Molecular Variability and Host Distribution of ‘Candidatus Phytoplasma solani’ Strains from Different Geographic Origins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sources of Nucleic Acid
2.2. Amplification of ‘Ca. P. solani’ Strains
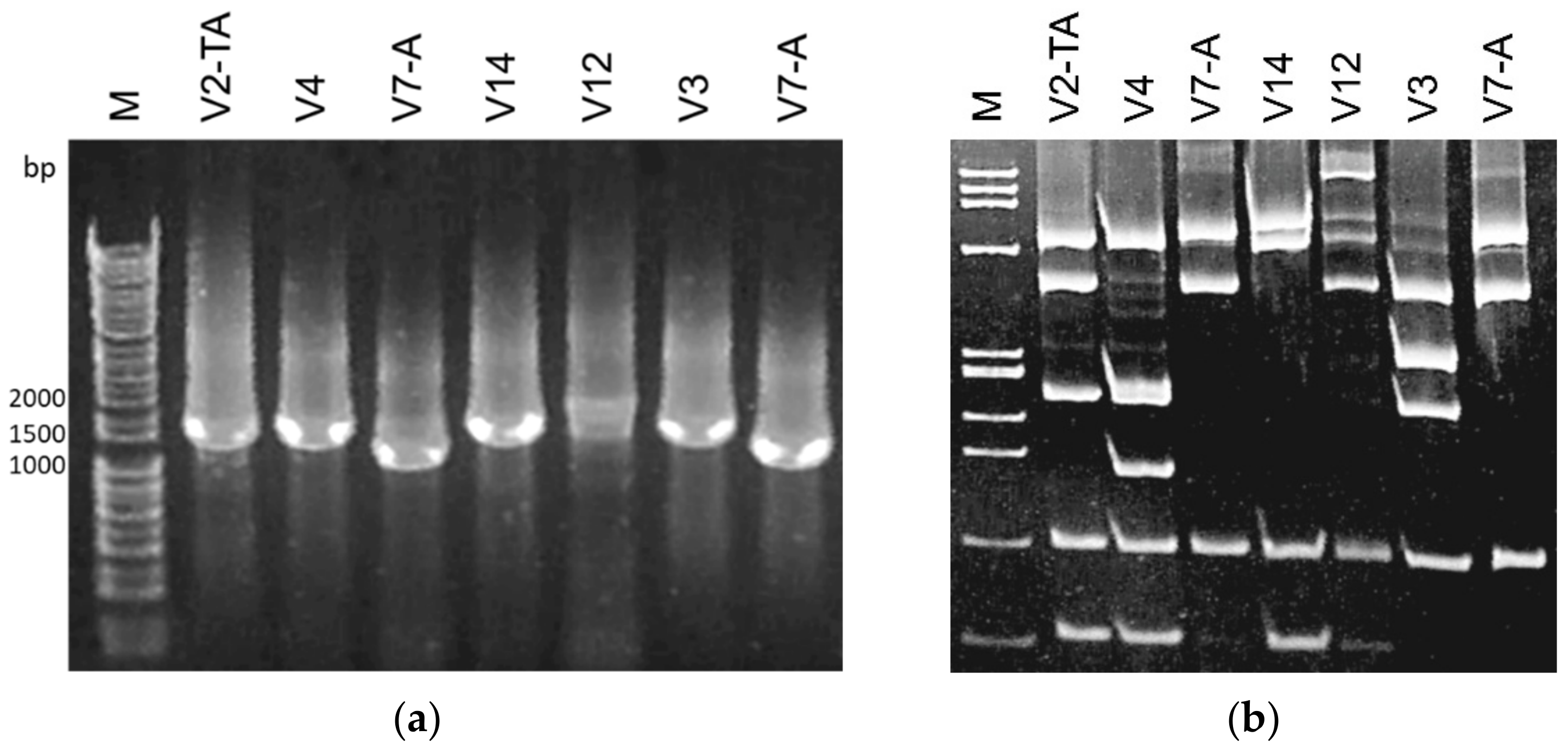
2.3. Restriction Fragment Length Polymorphism (RFLP) Analyses
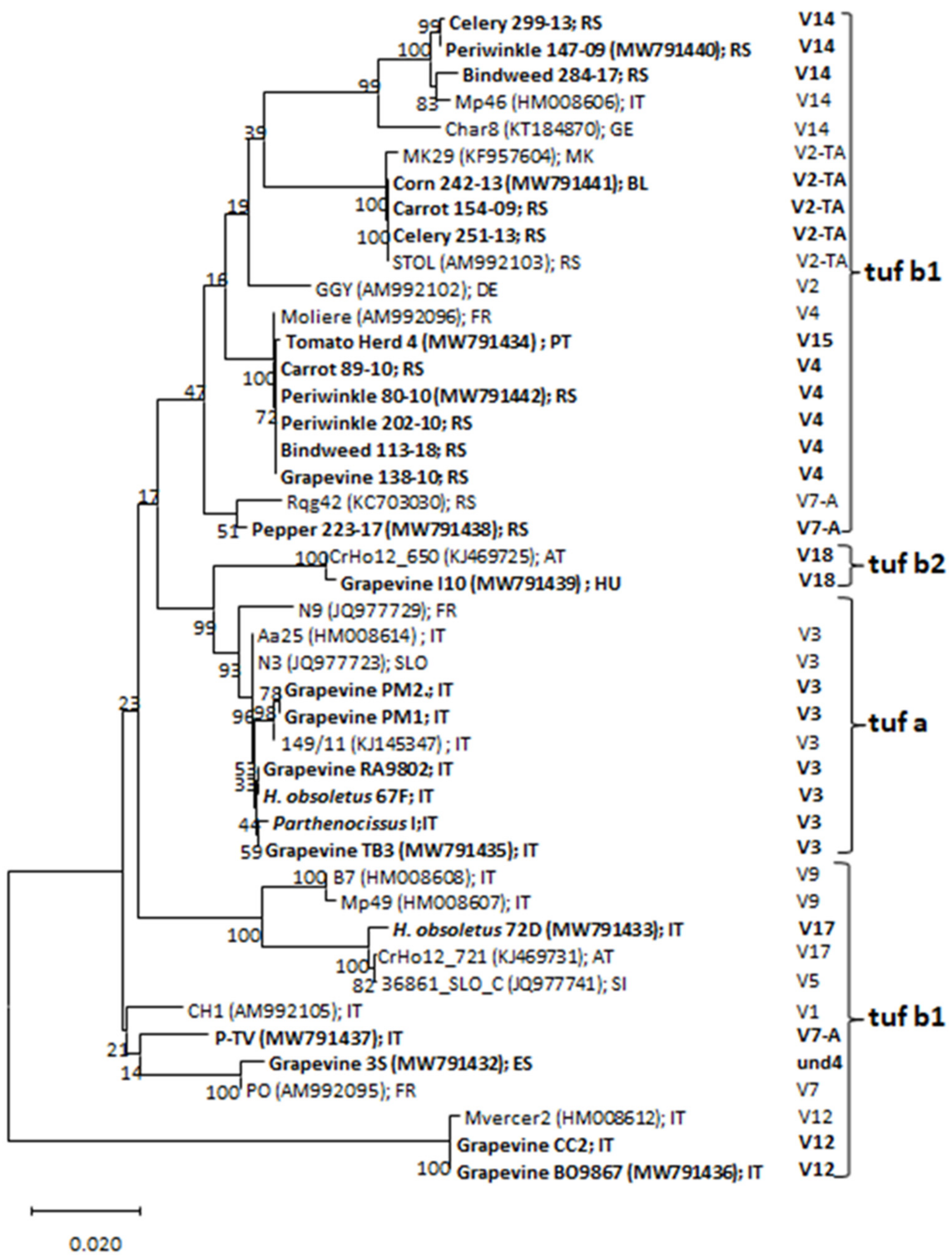
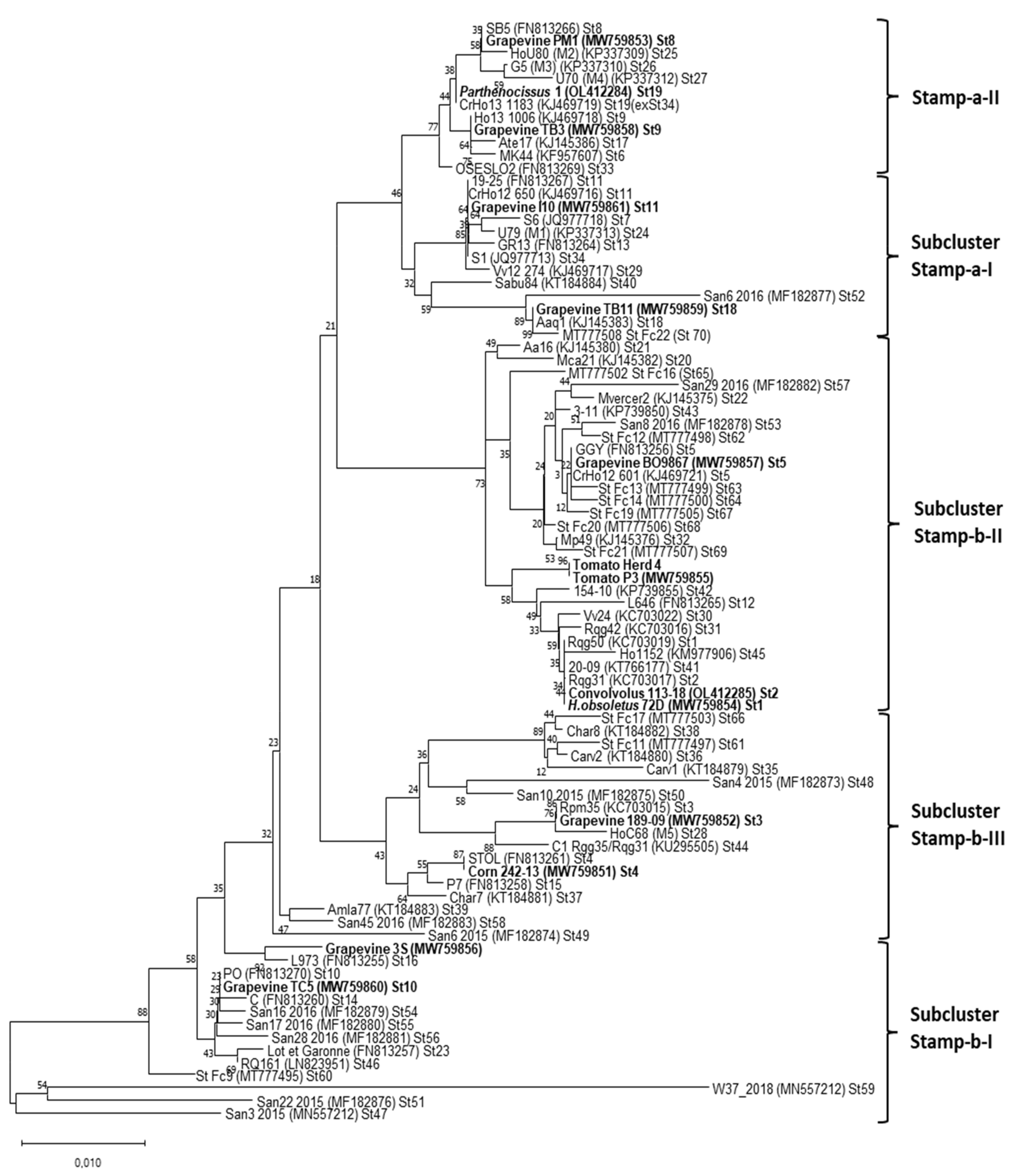
2.4. Sequencing and Phylogenetic Analyses
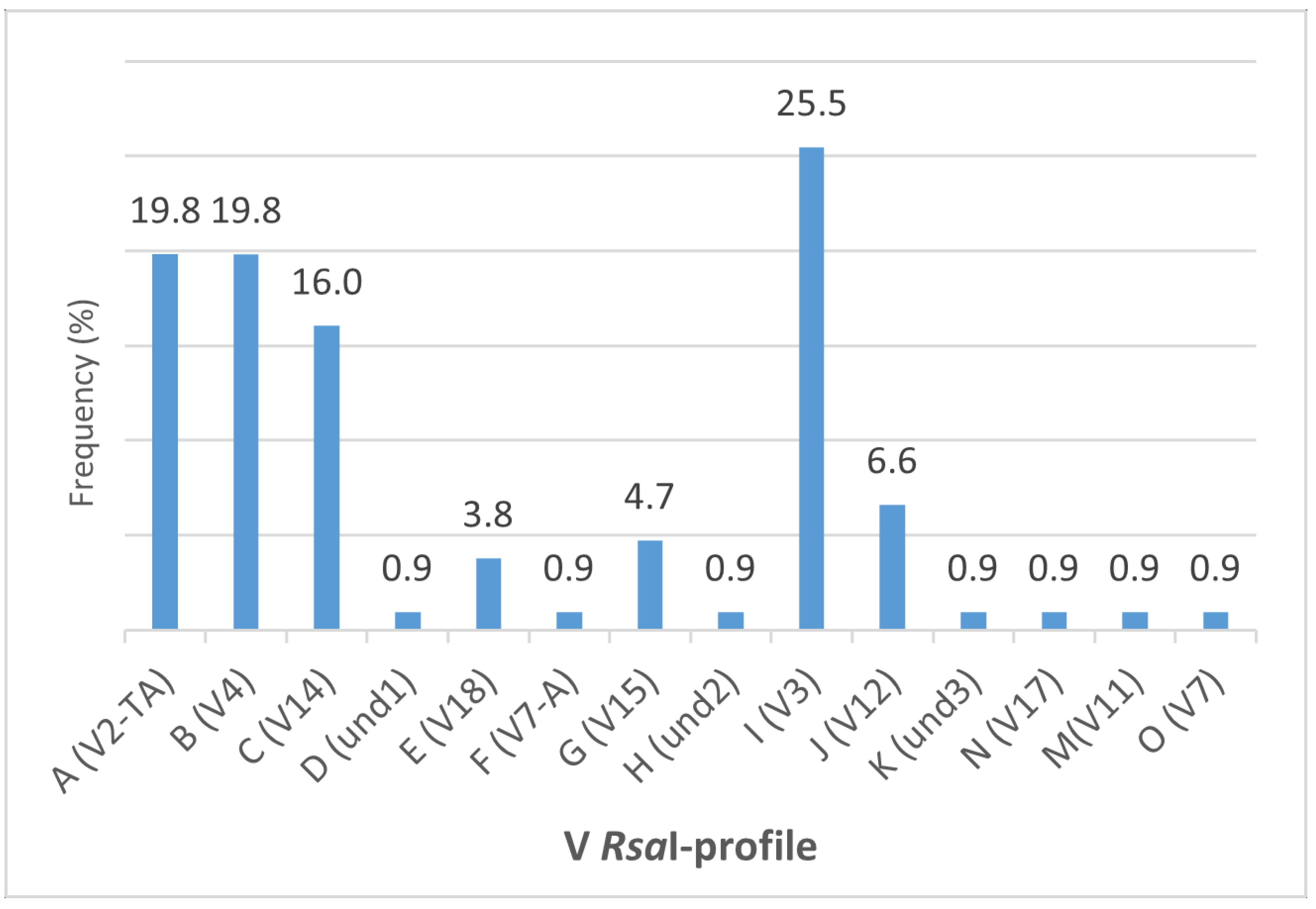
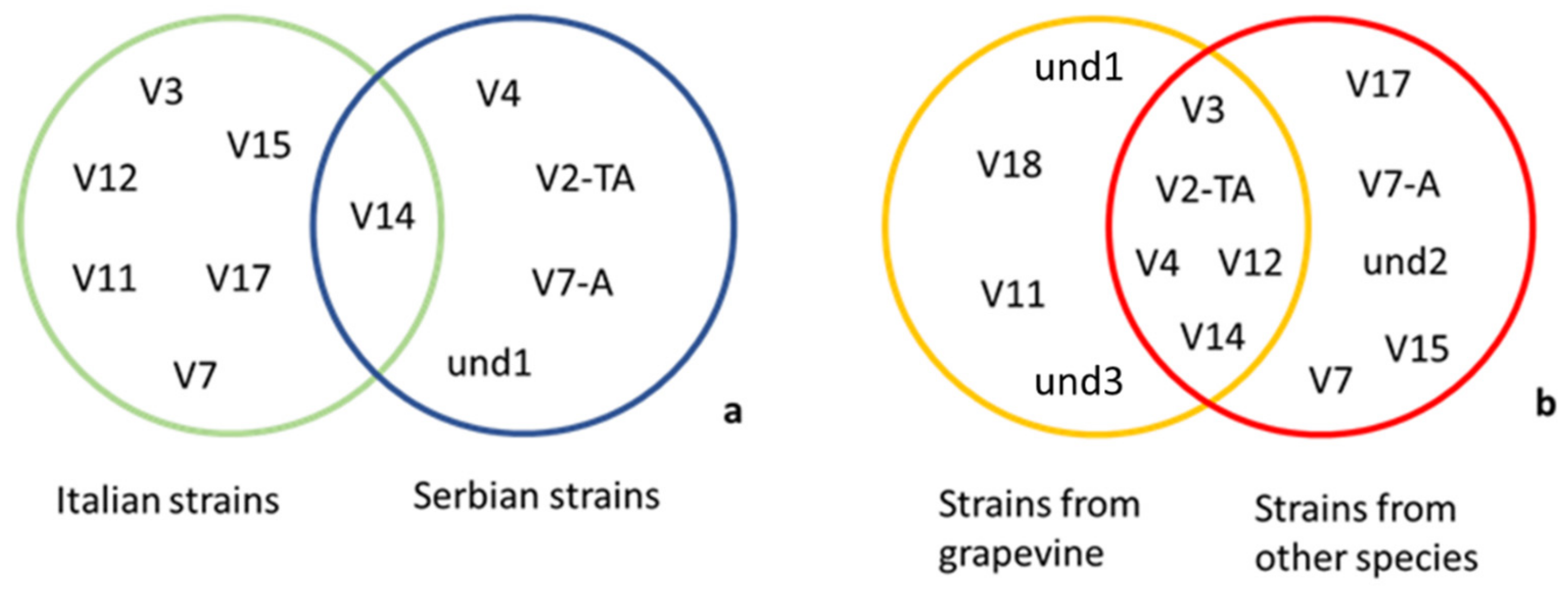
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quaglino, F.; Zhao, Y.; Casati, P.; Bulgari, D.; Bianco, P.A.; Wei, W.; Davis, R.E. ‘Candidatus Phytoplasma solani’, a novel taxon associated with “stolbur” and “bois noir”-related diseases of plants. Int. J. Syst. Evol. Microbiol. 2013, 63, 2879–2894. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.-M.; Gundersen-Rindal, D.E.; Bertaccini, A. Phytoplasma: Ecology and Genomic Diversity. Phytopathology 1998, 88, 1359–1366. [Google Scholar] [CrossRef] [Green Version]
- Weintraub, P.G.; Beanland, L. Insect vectors of phytoplasmas. Annu. Rev. Èntomol. 2006, 51, 91–111. [Google Scholar] [CrossRef]
- Carraro, L.; Ferrini, F.; Martini, M.; Ermacora, P.; Loi, N. A serious epidemic of “stolbur” on celery. J. Plant Pathol. 2008, 90, 131–135. [Google Scholar]
