Plant Growth Promoting Rhizobacteria, Arbuscular Mycorrhizal Fungi and Their Synergistic Interactions to Counteract the Negative Effects of Saline Soil on Agriculture: Key Macromolecules and Mechanisms
Abstract
:1. Introduction
2. Effect of Soil Saltiness on Plant Development
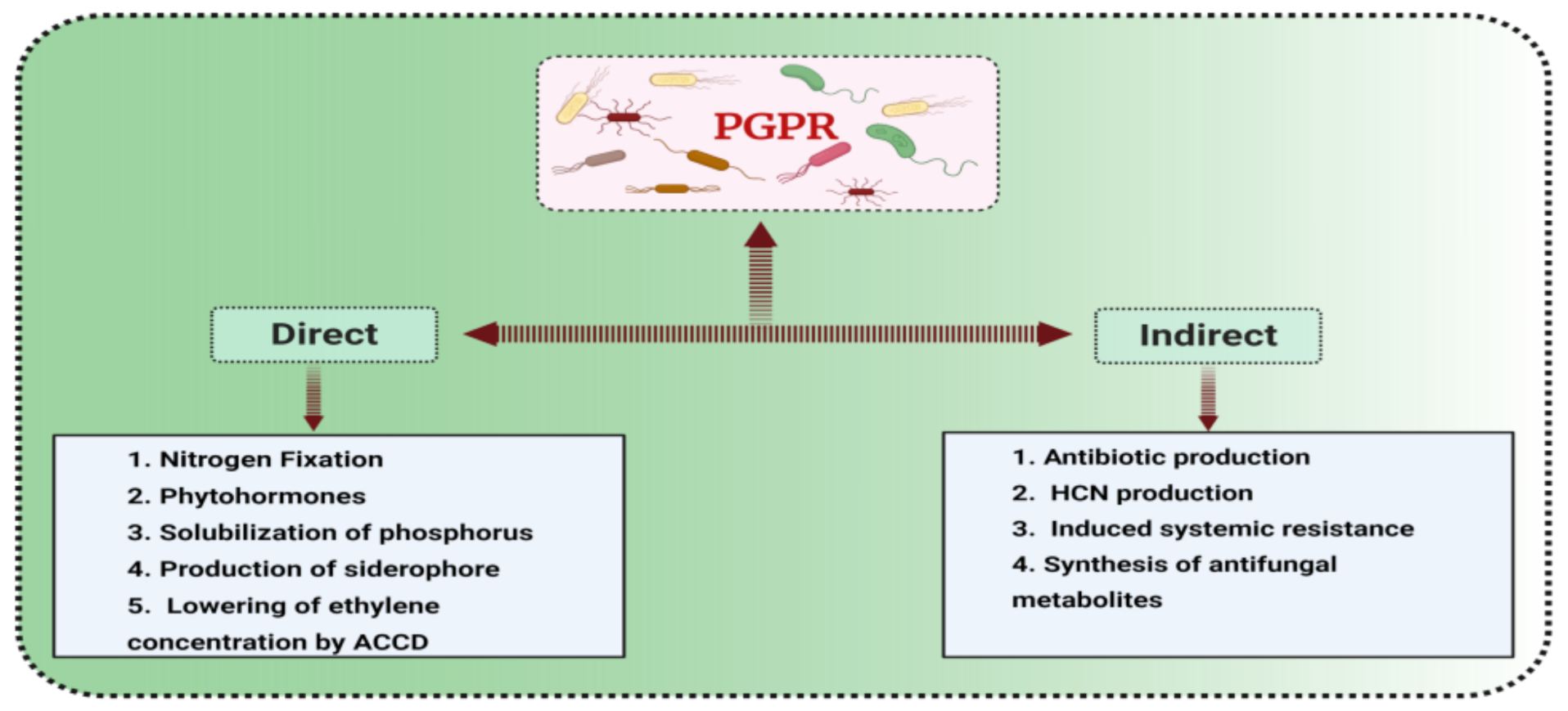
3. Plant Growth Promoting Bacteria
3.1. Classification and Mode of Action of PGPR
3.2. PGPR as a Major Player in Crop Production Enhancement under Salinity Stress
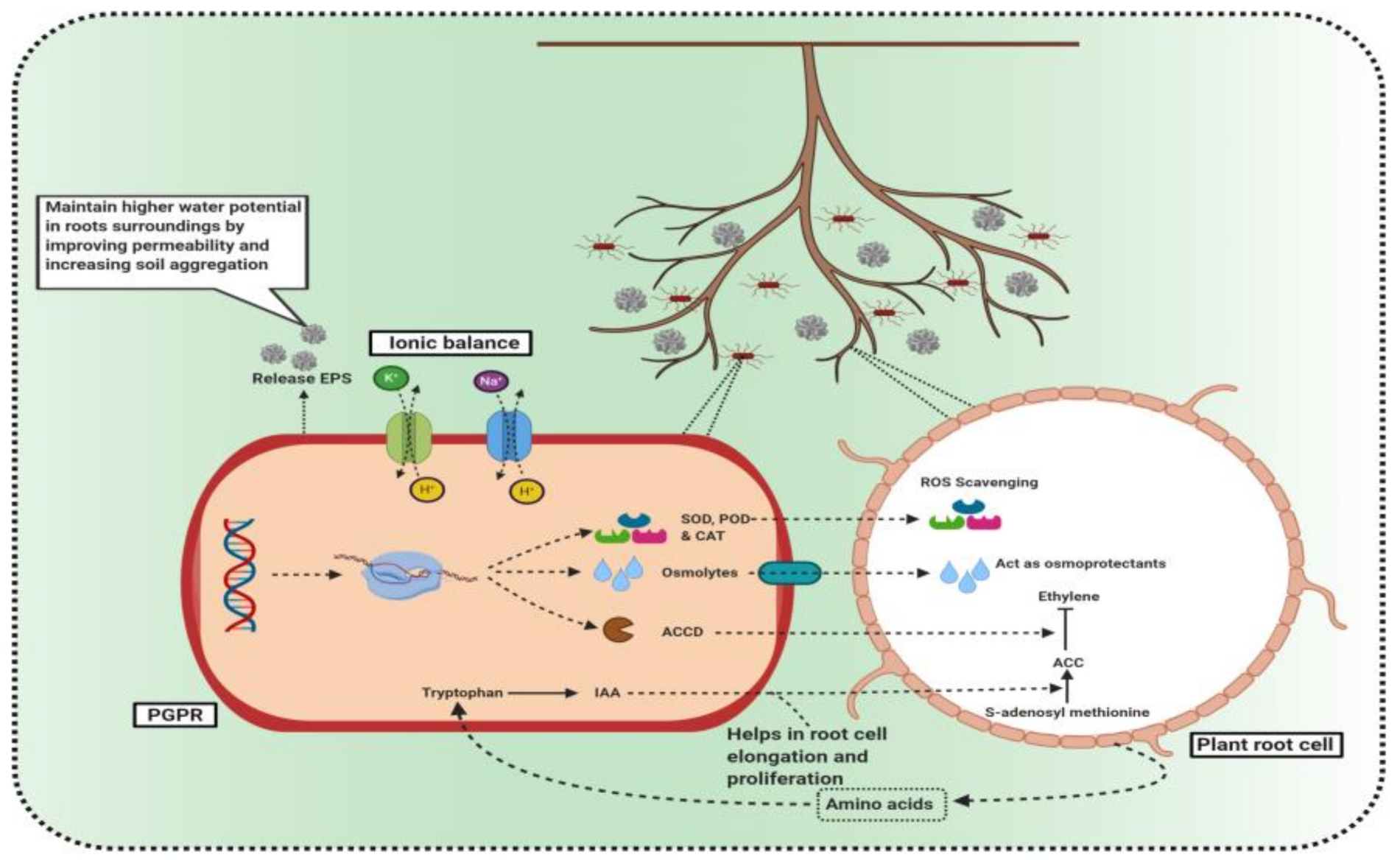
3.3. ACC Deaminase Production by PGPR as a Weapon to Fight Salt Stress
4. Arbuscular Mycorrhizal Fungi (AMF) as Complementary Microorganisms to PGPR to Overcome Salinity Stress
4.1. Mechanisms Employed by AMF for Salt Stress Amelioration
4.1.1. Increased Mineral Nutrition
4.1.2. Enhanced Water Uptake
4.1.3. Ionic Homeostasis
4.1.4. Phytohormone Synthesis
4.1.5. Improved Photosynthesis
4.1.6. Antioxidant Production
5. Co-Inoculation of AMF and PGPR Can Mitigate the Effects of Salinity in Plants
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Etesami, H.; Glick, B.R. Halotolerant plant growth-promoting bacteria: Prospects for alleviating salinity stress in plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Ramadoss, D.; Lakkineni, V.K.; Bose, P.; Ali, S.; Annapurna, K. Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. SpringerPlus 2013, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salwan, R.; Sharma, A.; Sharma, V. Microbes mediated plant stress tolerance in saline agricultural ecosystem. Plant Soil 2019, 442. [Google Scholar] [CrossRef]
- Parray, J.A.; Jan, S.; Kamili, A.N.; Qadri, R.A.; Egamberdieva, D.; Ahmad, P. Current perspectives on plant growth-promoting rhizobacteria. J. Plant Growth Regul. 2016, 35, 877–902. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Jia, Q.; Ibrahim, A.K.; Niyitanga, S.; Zhang, L. Mechanisms and signaling pathways of salt tolerance in crops: Understanding from the transgenic plants. Trop. Plant Biol. 2020, 13, 297–320. [Google Scholar] [CrossRef]
- Kushwaha, P.; Kashyap, P.L.; Bhardwaj, A.K.; Kuppusamy, P.; Srivastava, A.K.; Tiwari, R.K. Bacterial endophyte mediated plant tolerance to salinity: Growth responses and mechanisms of action. World J. Microbiol. Biotechnol. 2020, 36, 26. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Vaishnav, A.; Shukla, A.K.; Sharma, A.; Kumar, R.; Choudhary, D.K. Endophytic bacteria in plant salt stress tolerance: Current and future prospects. J. Plant Growth Regul. 2019, 38, 650–668. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Murzello, C.; Sun, Y.; Kim, M.S.; Xie, X.; Jeter, R.M.; Paré, P.W. Choline and osmotic-stress tolerance induced in Arabidopsis by the soil microbe Bacillus subtilis (GB03). Mol. Plant Microb. Interact. 2010, 23, 1097–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kronzucker, H.J.; Britto, D.T. Sodium transport in plants: A critical review. New Phytol. 2011, 18, 54–81. [Google Scholar] [CrossRef] [PubMed]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef] [Green Version]
- Jha, U.C.; Bohra, A.; Jha, R.; Parida, S.K. Salinity stress response and ‘omics’ approaches for improving salinity stress tolerance in major grain legumes. Plant Cell Rep. 2019, 38, 255–277. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Katiyar, D.; Hemantaranjan, A.; Singh, B. Plant growth promoting Rhizobacteria-an efficient tool for agriculture promotion. Adv. Plants Agric. Res. 2016, 4, 426–434. [Google Scholar] [CrossRef]
- Ahkami, A.H.; White, R.A., III; Handakumbura, P.P.; Jansson, C. Rhizosphere engineering: Enhancing sustainable plant ecosystem productivity. Rhizosphere 2017, 3, 233–243. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Kloepper, J.W.; Schroth, M.N. Plant growth promoting rhizobacteria on radishes. In Station de Pathologie Vegetale et Phytobacteriologie, Proceedings of the 4th International Conference on Plant Pathogenic Bacteria II, Angers, France, 27 August–2 September 1978; Gilbert-Clary: Clary, France, 1978; pp. 879–882. [Google Scholar]
- De-Bashan, L.E.; Hernandez, J.P.; Bashan, Y. The potential contribution of plant growth-promoting bacteria to reduce environmental degradation—A comprehensive evaluation. Appl. Soil Ecol. 2012, 61, 171–189. [Google Scholar] [CrossRef]
- De Figueiredo, M.V.B.; Bonifacio, A.; Rodrigues, A.C.; de Araujo, F.F. Plant growth-promoting rhizobacteria: Key mechanisms of action. In Microbial-Mediated Induced Systemic Resistance in Plants; Springer: Berlin/Heidelberg, Germany, 2016; pp. 23–37. [Google Scholar]
- Ahmad, M.; Zahir, Z.A.; Khalid, M.; Nazli, F.; Arshad, M. Efficacy of Rhizobium and Pseudomonas strains to improve physiology, ionic balance and quality of mung bean under salt-affected conditions on farmer’s fields. Plant Physiol. Biochem. 2013, 63, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Olivares, F.L.; Busato, J.G.; de Paula, A.M.; da Lima, L.S.; Aguiar, N.O.; Canellas, L.P. Plant growth promoting bacteria and humic substances: Crop promotion and mechanisms of action. Chem. Biol. Technol. Agric. 2017, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Hassen, W.; Cherif, H.; Souissi, Y.; Raddedi, N.; Neifar, M.; Cherif, A. Rhizobacteria and their metabolites as a promising green approach for the treatment of pesticide contaminated agricultural soils. MOJ Ecol. Environ. Sci. 2020, 5, 244–254. [Google Scholar]
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.-C. Perspectives and challenges of microbial application for crop improvement. Front. Plant Sci. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, M.; Seldin, L.; de Araujo, F.; Mariano, R. Plant growth promoting rhizobacteria: Fundamentals and applications. In Plant Growth and Health Promoting Bacteria; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 21–43. [Google Scholar]
- Verma, J.P.; Yadav, J.; Tiwari, K.N.; Singh, L.V. Impact of plant growth promoting rhizobacteria on crop production. Int. J. Agric. Res. 2010, 5, 954–983. [Google Scholar] [CrossRef] [Green Version]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Orozco-Mosqueda, M.C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Kannahi, M.; Senbagam, N. Studies on siderophore production by microbial isolates obtained from rhizosphere soil and its antibacterial activity. J. Chem. Pharm. Res. 2014, 6, 1142–1145. [Google Scholar]
