Intensification of Nickel Bioleaching with Neutrophilic Bacteria Guyparkeria halophila as an Approach to Limitation of Sulfuric Acid Pollution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mineral Materials
2.2. Microorganisms and Culturing Media
2.3. Leaching Experiments
2.4. Nickel Analyzes
2.5. Enzyme Activity Analyzes
2.6. Optical Density Analyses
2.7. Statistics
3. Results and Discussion
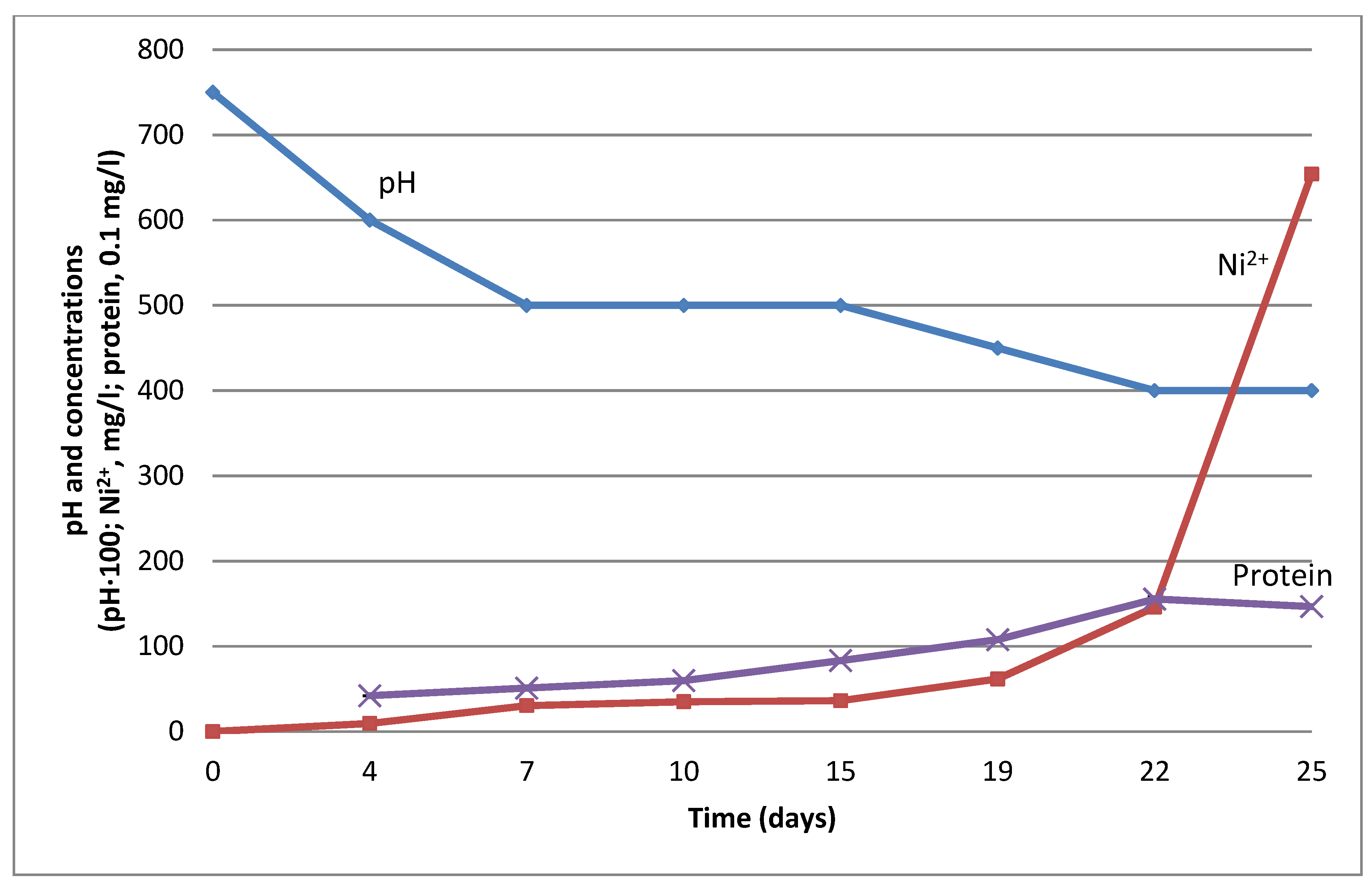
3.1. Stimulation of Bacterial Growth with Formate