- Navrátil, M.; Válová, P.; Fialová, R.; Lauterer, P.; Šafářová, D.; Starý, M. The incidence of stolbur disease and associated yield losses in vegetable crops in South Moravia (Czech Republic). Crop. Sci. 2009, 28, 898–904. [Google Scholar] [CrossRef]
- Suchov, K.C.; Vovk, A.M. Biology of the leafhopper Hyalesthes obsoletus Signoret, vector of the “stolbur” virus. Trudy Inst. Gen. 1948, 15, 193–202. [Google Scholar]
- Fos, A.; Danet, J.-L.; Zreik, L.; Garnier, M.; Bové, J.-M. Use of a monoclonal antibody to detect the “stolbur” mycoplasma-like organism in plants and insects and to identify a vector in France. Plant Dis. 1992, 76, 1092–1096. [Google Scholar] [CrossRef]
- Maixner, M. Transmission of German grapevine yellows (“Vergilbungskrankheit”) by the planthopper Hyalesthes obsoletus (Auchenorrhyncha: Cixiidae). Vitis 1994, 33, 103–104. [Google Scholar]
- Sforza, R.; Clair, D.; Daire, X.; Larrue, J.; Boudon-Padieu, E. The role of Hyalesthes obsoletus (Hemiptera: Cixiidae) in the oc-currence of “bois noir” of grapevines in France. J. Phytopath. 1998, 146, 549–556. [Google Scholar] [CrossRef]
- Mori, N.; Mitrović, J.; Smiljković, M.; Duduk, N.; Paltrinieri, S.; Bertaccini, A.; Duduk, B. Hyalesthes obsoletus in Serbia and its role in the epidemiology of corn reddening. Bull. Insectol. 2013, 66, 245–250. [Google Scholar]