- Zheng, B.-X.; Hao, X.-L.; Ding, K.; Zhou, G.-W.; Chen, Q.-L.; Zhang, J.-B.; Zhu, Y.-G. Long-term nitrogen fertilization decreased the abundance of inorganic phosphate solubilizing bacteria in an alkaline soil. Sci. Rep. 2017, 7, 42284. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Noya, Y.E.; Martínez-Romero, E.; Hernández-Rodríguez, C. Potential plant-growth-promoting and nitrogen-fixing bacteria associated with pioneer plants growing on mine tailings. In Molecular Microbial Ecology of the Rhizosphere; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; Volume 2, pp. 1003–1011. [Google Scholar]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [Green Version]
- Del Orozco-Mosqueda, M.C.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef] [PubMed]
- Duffy, B.; Keel, C.; Defago, G. Potential role of pathogen signaling in multitrophic plant-microbe interactions involved in disease protection. Appl. Environ. Microbiol. 2004, 70, 1836–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, U.; Chakraborty, B.N.; Basnet, M.; Chakraborty, A.P. Evaluation of Ochrobactrum anthropic TRS-2 and its talc based formulation for enhancement of growth of tea plants and management of brown root rot disease. J. Appl. Microbiol. 2009, 107, 625–634. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z.; Ahmad, H. Plant growth promoting bacteria: Role in soil improvement, abiotic and biotic stress management of crops. Plant Cell Rep. 2018, 37, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.-H.; Kim, J.-S.; Lee, C.-H.; Seo, H.-S.; Chun, S.-C.; Oh, J.; Choi, E.-H.; Park, G. Enhancement of vitality and activity of a plant growth-promoting bacteria (PGPB) by atmospheric pressure non-thermal plasma. Sci. Rep. 2019, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Jha, P.N. Alleviation of salinity-induced damage on wheat plant by an ACC deaminase-producing halophilic bacterium Serratia sp. SL-12 isolated from a salt lake. Symbiosis 2016, 69, 101–111. [Google Scholar] [CrossRef]
- Upadhyay, S.; Singh, D. Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 2015, 17, 288–293. [Google Scholar] [CrossRef]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef]
- Kumari, S.; Varma, A.; Tuteja, N.; Choudhary, D.K. Bacterial ACC-deaminase: An eco-friendly strategy to cope abiotic stresses for sustainable agriculture. In Plant-Microbe Interaction: An Approach to Sustainable Agriculture; Springer: Singapore, 2016; pp. 165–185. [Google Scholar]
- Li, H.; Jiang, X. Inoculation with plant growth-promoting bacteria (PGPB) improves salt tolerance of maize seedling. Russ. J. Plant Physiol. 2017, 64, 235–241. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- Jha, Y.; Subramanian, R. Paddy plants inoculated with PGPR show better growth physiology and nutrient content under saline condition. Chil. J. Agric. Res. 2013, 73, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Nautiyal, C.S.; Srivastava, S.; Chauhan, P.S.; Seem, K.; Mishra, A.; Sopory, S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 2013, 66. [Google Scholar] [CrossRef]
- Shah, G.; Jan, M.; Afreen, M.; Anees, M.; Rehman, S.; Daud, M.K.; Malook, I.; Jamil, M. Halophilic bacteria mediated phytoremediation of salt-affected soils cultivated with rice. J. Geochem. Explor. 2017, 174, 59–65. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Maurya, S.K.; Singh, D.P. Salinity tolerance in free-living plant growth promoting rhizobacteria. Indian J. Res. 2012, 3, 73–78. [Google Scholar]
- Rajput, L.; Imran, A.; Mubeen, F.; Hafeez, F.Y.; Fauzia, A.; Hafeez, Y.; Hafeez, F.Y. Salt-tolerant PGPR strain Planococcus rifietoensis promotes the growth and yield of wheat (Triticum aestivum L.) cultivated in saline soil. Pak. J. Bot. 2013, 45, 1955–1962. [Google Scholar]
- Orhan, F. Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum). Braz. J. Microbiol. 2016, 47, 621–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.P.; Jha, P.; Jha, P.N. Bio-inoculation of plant growth-promoting rhizobacterium Enterobacter cloacae ZNP-3 increased resistance against salt and temperature stresses in wheat plant (Triticum aestivum L.). J. Plant Growth Regul. 2017, 36, 783–798. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. Analysis of fatty acid composition of PGPR Klebsiella sp. SBP-8 and its role in ameliorating salt stress in wheat. Symbiosis 2017, 73, 213–222. [Google Scholar] [CrossRef]
- Sagar, A.; Dhusiya, K.; Shukla, P.K.; Ramteke, P.W. Salt tolerance plant growth promoting bacterium Enterobacter cloacae (KP226569) in sustainable maize production under salt stress. In Proceedings of the International Conference on Advancing Frontiers in Biotechnology for Sustainable Agriculture and Health (AFBSAH), Allahabad, India, 25–26 February 2016. [Google Scholar]
- Akram, M.S.; Shahid, M.; Tariq, M.; Azeem, M.; Javed, M.T.; Saleem, S.; Riaz, S. Deciphering Staphylococcus sciuri SAT-17 mediated antioxidative defense mechanisms and growth modulations in salt stressed maize (Zea mays L.). Front. Microbiol. 2016, 7, 86714. [Google Scholar] [CrossRef] [PubMed]
- Anzuay, M.S.; Ciancio, M.G.R.; Ludueña, L.M.; Angelini, J.G.; Barros, G.; Pastor, N.; Taurian, T. Growth promotion of peanut (Arachis hypogaea L.) and maize (Zea mays L.) plants by single and mixed cultures of efficient phosphate solubilizing bacteria that are tolerant to abiotic stress and pesticides. Microbiol. Res. 2017, 199, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Ratering, S.; Suarez, C.; Montoya, A.M.Z.; Geissler-Plaum, R.; Schnell, S.; Maria, A.; Montoya, Z.; Geissler-Plaum, R.; Schnell, S. Paradox of plant growth promotion potential of rhizobacteria and their actual promotion effect on growth of barley (Hordeum vulgare L.) under salt stress. Microbiol. Res. 2015, 181, 22–32. [Google Scholar] [CrossRef]
- Tank, N.; Saraf, M. Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J. Plant Interact. 2010, 5, 51–58. [Google Scholar] [CrossRef]
- Yoo, S.J.; Weon, H.Y.; Song, J.; Sang, M.K. Induced tolerance to salinity stress by halotolerant bacteria Bacillus aryabhattai H19-1 and B. mesonae H20-5 in tomato plants. J. Microbiol. Biotechnol. 2019, 29, 1124–1136. [Google Scholar] [CrossRef]
- Naz, I.; Bano, A.; Ul-Hassan, T. Isolation of phytohormones producing plant growth promoting rhizobacteria from weeds growing in Khewra salt range, Pakistan and their implication in providing salt tolerance to Glycine max L. Afr. J. Biotechnol. 2009, 8, 5762–5766. [Google Scholar]