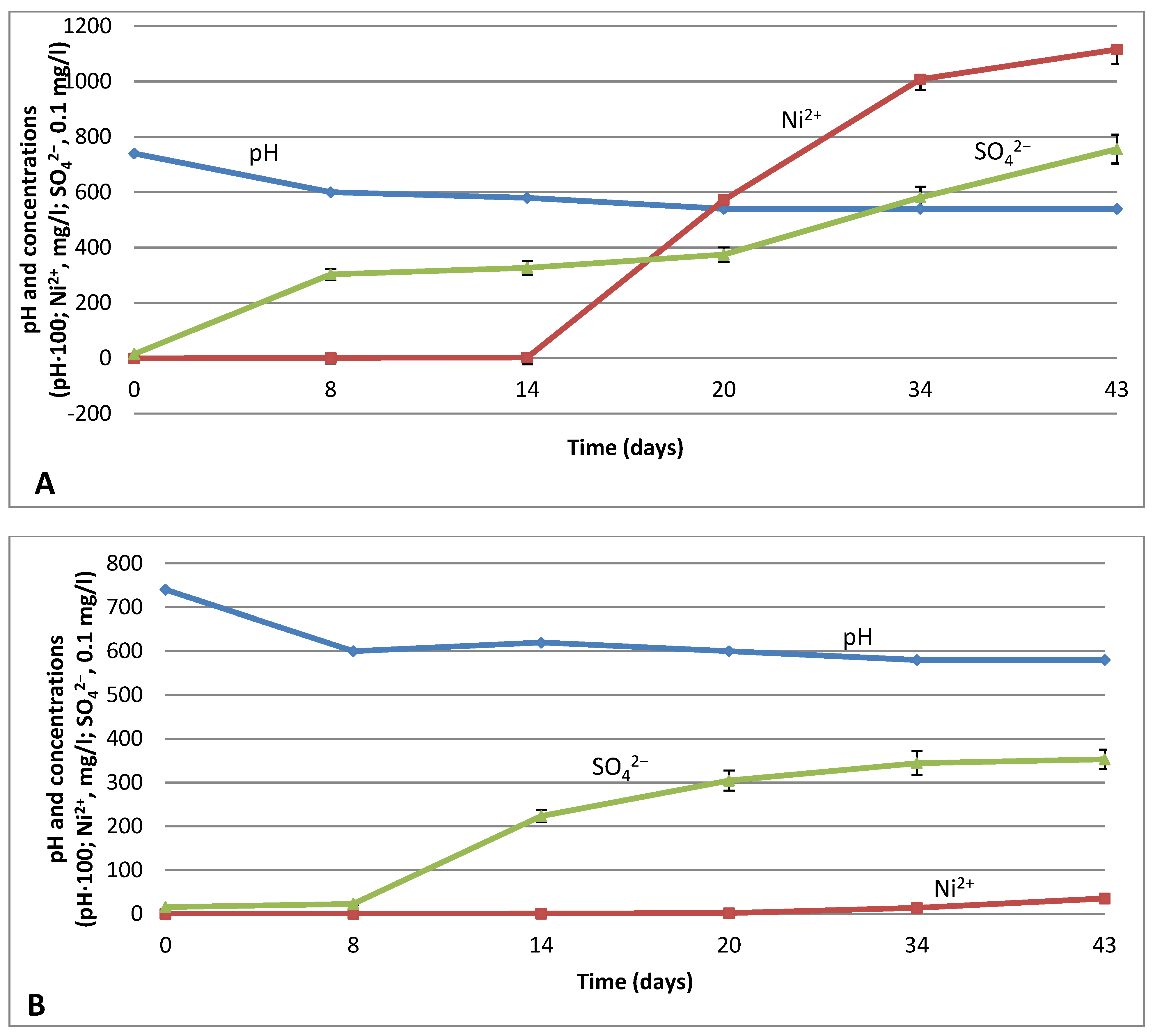
3.2. Stimulation of Ni Leaching by G. halophila with Formic Acid
3.3. Subsequent Leaching with Neutrophilic Bacteria G. halophila and Acidophilic Bacteria Acidithiobacillus sp.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuchs, W. Process of Leaching Sulfide Containing Materals with Hot, Strong Sulfuric Acid. U.S. Patent 3,726,667, 10 April 1973. Available online: https://patents.google.com/patent/US3726667A/en (accessed on 15 November 2021).