- Cvrković, T.; Jović, J.; Mitrović, M.; Krstić, O.; Toševski, I. Experimental and molecular evidence of Reptalus panzeri as a nat-ural vector of “bois noir”. Plant Pathol. 2014, 63, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Chuche, J.; Danet, J.-L.; Salar, P.; Thiery, D. Transmission of ‘Candidatus Phytoplasma solani’ by Reptalus quinquecostatus (Hemiptera: Cixiidae). Ann. Appl. Biol. 2016, 169, 214–223. [Google Scholar] [CrossRef]
- Riedle-Bauer, M.; Sára, A.; Regner, F. Transmission of a Stolbur Phytoplasma by the Agalliinae Leafhopper Anaceratagallia ribauti(Hemiptera, Auchenorrhyncha, Cicadellidae). J. Phytopathol. 2008, 156, 687–690. [Google Scholar] [CrossRef]
- Quaglino, F.; Sanna, F.; Moussa, A.; Faccincani, M.; Passera, A.; Casati, P.; Bianco, P.A.; Mori, N. Identification and ecology of alternative insect vectors of ’Candidatus Phytoplasma solani’ to grapevine. Sci. Rep. 2019, 9, 19522. [Google Scholar] [CrossRef]
- Fabre, A.; Danet, J.-L.; Foissac, X. The “stolbur” phytoplasma antigenic membrane protein gene stamp is submitted to diversifying positive selection. Gene 2011, 472, 37–41. [Google Scholar] [CrossRef]
- Murolo, S.; Marcone, C.; Prota, V.; Garau, R.; Foissac, X.; Romanazzi, G. Genetic variability of the “stolbur” phytoplasma vmp1 gene in grapevines, bindweeds and vegetables. J. Appl. Microbiol. 2010, 109, 2049–2059. [Google Scholar] [CrossRef]