- Baha, N.; Bekki, A. An approach of improving plant salt tolerance of Lucerne (Medicago sativa) grown under salt stress: Use of Bio-inoculants. J. Plant Growth. Regul. 2015, 34, 169–182. [Google Scholar] [CrossRef]
- Sukweenadhi, J.; Balusamy, S.R.; Kim, Y.-J.; Lee, C.H.; Kim, Y.-J.; Koh, S.C.; Yang, D.C. A growth-promoting bacteria, Paenibacillus yonginensis DCY84T enhanced salt stress tolerance by activating defense-related systems in Panax ginseng. Front. Plant Sci. 2018, 9, 813. [Google Scholar] [CrossRef] [Green Version]
- Hussein, K.A.; Joo, J.H. Plant growth-promoting rhizobacteria improved salinity tolerance of Lactuca sativa and Raphanus sativus. J. Microbiol. Biotechnol. 2018, 28, 938–945. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Lei, P.; Pang, X.; Li, S.; Xu, H.; Xu, Z.; Feng, X. Enhanced tolerance to salt stress in canola (Brassica napus L.) seedlings inoculated with the halotolerant Enterobacter cloacae HSNJ4. Appl. Soil Ecol. 2017, 119, 26–34. [Google Scholar] [CrossRef]
- Rao, K.P.; Lal, A.M.; Abraham, G.; Ramteke, P.W. Threshold capacity of strawberry cultivars to salinity and rhizosphere bacterial population for tolerance. Indian J. Agric. Biochem. 2018, 31, 65–70. [Google Scholar] [CrossRef]
- Kong, Z.; Glick, B.R. The role of plant growth-promoting bacteria in metal phytoremediation. Adv. Microb. Physiol. 2017, 71, 97–132. [Google Scholar] [PubMed]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Numana, M.; Bashira, S.; Khana, Y.; Mumtaza, R.; Khan, Z.; Khanb, A.L.; Khanb, A.; Harrasi, A.A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, S.K.; Singh, M.K.; Singh, V.K.; Modi, A.; Singh, P.K.; Kumar, A. Plant growth-promoting rhizobacteria and their functional role in salinity stress management. Abat. Environ. Pollut. 2020, 151–160. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review. Microbiol. Res. 2017, 207, 41–52. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur. J. Plant. Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Saghafi, D.; Ghorbanpour, M.; Lajayer, B.A. Efficiency of Rhizobium strains as plant growth promoting rhizobacteria on morpho-physiological properties of Brassica napus L. under salinity stress. J. Soil Sci. Plant Nutr. 2018, 18, 253–268. [Google Scholar] [CrossRef] [Green Version]
- Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Salt stress alleviation in citrus plants by plant growth-promoting rhizobacteria Pseudomonas putida and Novosphingobium sp. Plant Cell Rep. 2018, 37, 1557–1569. [Google Scholar] [CrossRef]
- Glick, B.R.; Penrose, D.; Li, J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Shelke, G.M.; Kumar, A.; Jha, P.N. Biochemistry and genetics of ACC deaminase: A weapon to “stress ethylene” produced in plants. Front. Microbiol. 2015, 6, 937. [Google Scholar] [PubMed]
- Camoni, L.; Visconti, S.; Aducci, P.; Marra, M. 14-3-3 proteins in plant hormone signaling: Doing several things at once. Front. Plant Sci. 2018, 9, 297. [Google Scholar] [CrossRef]
- Paul, D.; Nair, S. Stress adaptations in a plant growth promoting rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils. J. Basic Microbiol. 2008, 48, 378–384. [Google Scholar] [CrossRef]
- Bal, H.B.; Das, S.; Dangar, T.K.; Adhya, T.K. ACC deaminase and IAA producing growth promoting bacteria from the rhizosphere soil of tropical rice plants. J. Basic Microbiol. 2013, 53, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Bal, H.B.; Nayak, L.; Das, S.; Adhya, T.K. Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil 2013, 366, 93–105. [Google Scholar] [CrossRef]
- Etesami, H.; Mirseyed, H.; Hossein, H.; Alikhani, A. Bacterial biosynthesis of 1-aminocyclopropane-1-caboxylate (ACC) deaminase, a useful trait to elongation and endophytic colonization of the roots of rice under constant flooded conditions. Physiol. Mol. Biol. Plants 2014, 20, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Bhise, K.K.; Dandge, P.B. Alleviation of salinity stress in rice plant by encapsulated salt tolerant plant growth promoting bacteria Pantoea agglomerans strain KL and its root colonization ability. Arch. Agron. Soil Sci. 2019, 65, 1955–1968. [Google Scholar] [CrossRef]
- Sagar, A.; Shukla, P.K.; Sayyad, R.Z.; Ramteke, P.W. Stimulation of seed germination and growth parameters of rice var. Sahbhagi by Enterobacter cloacae (KP226569) PR4 in presence of ammonia sulphate as substitute of ACC. In Plant Growth Promoting Rhizobacteria (PGPR): Prospects in Sustainable Agriculture; Sayyed, R.Z., Reddy, M.S., Antonius, S., Eds.; Springer Nature: Singapore, 2019; pp. 117–124. [Google Scholar]
- Sagar, A.; Sayyed, R.Z.; Ramteke, P.W.; Sharma, S.; Marraiki, N.; Elgorban, A.M.; Syed, A. ACC deaminase and antioxidant enzymes producing halophilic Enterobacter sp. ameliorates salt stress and promotes the growth of rice and millets under salt stress. Physiol. Mol. Biol. Plants 2020, 26, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Zahir, Z.A.; Ghani, U.; Naveed, M.; Nadeem, S.M.; Asghar, H.N. Comparative effectiveness of Pseudomonas and Serratia sp containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.) under salt-stressed conditions. Arch. Microbiol. 2009, 191, 415–424. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Asghar, H.N.; Arshad, M. Rhizobacteria capable of producing ACC-deaminase may mitigate salt stress in wheat. Soil Sci. Soc. Am. J. 2010, 74, 533–542. [Google Scholar] [CrossRef]
- Nia, S.H.; Zarea, M.J.; Rejali, F.; Varma, A. Yield and yield components of wheat as affected by salinity and inoculation with Azospirillum strains from saline or non-saline soil. J. Saudi Soc. Agric. Sci. 2012, 11, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Nawaz, S. Mitigation of salinity-induced negative impact on the growth and yield of wheat by plant growth-promoting rhizobacteria in naturally saline conditions. Ann. Microbiol. 2013, 63, 225–232. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The plant-growth-promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J. Plant Physiol. 2015, 184, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Jha, P.N. A halotolerant bacterium Bacillus licheniformis HSW-16 augments induced systemic tolerance to salt stress in wheat plant (Triticum aestivum). Front. Plant Sci. 2016, 7, 1890. [Google Scholar] [CrossRef] [PubMed]