- Liu, F.; Qiao, X.; Zhou, L.; Zhang, J. Migration and Fate of Acid Mine Drainage Pollutants in Calcareous Soil. Int. J. Environ. Res. Public Health 2018, 15, 1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerley, S.; Wilson, D.; Prater, J. Cyclic Leaching Process Employing Iron-Oxidizing Bacteria. U.S. Patent 2,829,964, 8 April 1958. Available online: https://patents.google.com/patent/US2829964A/en (accessed on 15 November 2021).
- Campodonico, M.A.; Vaisman, D.; Castro, J.F.; Razmilic, V.; Mercado, F.; Andrews, B.A.; Feist, A.M.; Asenjo, J.A. Acidithiobacillus ferrooxidans’s comprehensive model driven analysis of the electron transfer metabolism and synthetic strain design for biomining applications. Metab. Eng. Commun. 2016, 3, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Monachon, M.; Albelda-Berenguer, M.; Joseph, E. Chapter One—Biological oxidation of iron sulfides. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 107, pp. 1–27. [Google Scholar] [CrossRef]
- Roshani, M.; Shojaosadati, S.; Safdari, S.; Vasheghani-Farahani, E.; Mirjalili, K.; Manafi, Z. Bioleaching of molybdenum by two new thermophilic strains isolated and characterized. Iran. J. Chem. Chem. Eng. 2017, 36, 183–194. [Google Scholar] [CrossRef]
- Ai, C.; McCarthy, S.; Liang, Y.; Rudrappa, D.; Qiu, G.; Blum, P. Evolution of copper arsenate resistance for enhanced enargite bioleaching using the extreme thermoacidophile Metallosphaera sedula. J. Ind. Microbiol. Biotechnol. 2017, 44, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Jalali, F.; Fakhar, J.; Zolfaghari, A. On using a new strain of Acidithiobacillus ferridurans for bioleaching of low-grade uranium. Sep. Sci. Technol. 2019, 55, 994–1004. [Google Scholar] [CrossRef]
- Meng, X.; Zhao, H.; Sun, M.; Zhang, Y.; Zhang, Y.; Lv, X.; Kim, H.; Vainshtein, M.; Wang, S.; Qiu, G. The role of cupric ions in the oxidative dissolution process of marmatite: A dependence on Cu2+ concentration. Sci. Total Environ. 2019, 675, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, H.; Abashina, T.; Vainshtein, M. Review on arsenic removal from sulfide minerals: An emphasis on enargite and arsenopyrite. Miner. Eng. 2021, 172, 107133. [Google Scholar] [CrossRef]
- You, J.; Solongo, S.K.; Gomez-Flores, A.; Choi, S.; Zhao, H.; Urík, M.; Ilyas, S.; Kim, H. Intensified bioleaching of chalcopyrite concentrate using adapted mesophilic culture in continuous stirred tank reactors. Bioresour. Technol. 2020, 307, 123181. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, S.; Bhatti, H.N.; Bhatti, I.A.; Sheikh, M.A.; Ghauri, M.A. Bioleaching of metal ions from low grade sulphide ore: Process optimization by using orthogonal experimental array design. Afr. J. Biotechnol. 2010, 9, 2801–2810. [Google Scholar] [CrossRef]
- Ilyas, S.; Chi, R.; Bhatti, H.N.; Bhatti, I.A.; Ghauri, M.A. Column bioleaching of low-grade mining ore containing high level of smithsonite, talc, sphaerocobaltite and azurite. Bioprocess Biosyst. Eng. 2011, 35, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Pronk, J.T.; Meijer, W.M.; Hazeu, W.; van Dijken, J.P.; Bos, P.; Kuenen, J.G. Growth of Thiobacillus ferrooxidans on Formic Acid. Appl. Environ. Microbiol. 1991, 57, 2057–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pronk, J.T.; van Dijken, J.P.; Bos, P.; Kuenen, J.G. High Tield Method of Growing Thiobacillus ferrooxidans on Formate. Patent of South Africa ZA 923117B, 30 December 1992. Available online: https://patents.google.com/patent/ZA923117B/ru (accessed on 15 November 2021).