- Quaglino, F.; Maghradze, D.; Casati, P.; Chkhaidze, N.; Lobjanidze, M.; Ravasio, A.; Passera, A.; Venturini, G.; Failla, O.; Bianco, P.A. Identification and Characterization of New ‘Candidatus Phytoplasma solani’ Strains Associated with Bois Noir Disease in Vitis vinifera L. Cultivars Showing a Range of Symptom Severity in Georgia, the Caucasus Region. Plant Dis. 2016, 100, 904–915. [Google Scholar] [CrossRef] [Green Version]
- Murolo, S.; Mancini, V.; Romanazzi, G. Spatial and temporal “stolbur” population structure in a cv. Chardonnay vineyard according to vmp1 gene characterisation. Plant Pathol. 2014, 63, 700–707. [Google Scholar] [CrossRef]
- Langer, M.; Maixner, M. Molecular characterisation of grapevine yellows associated phytoplasmas of the “stolbur”-group based on RFLP-analysis of non-ribosomal DNA. Vitis 2004, 43, 191–199. [Google Scholar]
- Aryan, A.; Brader, G.; Mörtel, J.; Pastar, M.; Riedle-Bauer, M. An abundant ‘Candidatus Phytoplasma solani’ tuf b strain is associated with grapevine, stinging nettle and Hyalesthes obsoletus. Eur. J. Plant Pathol. 2014, 140, 213–227. [Google Scholar] [CrossRef] [Green Version]
- Kosovac, A.; Radonjić, S.; Hrnčić, S.; Krstić, O.; Toševski, I.; Jović, J. Molecular tracing of the transmission routes of “bois noir” in Mediterranean vineyards of Montenegro and experimental evidence for the epidemiological role of Vitex agnuscastus (Lamiaceae) and associated Hyalesthes obsoletus (Cixiidae). Plant Pathol. 