- Bhise, K.K.; Bhagwat, P.K.; Dandge, P.B. Synergistic effect of Chryseobacterium gleum sp. SUK with ACC deaminase activity in alleviation of salt stress and plant growth promotion in Triticum aestivum L. 3 Biotech 2017, 7, 105. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Arshad, M. Rhizobacteria containing ACC-deaminase confers salt tolerance in maize grown on salt affected fields. Can. J. Microbiol. 2009, 55, 1302–1309. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Farooq, H.M.; Zahir, Z.A.; Hussain, M.; Hussain, A. Application of ACC-deaminase containing rhizobacteria with fertilizer improves maize production under drought and salinity stress. Int. J. Agric. Biol. 2014, 16, 591–596. [Google Scholar]
- Sagar, A.; Shukla, P.K.; Ramteke, P.W. 1-aminocyclopropane-1-carboxylate deaminase (ACCD) containing Enterobacter cloacae (KP226569) enhanced the seed germination and growth parameters of maize var SHIATS MS-2 in presence of ammonia sulphate as substitute of ACC. In Proceedings of the International Conference on Technological Advancement for Sustainable Agriculture and Rural Development (TASARD), Noida, India, 20–22 February 2017; Society for Plant Research in Collaboration with African-Asian Rural Development: New Delhi, India, 2017. [Google Scholar]
- Sagar, A.; Kuddus, M.; Singh, B.P.; Labhane, N.M.; Srivastava, S.; Ramteke, P.W. Plant growth promotion of millets under abiotic stress using Enterobacter cloacae PR10 (KP226575). J. Indian Bot. Soc. 2020, 100, 30–41. [Google Scholar]
- Ahmad, M.; Zahir, Z.A.; Asghar, H.N.; Asghar, M. Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase. Can. J. Microbiol. 2011, 57, 578–589. [Google Scholar] [CrossRef]
- Siddikee, M.A.; Chauhan, P.S.; Sa, T. Regulation of ethylene biosynthesis under salt stress in red pepper (Capsicum annuum L.) by 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase- producing halotolerant bacteria. J. Plant Growth Regul. 2012, 31, 265–272. [Google Scholar] [CrossRef]
- Chang, P.; Gerhardt, K.E.; Huang, X.-D.; Yu, X.-M.; Glick, B.R.; Gerwing, P.D.; Greenberg, B.M. Plant growth-promoting bacteria facilitate the growth of barley and oats in salt-impacted soil: Implications for phytoremediation of saline soils. Int. J. Phytoremed. 2014, 16, 1133–1147. [Google Scholar] [CrossRef]
- Goswami, D.; Parmar, S.; Vaghela, H.; Dhandhukia, P.; Thakker, J.N. Describing Paenibacillus mucilaginosus strain N3 as an efficient plant growth promoting rhizobacteria (PGPR). Cogent Food Agric. 2015, 1, 1000714. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Naveed, M.; Imran, M.; Zahir, Z.A.; Crowley, D.E. Relationship between in vitro characterization and comparative efficacy of plant growth-promoting rhizobacteria for improving cucumber salt tolerance. Arch. Microbiol. 2016, 198, 379–387. [Google Scholar] [CrossRef]
- Saravanakumar, D.; Samiyappan, R. ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J. Appl. Microbiol. 2007, 102, 1283–1292. [Google Scholar] [CrossRef]
- Kang, S.-M.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.-G.; Lee, K.-E.; Lee, I.-J. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019, 19, 80. [Google Scholar] [CrossRef] [Green Version]
- Hajiboland, R. Role of arbuscular mycorrhiza in amelioration of salinity. In Salt Stress Plants: Signalling, Omics and Adaptations; Springer: New York, NY, USA, 2013; pp. 301–354. [Google Scholar]
- Wu, Q.-S.; Zou, Y.-N.; Abd-Allah, E.F. Mycorrhizal association and ROS in plants. In Oxidative Damage to Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 453–475. [Google Scholar]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ahmed, N.; Ashraf, M.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santander, C.; Sanhueza, M.; Olave, J.; Borie, F.; Valentine, A.; Cornejo, P. Arbuscular mycorrhizal colonization promotes the tolerance to salt stress in lettuce plants through an efficient modification of ionic balance. J. Soil Sci. Plant Nutr. 2019, 19, 321–331. [Google Scholar] [CrossRef]
- Balliu, A.; Sallaku, G.; Rewald, B. AMF inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 2015, 7, 15967–15981. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Dames, J.F.; Gupta, A.; Sharma, S.; Gilbert, J.A.; Ahmad, P. Current developments in arbuscular mycorrhizal fungi research and its role in salinity stress alleviation: A biotechnological perspective. Crit. Rev. Biotechnol. 2015, 35, 461–474. [Google Scholar] [CrossRef]
- Eroğlu, Ç.G.; Cabral, C.; Ravnskov, S.; Topbjerg, H.B.; Wollenweber, B. Arbuscular mycorrhiza influences carbon-use efficiency and grain yield of wheat grown under pre-and post-anthesis salinity stress. Plant Biol. 2020, 22, 863–871. [Google Scholar] [CrossRef]
- Estrada, B.; Beltrán-Hermoso, M.; Palenzuela, J.; Iwase, K.; Ruiz-Lozano, J.M.; Barea, J.; Oehl, F. Diversity of arbuscular mycorrhizal fungi in the rhizosphere of Asteriscus maritimus (L.) Less., a representative plant species in arid and saline Mediterranean ecosystems. J. Arid Environ. 2013, 97, 170–175. [Google Scholar] [CrossRef]
- Mardukhi, B.; Rejali, F.; Daei, G.; Ardakani, M.R.; Malakouti, M.J.; Miransari, M. Arbuscular mycorrhizas enhance nutrient uptake in different wheat genotypes at high salinity levels under field and greenhouse conditions. C. R. Biol. 2011, 334, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Campanelli, A.; Ruta, C.; De Mastro, G.; Morone-Fortunato, I. The role of arbuscular mycorrhizal fungi in alleviating salt stress in Medicago sativa L. var. icon. Symbiosis 2013, 59, 65–76. [Google Scholar] [CrossRef]
- Montesinos-Navarro, A.; Segarra-Moragues, J.G.; Valiente-Banuet, A.; Verdú, M. The network structure of plant-arbuscular mycorrhizal fungi. New Phytol. 2012, 194, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Hajiboland, R.; Aliasgharzadeh, A.; Laiegh, S.F.; Poschenrieder, C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 2010, 331, 313–327. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zon, Y.N.; Liu, W.; Ye, X.E.; Zai, H.E.; Zhao, L.J. Alleviation of salt stress in citrus seedlings inoculated with mycorrhiza: Changes in leaf antioxidant defense systems. Plant Soil Environ. 2010, 56, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Porras-Soriano, A.; Soriano-Martin, M.L.; Porras-Piedra, A.; Azcón, R. Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. J. Plant Physiol. 2009, 166, 1350–1359. [Google Scholar] [CrossRef]
