StyleLee, K., Missaoui, A., Mahmud, K., Presley, H., & Lonnee, M. (2021). Interaction between Grasses and Epichloë Endophytes and Its Significance to Biotic and Abiotic Stress Tolerance and the Rhizosphere. Microorganisms, 9(11), 2186. https://doi.org/10.3390/microorganisms9112186