2016, 65, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Kosovac, A.; Jakovljević, M.; Krstić, O.; Cvrković, T.; Mitrović, M.; Toševski, I.; Jović, J. Role of plant-specialized Hyalesthes obsoletus associated with Convolvulus arvensis and Crepis foetida in the transmission of ‘Candidatus Phytoplasma solani’-inflicted “bois noir” disease of grapevine in Serbia. Eur. J. Plant Pathol. 2019, 153, 183–195. [Google Scholar] [CrossRef]
- Ćurčić, Ž.; Stepanović, J.; Zübert, C.; Taški-Ajduković, K.; Kosovac, M.A.; Rekanović, E.; Kube, M.; Duduk, B. Rubbery Taproot Disease of Sugar Beet in Serbia Associated with ‘Candidatus Phytoplasma solani’. Plant Dis. 2021, 105, 255–263. [Google Scholar] [CrossRef]
- Johannesen, J.; Foissac, X.; Kehrli, P.; Maixner, M. Impact of Vector Dispersal and Host-Plant Fidelity on the Dissemination of an Emerging Plant Pathogen. PLoS ONE 2012, 7, e51809. [Google Scholar] [CrossRef] [Green Version]
- Balakishiyeva, G.; Bayramova, J.; Mammadov, A.; Salar, P.; Danet, J.-L.; Ember, I.; Verdin, E.; Foissac, X.; Huseynova, I. Im-portant genetic diversity of ‘Candidatus Phytoplasma solani’ related strains associated with “bois noir” grapevine yellows and planthoppers in Azerbaijan. Eur. J. Plant Pathol. 2018, 151, 937–946. [Google Scholar] [CrossRef]
- Pierro, R.; Panattoni, A.; Passera, A.; Materazzi, A.; Luvisi, A.; Loni, A.; Ginanni, M.; Lucchi, A.; Bianco, P.A.; Quaglino, F. Proposal of A New Bois Noir Epidemiological Pattern Related to ‘Candidatus Phytoplasma Solani’ Strains Characterized by A Possible Moderate Virulence in Tuscany. Pathogens 2020, 9, 268. [Google Scholar] [CrossRef] [Green Version]