- Zuccarini, P.; Okurowska, P. Effects of mycorrhizal colonization and fertilization on growth and photosynthesis of sweet basil under salt stress. J. Plant Nutr. 2008, 31, 497–513. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Sonmez, O.; Aydemir, S.; Tuna, A.L.; Cullu, M.A. The influence of arbuscular mycorrhizal colonisation on key growth parameters and fruit yield of pepper plants grown at high salinity. Sci. Hortic. 2009, 121. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N. Adaptive responses of birch-leaved pear (Pyrus betulaefolia) seedlings to salinity stress. Not. Bot. Horti Agrobot. 2009, 37, 133–138. [Google Scholar]
- Hashem, A.; Allah, E.F.A.; Alqarawi, A.A.; Egamberdieva, D. Arbuscular mycorrhizal fungi and plant stress tolerance. In Plant Microbiome: Stress Response, Microorganisms for Sustainability; Springer Nature: Singapore, 2018; pp. 81–103. [Google Scholar]
- Khalloufi, M.; Martínez-Andújar, C.; Lachaâl, M.; Karray-Bouraoui, N.; Pérez-Alfocea, F.; Albacete, A. The interaction between foliar GA3 application and arbuscular mycorrhizal fungi inoculation improves growth in salinized tomato (Solanum lycopersicum L.) plants by modifying the hormonal balance. J. Plant Physiol. 2017, 214, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Redondo-Gomez, S.; Mateos-Naranjo, E.; Aroca, R.; Garcia, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J. Plant Physiol. 2015, 185, 75–83. [Google Scholar] [CrossRef]
- Hajiboland, R.; Dashtebani, F.; Aliasgharzad, N. Physiological responses of halophytic C4 grass Aeluropus littoralis to salinity and arbuscular mycorrhizal fungi colonization. Photosynthetica 2015, 53, 572–584. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Mishra, S. Influence of arbuscular mycorrhizal (AM) fungi and salinity on seedling growth, solute accumulation and mycorrhizal dependency of Jatropha curcas L. J. Plant Growth Regul. 2010, 29, 297–306. [Google Scholar] [CrossRef]
- Mathur, N.; Singh, J.; Bohra, S.; Bohra, A.; Vyas, A. Arbuscular mycorrhizal fungi alleviate salt stress of Trichosanthes dioica Roxb. Soil Plant Sci. 2010, 60, 510–516. [Google Scholar]
- Daei, G.; Ardekani, M.R.; Rejali, F.; Teimuri, S.; Miransari, M. Alleviation of salinity stress on wheat yield, yield components, and nutrient uptake using arbuscular mycorrhizal fungi under field conditions. J. Plant Physiol. 2009, 166, 617–625. [Google Scholar] [CrossRef]
- Garg, N.; Manchanda, G. Effect of arbuscular mycorrhizal inoculation of salt-induced nodule senescence in Cajanus cajan (pigeonpea). J. Plant Growth Regul. 2008, 27, 115–124. [Google Scholar] [CrossRef]
- Jahromi, F.; Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Influence of salinity on the in vitro development of Glomus intraradices and on the in vivo physiological and molecular responses of mycorrhizal lettuce plants. Microb. Ecol. 2008, 55, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, Y.; Shi, P.; Chen, X.; Lin, H.; Zhao, B. The differential behavior of arbuscular mycrorrhizal fungi in interaction with Astragalus sinicus L. under salt stress. Mycorrhiza 2011, 21, 27–33. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhiza on organic solutes in maize leaves under salt stress. Mycorrhiza 2011, 21, 423–430. [Google Scholar] [CrossRef]
- Dudhane, M.P.; Borde, M.Y.; Jite, P.K. Effect of arbuscular mycorrhizal fungi on growth and antioxidant activity in Gmelina arborea Roxb. under salt stress condition. Not. Sci. Biol. 2011, 3, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, M.; Boughattas, S.; Hu, S.; Oh, S.-H.; Sa, T. A meta-analysis of arbuscular mycorrhizal effects on plants grown under salt stress. Mycorrhiza 2014, 24, 611–625. [Google Scholar] [CrossRef]
- Borde, M.; Dudhane, M.; Kulkarni, M. Role of arbuscular mycorrhizal fungi (AMF) in salinity tolerance and growth response in plants under salt stress conditions. In Mycorrhiza-Eco-Physiology, Secondary Metabolites, Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2017; pp. 71–86. [Google Scholar]
- Bothe, H. Arbuscular mycorrhiza and salt tolerance of plants. Symbiosis 2012, 58, 7–16. [Google Scholar] [CrossRef]
- Saxena, B.; Shukla, K.; Giri, B. Arbuscular mycorrhizal fungi and tolerance of salt stress in plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Springer: Singapore, 2017; pp. 67–97. [Google Scholar]
- Li, T.; Hu, Y.-J.; Hao, Z.-P.; Li, H.; Chen, B.-D. Aquaporin genes GintAQPF1 and GintAQPF2 from Glomus intraradices contribute to plant drought tolerance. Plant Signal. Behav. 2013, 8, e24030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Zhao, X.; Bao, E.; Cao, K.; Zou, Z. Effects of arbuscular mycorrhizal fungi on watermelon growth, elemental uptake, antioxidant, and photosystem II activities and stress-response gene expressions under salinity-alkalinity stresses. Front. Plant Sci. 2019, 10, 863. [Google Scholar] [CrossRef] [Green Version]
- Porcel, R.; Aroca, R.; Azcon, R.; Ruiz-Lozano, J.M. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza 2016, 26, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Pons, S.; Fournier, S.; Chervin, C.; Bécard, G.; Rochange, S.; Frey, N.F.D.; Pagès, V.P. Phytohormone production by the arbuscular mycorrhizal fungus Rhizophagus irregularis. PLoS ONE 2020, 15, e0240886. [Google Scholar] [CrossRef]
- Basu, S.; Rabara, R.C.; Negi, S. AMF: The future prospect for sustainable agriculture. Physiol. Mol. Plant Pathol. 2018, 102, 36–45. [Google Scholar] [CrossRef]
- Al-Arjani, A.B.F.; Hashem, A.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi modulate dynamics tolerance expression to mitigate drought stress in Ephedra foliata Boiss. Saudi J. Biol. Sci. 2020, 27, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Motaleb, N.A.; Elhady, S.A.; Ghoname, A. AMF and Bacillus megaterium neutralize the harmful effects of salt stress on bean plants. Gesunde Pflanz. 2020, 72, 29–39. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front. Plant Sci. 2017, 8, 1739. [Google Scholar] [CrossRef] [PubMed]
- Al-Khaliel, A. Effect of salinity stress on mycorrhizal association and growth response of peanut infected by Glomus mosseae. Plant Soil Environ. 2010, 56, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Al-Karaki, G.N. Growth of mycorrhizal tomato and mineral acquisition under salt stress. Mycorrhiza 2000, 10, 51–54. [Google Scholar] [CrossRef]