- Boden, R. Reclassification of Halothiobacillus hydrothermalis and Halothiobacillus halophilus to Guyparkeria gen. nov. in the Thioalkalibacteraceae fam. nov., with emended descriptions of the genus Halothiobacillus and family Halothiobacillaceae. Int. J. Syst. Evol. Microbiol. 2017, 67, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.A.; Quayle, J.R.; Kemp, M.; Large, P.; Peel, D.; Heptinstall, J. Microbial growth on C1 compounds. 6. Oxidation of methanol, formaldehyde and formate by methanol-grown Pseudomonas AM 1. Biochem. J. 1964, 93, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schacterle, G.R.; Pollack, R.L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal. Biochem. 1973, 51, 654–655. [Google Scholar] [CrossRef]
- Yanishevskya, E.; Fokina, N.; Selivanova, E.; Kompanchenko, A.; Makarov, D.; Goryachev, A. Processing of Sulfide Copper-Nickel Ores from the Deposits in Murmansk Region by Heap Leaching. Minerals 2021, 11, 820. [Google Scholar] [CrossRef]

| Nos. | Minerals | Concentration, wt% | |
|---|---|---|---|
| Name | Composition | ||
| 1 | pyrrhotite | Fe(1−x)S | 33.0 |
| 2 | plagioclase | (Na, Ca)AlSi3O8 | 22.0 |
| 3 | hornblende | Ca2(Mg)O–2(Fe)2–4(Al, Fe)(Si7Al)O22(OH)2 | 13.0 |
| 4 | hypersthene | (Mg, Fe)SiO3 | 5.0 |
| 5 | pentlandite | (Fe, Ni)9S8 | 3.0 |
| 6 | chalcopyrite | CuFeS2 | 0.7 |
| Enzyme | Cofactors | Activity, nM/min·mg of Protein | |
|---|---|---|---|
| A. ferrooxidans ATCC 21834 [14] | G. halophila VKM B-2757D | ||
| Formate dehydrogenase | PMS | 80 (max.) | 18 |
| NAD+ | 0 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abashina, T.; Yachkula, A.; Kaparullina, E.; Vainshtein, M. Intensification of Nickel Bioleaching with Neutrophilic Bacteria Guyparkeria halophila as an Approach to Limitation of Sulfuric Acid Pollution. Microorganisms 2021, 9, 2461. https://doi.org/10.3390/microorganisms9122461
Abashina T, Yachkula A, Kaparullina E, Vainshtein M. Intensification of Nickel Bioleaching with Neutrophilic Bacteria Guyparkeria halophila as an Approach to Limitation of Sulfuric Acid Pollution. Microorganisms. 2021; 9(12):2461. https://doi.org/10.3390/microorganisms9122461
Chicago/Turabian StyleAbashina, Tatiana, Alyona Yachkula, Elena Kaparullina, and Mikhail Vainshtein. 2021. "Intensification of Nickel Bioleaching with Neutrophilic Bacteria Guyparkeria halophila as an Approach to Limitation of Sulfuric Acid Pollution" Microorganisms 9, no. 12: 2461. https://doi.org/10.3390/microorganisms9122461