- Kostadinovska, E.; Quaglino, F.; Mitrev, S.; Casati, P.; Bulgari, D.; Bianco, P.A. Multiple gene analyses identify distinct “bois noir” phytoplasma genotypes in the Republic of Macedonia. Phytopath. Medit 2014, 53, 300–310. [Google Scholar]
- Atanasova, B.; Jakovljevic, M.; Spasov, D.; Jovic, J.; Mitrović, M.; Tosevski, I.; Cvrkovic, T. The molecular epidemiology of bois noir grapevine yellows caused by ‘Candidatus Phytoplasma solani’ in the Republic of Macedonia. Eur. J. Plant Pathol. 2015, 142, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Delić, D.; Balech, B.; Radulović, M.; Lolić, B.; Karačić, A.; Vukosavljević, V.; Cvetković, T.J. Vmp1 and stamp genes variability of ‘Candidatus Phytoplasma solani’ in Bosnian and Herzegovinian grapevine. Eur. J. Plant Pathol. 2016, 145, 221–225. [Google Scholar] [CrossRef]
- Plavec, J.; Križanac, I.; Budinšćak, Ž.; Škorić, D.; Seruga-Musić, M. A case study of FD and BN phytoplasma variability in Croatia: Multigene sequence analysis approach. Eur. J. Plant Pathol. 2015, 142, 591–601. [Google Scholar] [CrossRef]
- Foissac, X.; Carle, P.; Fabre, A.; Salar, P.; Danet, J.-L. ‘Candidatus Phytoplasma solani’ genome project and genetic diversity in the Euro-Mediterranean basin Invited conference. In Proceedings of the Third European Bois Noir Workshop, Barcelona, Spain, 20–21 March 2013. [Google Scholar]