- Li, Z.; Wu, N.; Meng, S.; Wu, F.; Liu, T. Arbuscular mycorrhizal fungi (AMF) enhance the tolerance of Euonymus maackii Rupr. at a moderate level of salinity. PLoS ONE 2020, 15, e0231497. [Google Scholar] [CrossRef]
- Hidri, R.; Mahmoud, O.M.-B.; Farhat, N.; Cordero, I.; Pueyo, J.J.; Debez, A.; Barea, J.-M.; Abdelly, C.; Azcon, R. Arbuscular mycorrhizal fungus and rhizobacteria affect the physiology and performance of Sulla coronaria plants subjected to salt stress by mitigation of ionic imbalance. J. Plant Nutr. Soil Sci. 2019, 182, 451–462. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.-M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Gamalero, E.; Berta, G.; Massa, N.; Glick, B.R.; Lingua, G. Interactions between Pseudomonas putida UW4 and Gigaspora rosea BEG9 and their consequences for the growth of cucumber under salt-stress conditions. J. Appl. Microbiol. 2010, 108, 236–245. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Rathore, P.; Kumari, R.; Yadav, R. Brown gold of marginal soil: Plant growth promoting bacteria to overcome plant abiotic stress for agriculture, biofuels and carbon sequestration. Sci. Total Environ. 2020, 711, 135062. [Google Scholar] [CrossRef]
- Mathimaran, N.; Sekar, J.; Nanjundegowda, T.M.; Prabavathy, V.; Ramalingam, P.V.; Perisamy, V.; Raju, K.; Natesan, S.M.; Narayanswamy, M.B.; Chikkegowda, B.N.; et al. Intercropping transplanted pigeon pea with finger millet: Arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria boost yield while reducing fertilizer input. Front. Sustain. Food Syst. 2020, 4, 88. [Google Scholar] [CrossRef]
- Pan, J.; Huang, C.; Peng, F.; Zhang, W.; Luo, J.; Ma, S.; Xue, X. Effect of arbuscular mycorrhizal fungi (AMF) and plant growth-promoting bacteria (PGPR) inoculations on Elaeagnus angustifolia L. in saline soil. Appl. Sci. 2020, 10, 945. [Google Scholar] [CrossRef] [Green Version]
- Hashem, A.; Allah, E.F.A.; Alqarawi, A.A.; Al-Huqail, A.A.; Wirth, S.; Egamberdieva, D. The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front. Microbiol. 2016, 7, 1089. [Google Scholar] [CrossRef] [Green Version]
- Baradar, A.; Saberi-Riseh, R.; Sedaghati, E.; Akhgar, A. Effect of some bacteria and iron chelators on potato colonization by arbuscular mycorrhiza fungi inoculated by Rhizoctonia. Ind. J. Sci. Technol. 2015, 8, 1–5. [Google Scholar] [CrossRef]
- Lee, Y.; Krishnamoorthy, R.; Selvakumar, G.; Kim, K.; Sa, T. Alleviation of salt stress in maize plant by co-inoculation of arbuscular mycorrhizal fungi and Methylobacterium oryzae CBMB20. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 533–540. [Google Scholar] [CrossRef]
- Hassani, F.; Ardakani, M.; Asgharzade, A.; Paknezhad, F.; Hamidi, A. Efficiency of mycorrhizal fungi and phosphate solubilizing bacteria on phosphorus uptake and chlorophyll index in potato plants. Int. J. Biosci. 2014, 4, 244–251. [Google Scholar]
- Younesi, O.; Moradi, A. Effects of plant growth-promoting rhizobacterium (PGPR) and arbuscular mycorrhizal fungus (AMF) on antioxidant enzyme activities in salt-stressed bean (Phaseolus vulgaris L.). Agriculture 2014, 60, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Mdel, M.A.; Kohler, J.; Caravaca, F.; Roldán, A. Differential effects of Pseudomonas mendocina and Glomus intraradices on lettuce plants physiological response and aquaporin PIP2 gene expression under elevated atmospheric CO2 and drought. Microb. Ecol. 2009, 58, 942–951. [Google Scholar]
- Dhawi, F.; Datta, R.; Ramakrishna, W. Mycorrhiza and heavy metal resistant bacteria enhance growth, nutrient uptake and alter metabolic profile of sorghum grown in marginal soil. Chemosphere 2016, 157, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Ror, P.; Rathore, P.; Ramakrishna, W. Bacteria from native soil in combination with arbuscular mycorrhizal fungi augment wheat yield and biofortification. Plant Physiol. Biochem. 2020, 150, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Ror, P.; Rathore, P.; Kumar, S.; Ramakrishna, W. Bacillus subtilis CP4, isolated from native soil in combination with arbuscular mycorrhizal fungi promotes biofortification, yield and metabolite production in wheat under field conditions. J. Appl. Microbiol. 2020. [Google Scholar] [CrossRef]
- Diagne, N.; Ndour, M.; Djighaly, P.I.; Ngom, D.; Ngom, M.C.N.; Ndong, G.; Svistoonoff, S.; Cherif-Silini, H. Effect of plant growth promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) on salt stress tolerance of Casuarina obesa (Miq.). Front. Sustain. Food Syst. 2020, 4, 266. [Google Scholar] [CrossRef]
- Ashrafi, E.; Zahedi, M.; Razmjoo, J. Co-inoculations of arbuscular mycorrhizal fungi and rhizobia under salinity in alfalfa. Soil Sci. Plant Nutr. 2014, 60, 619–629. [Google Scholar] [CrossRef] [Green Version]
- Hashem, A.; Allah, E.F.A.; Alqarawi, A.A.; Wirth, S.; Egamberdieva, D. Comparing symbiotic performance and physiological responses of two soybean cultivars to arbuscular mycorrhizal fungi under salt stress. Saudi J. Biol. Sci. 2019, 26, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Nanjundappa, A.; Bagyaraj, D.J.; Saxena, A.K.; Kumar, M.; Chakdar, H. Interaction between arbuscular mycorrhizal fungi and Bacillus spp. in soil enhancing growth of crop plants. Fungal Biol. Biotechnol. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamalero, E.; Glick, B.R. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015, 169, 13–22. [Google Scholar] [CrossRef] [Green Version]
- López-Ráez, J.A.; Verhage, A.; Fernández, I.; García, J.M.; Azcón-Aguilar, C.; Flors, V.; Pozo, M.J. Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. J. Exp. Bot. 2010, 61, 2589–2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, N.K.; Fatima, T.; Mishra, J.; Mishra, I.; Verma, S.; Verma, R.; Verma, M.; Bhattacharya, A.; Verma, P.; Mishra, P.; et al. Halo-tolerant plant growth promoting rhizobacteria for improving productivity and remediation of saline soils. J. Adv. Res. 2020, 26, 69–82. [Google Scholar] [CrossRef]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef] [Green Version]
- Pérez-De-Luque, A.; Tille, S.; Johnson, I.; Pascual-Pardo, D.; Ton, J.; Cameron, D.D. The interactive effects of arbuscular mycorrhiza and plant growth-promoting rhizobacteria synergistically enhance host plant defences against pathogens. Sci. Rep. 2017, 7, 16409. [Google Scholar] [CrossRef]