- Murolo, S.; Romanazzi, G. In-vineyard population structure of ‘Candidatus Phytoplasma solani’ using multilocus sequence typing analysis. Infect. Genet. Evol. 2015, 31, 221–230. [Google Scholar] [CrossRef]
- Mori, N.; Quaglino, F.; Tessari, F.; Pozzebon, A.; Bulgari, D.; Casati, P.; Bianco, P.A. Investigation on ‘Bois Noir’ epidemiology in north-eastern Italian vineyards through a multidisciplinary approach. Ann. Appl. Biol. 2015, 166, 75–89. [Google Scholar] [CrossRef]
- Prince, J.P.; Davis, R.E.; Wolf, T.K.; Lee, I.-M.; Mogen, B.D.; Dally, E.L.; Bertaccini, A.; Credi, R.; Barba, M. Molecular detection of diverse mycoplasma-like organisms (MLOs) associated with grapevine yellows and their classification with aster yellows, X- disease and elm yellows MLOs. Phytopathology 1993, 83, 1130–1137. [Google Scholar] [CrossRef]
- Angelini, E.; Clair, D.; Borgo, M.; Bertaccini, A.; Boudon-Padieu, E. “Flavescence dorée” in France and Italy—occurrence of closely related phytoplasma isolates and their near relationships to Palatinate grapevine yellows and an alder yellows phyto-plasma. Vitis 2001, 40, 79–86. [Google Scholar]
- Bertaccini, A. International Phytoplasmologist Working Group Web. 2014. Available online: http://www.ipwgnet.org/collection (accessed on 15 September 2021).