- Cameron, D.D.; Neal, A.L.; van Wees, S.C.M.; Ton, J. Mycorrhiza-induced resistance: More than the sum of its parts? Trends Plant Sci. 2013, 18, 539–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, K.; Kimura, S.; Rokka, A.; Tran, H.C.; Toyota, M.; Kukkonen, J.P.; Wrzaczek, M. CRK2 enhances salt tolerance by regulating callose deposition in connection with PLDα1. Plant Physiol. 2019, 180, 2004–2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashem, A.; Allah, E.F.A.; Alqarawi, A.A.; Al-Huqail, A.A.; Shah, M.A. Induction of osmoregulation and modulation of salt stress in Acacia gerrardii Benth. by arbuscular mycorrhizal fungi and Bacillus subtilis (BERA 71). BioMed. Res. Int. 2016, 2016, 6294098. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| PGPR | Crop/Plant | Response | Reference |
|---|---|---|---|
| Bacillus cereus, Pseudomonas species | Rice | Increased N (26%), P (16%), K (31%) | [49] |
| Bacillus amyloliquefaciens | Rice | Increased plant growth | [50] |
| Thalassobacillus denorans, Oceanobacillus kapialis | Rice | Increased germination percentage and rate | [51] |
| Bacillus subtilis, Arthrobacter sp. | Wheat | Increased dry biomass, total soluble sugars, and proline content | [52] |
| Planococcus rifietoensis | Wheat | Enhanced growth and yield | [53] |
| Thalassobacillus, Bacillus, Halomonas, Oceanobacillus, Zhihengliuella sp. | Wheat | Increased the root and shoot length, and plant fresh weight | [54] |
| Enterobacter cloacae | Wheat | Improved growth parameters, biomass, and chlorophyll content | [55] |
| Klebsiella sp. | Wheat | Increased proline, total soluble sugar, and total protein content of treated plants | [56] |
| Enterobacter cloacae | Maize | Increased root and shoot growth | [57] |
| Staphylococcus sciuri | Maize | Enhanced nutrient, chlorophyll, and protein content | [58] |
| Phosphate solubilizing bacteria | Maize and Peanut | Increased seed germination, plant growth, and P content | [59] |
| Curtobacterium flaccumfaciens | Barley | Increased plant growth | [60] |
| P. aeruginosa, P. stutzeri | Tomato | Enhanced root and shoot length | [61] |
| Bacillus aryabhattai H19-1, B. mesonae H20-5 | Tomato | Significantly higher levels of proline, abscisic acid (ABA), and antioxidant enzyme activities were observed | [62] |
| B. arryabhattai H19-1, B. mesonae H20-5 | Soybean | Enhanced root and shoot length and dry biomass | [63] |
| Sinorhizobium meliloti, Paenibacillus yonginensis | Lucerne and Ginseng | Increased chlorophyll and carotenoid | [64,65] |
| A. chroococcum, Lactobacillus sp. | Lettuce | Increased root length at 50 and 100 mM NaCl | [66] |
| Enterobacter cloacae | Canola | Increased proline levels | [67] |
| Bacillus, Pseudomonas, Enterobacter, Azotobacter, Rhizobium | Strawberry | Increased plant height | [68] |
| PGPR | Crop | Response | Reference |
|---|---|---|---|
| P. fluorescens | Rice | Maintained root colonization potential by osmotolerance mechanisms | [84] |
| Bacillus, Microbacterium, Methylophaga, Agromyces, Paenibacillus | Rice | Enhanced yield | [85] |
| Alcaligenes, Bacillus, Ochrobactrum | Rice | Positive impact on germination percentage, shoot and root growth, and chlorophyll content | [86] |
| Pseudomonas putida, Pseudomonas fluorescens | Rice | Promoted rice growth by colonizing rice roots | [87] |
| Pantoea agglomerans strain KL | Rice | Increased length, biomass, and photosynthetic pigments | [88] |
| Enterobacter cloacae (KP226569) | Rice | Enhanced seed germination and growth | [89] |
| Enterobacter sp. PR14 | Rice and Millets | Enhanced seed germination, root and shoot length | [90] |
| P. putida, P. aeruginosa, S. Proteamaculans | Wheat | Increased plant height, root length, and grain yield | [91] |
| P. putida, Enterobacter cloacae, Serratia ficaria, P. Fluorescens | Wheat | Improved growth and yield | [92] |
| Azospirillum strains | Wheat | Increased shoot dry weight and grain yield | [93] |
| Pseudomonas putida, Pseudomonas fluorescens, Enterobacter cloacae, Serratia ficaria | Wheat | Enhanced germination rate and improved the nutrient status | [94] |
| Bacillus, Hallobacillus | Wheat | Enhanced plant growth | [2] |
| Klebsiella sp. | Wheat | Increased plant biomass and chlorophyll content | [95] |
| B. subtilis | Wheat | Increased growth and yield | [46] |
| Bacillus licheniformis | Wheat | Increased root and shoot length, fresh weight, and dry weight | [96] |
| Chryseobacterium gleum sp. SUK | Wheat | Increased yield | [97] |
| Pseudomonas putida (W2), P. fluorescens (W17) | Wheat | Increased growth and yield | [98] |
| P. syringae, P. bathycetes, E. aerogenes, F. ferrugineum, P. fluorescens | Maize | Improved growth, yield, and nutrition | [78] |
| Pseudomonas syringae, Pseudomonas fluorescens | Maize | Significantly improved yield | [99] |
| Enterobacter cloacae | Maize | Increased seed germination and elongation of root and shoot | [100] |
| Enterobacter cloacae (KP226575) | Millets | Increased seed germination and elongation of root and shoot | [101] |
| Pseudomonas syringae, Pseudomonas fluorescens, Rhizobium phaseoli | Mung bean | Improved seedling growth and nodulation | [102] |
| Rhizobium, Pseudomonas | Mung bean | Improved growth, physiology, and quality of seed | [103] |
| Brevibacterium epidermidis, Bacillus aryabhattai | Canola | Increased seed germination | [104] |
| Pseudomonas sp. | Barley and Oats | Enhanced root biomass | [105] |
| Aneurinibacillus aneurinilyticus, Paenibacillus sp. | French bean | Enhanced plant growth | [106] |
| Paenibacillus mucilaginosus strain N3 | Green gram | Increased overall dry biomass | [107] |
| Bacillus megaterium, Variovorax paradoxus | Cucumber | Increased growth | [108] |
| Pseudomonas strain | Groundnut | Increased total yield | [109] |
| Leclercia adecarboxylata | Tomato | Improved plant growth | [110] |
| AMF | Crop | Plant Response Under Salt Stress | Reference |
|---|---|---|---|
| Glomus mosseae, G. etunicatum, G. intraradices | Wheat | Significant enhancement of N, K, P, Ca, Mg, Mn, Cu, Fe, Zn uptake | [119] |
| Glomus viscosum H.T. Nicoson strain A6 | Alfalfa | Improved K uptake | [120] |
| Glomus intraradices | Carnation | Flower dry weight and the total number of flowers per plant increased; number of buds and flowers increased | [121] |
| Glomus intraradices | Tomato | Na uptake in inoculated plants lower compared to control; AMF plants had greater values for K/Na and Ca/Na in both shoots and roots | [122] |
| Glomus mosseae, Glomus versiforme | Orange | Accumulation of ROS and membrane damage reduced; SOD activity was largely induced | [123] |
| Glomus mosseae, Glomus intraradices | Olive | AMF colonization was more effective under saline condition; shoot and root dry weight increased; K concentration increased in shoot | [124] |
| Glomus intraradices | Sweet Basil | Reduced Na concentration in plants; treated plants grew faster | [125] |
| Glomus clarum | Pepper | Significantly improved shoot, root dry matter, and fruit yield; improved chlorophyll concentration; proline concentration was lower | [126] |
| Glomus mosseae, Paraglomus occultum | Citrus | Leaf number, leaf area, shoot and root dry weights increased; relative water content increased; root concentration of K+, Ca2+, and Mg2+ were higher | [127] |
| Glomus etunicatum, Glomus intraradices, Glomus mosseae | Cucumber | Increased biomass, photosynthetic pigment synthesis, and antioxidant enzymes | [128] |
| Rhizophagus irregularis | Tomato | Enhanced shoot FW, leaf area, leaf number, root FW, and levels of growth hormones | [129] |
| Claroideoglomus etunicatum | Rice | Improved quantum yield of PSII photochemistry, net photosynthetic rate, and stomatal conductance | [130] |
| Claroideoglomus etunicatum | Indian Walnut | Increased shoot and root dry mass, stomatal conductance, soluble sugars, free α-amino acids, and Na+ and K+ uptake | [131] |
| Glomus intraradices | Tomato | Improved dry matter, ion uptake, growth parameters, and chlorophyll content | [132] |
| AMF consortia | Physic nut | AMF lessen the deleterious effect of salt stress (up to 0.5% NaCl) on seedling growth parameters under salt levels | [133] |
| Glomus deserticola | Parwal | AMF improved yield and alleviated deleterious effects of salt | [134] |
| Glomus etunicatum, G. mosseae, G. intraradices | Wheat | Selection of the right combination of AMF species improved wheat cultivation under salinity stress | [135] |
| Glomus mosseae | Pigeon pea | AMF inoculation increased solute accumulation to maintain osmotic balance and antioxidant enzyme activity under stress | [136] |
| Glomus intraradices | Lettuce | Shoot dry weight and shoot water content increased, and transpiration rate decreased | [137] |
| Glomus mosseae, Glomus claroideum, Glomus intraradices | Milkvetch | G. intraradices performed better than two other fungi in root colonization and enzyme activity; synergistic interaction between fungi under NaCl stress also seen | [138] |
| Glomus mosseae | Maize | AMF symbiosis improved solute accumulation in maize leaves to mitigate the negative impact of soil salinity | [139] |
| Glomus fasciculatum | English beechwood | AMF was very effective in strengthening the tolerance of Gmelina arborea grown in arid and semiarid areas | [140] |
| Plant Species | AMF Partner | PGPR Partner | Application | Ref. |
|---|---|---|---|---|
| Pigeon pea and finger millet | AMF | Pseudomonas | 128% yield increase was observed in finger millet and pigeon pea intercropping system at Kolli Hills but not at Bangalore site | [165] |
| Common bean | Glomus irradicans | Bacillus megaterium | Enhanced chlorophyll and antioxidant enzymatic activity at all tested salinity levels | [171] |
| Russian Olive | Glomus mosseae | Bacillus amyloliquefaciens | Enhanced seedlings growth and improved soil nutrient uptake | [172] |
| French honeysuckle | Rhizophagus intraradices | Pseudomonas sp., Bacillus subtilis | Soil quality improvement by modulating enzymes involved in the cycling of carbon, nitrogen, and phosphorus | [156] |
| Talh tree | Claroideoglomus etunicatum, Rhizophagus intraradices, Funneliformis mosseae | B. subtilis | Increased plant biomass, nodulation, leghemoglobin, crude protein content, and photosynthetic pigments | [148] |
| Potato | Glomus intraradices, G. mosseae | P. fluorescens T17-4, P. fluorescens VUPf5, P. fluorescens F140 | Increased fresh and dry weight, other growth factors and chlorophyll | [173] |
| Maize | Glomus etunicatum | Methylobacterium oryzae CBMB20 | Increased dry biomass, AMF root colonization, and nutrients in plants under salt stress; Na+ uptake reduced by 41% | [174] |
| Potato | Glomus mosseae, G. fasciculatum | Two strains of Pseudomonas (P116 and P173) and Bacillus (Bacillus subtilis and B. megaterium) | Significant effect on chlorophyll index and phosphorus absorption | [175] |
| Common bean | Glomus mosseae | Pseudomonas florescens | Increased proline content, CAT, and POX activity | [164] |
| Cucumber | Gigaspora rosea BEG9 | Pseudomonas putida UW4 | Increased leaf area and photosynthetic efficiency | [158] |
| Lettuce | Glomus spp. | Pseudomonas mendocina | Enhanced plant biomass | [176] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagar, A.; Rathore, P.; Ramteke, P.W.; Ramakrishna, W.; Reddy, M.S.; Pecoraro, L. Plant Growth Promoting Rhizobacteria, Arbuscular Mycorrhizal Fungi and Their Synergistic Interactions to Counteract the Negative Effects of Saline Soil on Agriculture: Key Macromolecules and Mechanisms. Microorganisms 2021, 9, 1491. https://doi.org/10.3390/microorganisms9071491
Sagar A, Rathore P, Ramteke PW, Ramakrishna W, Reddy MS, Pecoraro L. Plant Growth Promoting Rhizobacteria, Arbuscular Mycorrhizal Fungi and Their Synergistic Interactions to Counteract the Negative Effects of Saline Soil on Agriculture: Key Macromolecules and Mechanisms. Microorganisms. 2021; 9(7):1491. https://doi.org/10.3390/microorganisms9071491
Chicago/Turabian StyleSagar, Alka, Parikshita Rathore, Pramod W. Ramteke, Wusirika Ramakrishna, Munagala S. Reddy, and Lorenzo Pecoraro. 2021. "Plant Growth Promoting Rhizobacteria, Arbuscular Mycorrhizal Fungi and Their Synergistic Interactions to Counteract the Negative Effects of Saline Soil on Agriculture: Key Macromolecules and Mechanisms" Microorganisms 9, no. 7: 1491. https://doi.org/10.3390/microorganisms9071491
APA StyleSagar, A., Rathore, P., Ramteke, P. W., Ramakrishna, W., Reddy, M. S., & Pecoraro, L. (2021). Plant Growth Promoting Rhizobacteria, Arbuscular Mycorrhizal Fungi and Their Synergistic Interactions to Counteract the Negative Effects of Saline Soil on Agriculture: Key Macromolecules and Mechanisms. Microorganisms, 9(7), 1491. https://doi.org/10.3390/microorganisms9071491