- Schneider, B.; Gibbs, K.S.; Seemüller, E. Sequence and RFLP analysis of the elongation factor Tu gene used in differentiation and classification of phytoplasmas. Microbiology 1997, 143, 3381–3389. [Google Scholar] [CrossRef] [Green Version]
- Cimerman, A.; Pacifico, D.; Salar, P.; Marzachì, C.; Foissac, X. Striking diversity of vmp1, a variable gene encoding a putative membrane protein of the “stolbur” phytoplasma. Appl. Environ. Microbiol. 2009, 75, 2951–2957. [Google Scholar] [CrossRef] [Green Version]
- Fialová, R.; Válová, P.; Balakishiyeva, G.; Danet, J.-L.; Šafárová, D.; Foissac, X.; Navratil, M. Genetic variability of “stolbur” phytoplasma in annual crop and wild plant species in South Moravia (Czech Republic). J. Plant Pathol. 2009, 91, 411–416. [Google Scholar]
- Staden, R.; Beal, K.F.; Bonfield, J.K. The Staden Package, 1998. In Bioinformatics Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2000; Volume 132, pp. 115–130. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence align-ment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Quaglino, F.; Passera, A.; Faccincani, M.; Moussa, A.; Pozzebon, A.; Sanna, F.; Casati, P.; Bianco, P.A.; Mori, N. Molecular and spatial analyses reveal new insights on Bois noir epidemiology in Franciacorta vineyards. Ann. Appl. Biol. 2021, 179, 151–168. [Google Scholar] [CrossRef]
- Pierro, R.; Passera, A.; Panattoni, A.; Casati, P.; Luvisi, A.; Rizzo, D.; Bianco, P.A.; Quaglino, F.; Materazzi, A. Molecular Typing of Bois Noir Phytoplasma Strains in the Chianti Classico Area (Tuscany, Central Italy) and Their Association with Symptom Severity in Vitis vinifera ‘Sangiovese’. Phytopathology 2018, 108, 362–373. [Google Scholar] [CrossRef] [Green Version]
- Jamshidi, E.; Murolo, S.; Salehi, M.; Romanazzi, G. Sequence analysis of new tuf molecular types of ’Candidatus Phytoplasma solani’ in Iranian vineyards. Pathogens 2020, 9, 508. [Google Scholar] [CrossRef]
- Oliveri, C.; Pacifico, D.; D’Urso, V.; La Rosa, R.; Marzachì, C.; Tessitori, M. “Bois noir” phytoplasma variability in a Mediter-ranean vineyard system: New plant host and putative vectors. Austral. Plant Pathol. 2015, 44, 235–244. [Google Scholar] [CrossRef]
- Pacifico, D.; Alma, A.; Bagnoli, B.; Foissac, X.; Pasquini, G.; Tessitori, M.; Marzachì, C. Characterization of “bois noir” isolates by restriction fragment length polymorphism of a “stolbur”-specific putative membrane protein gene. Phytopathology 2009, 99, 711–715. [Google Scholar] [CrossRef] [Green Version]
- Seruga-Music, M.; Pusic, P.; Fabre, A.; Skoric, D.; Foissac, X. Variability of “stolbur” phytoplasma strains infecting Croatian grapevine by multilocus sequence typing. Bull. Insectol. 2011, 64, S39–S40. [Google Scholar]
| Strain | Host | Country | GenBank Acc. No. |
|---|---|---|---|
| Mp46 | Grapevine | Italy | HM008606 |
| Char8 | Grapevine | Georgia | KT184870 |
| MK29 | Grapevine | Macedonia | KF957604 |
| STOL | Pepper | Serbia | AM992103 |
| GGY | Grapevine | Germany | AM992102 |
| Moliere | Prunus avium | France | AM992096 |
| Rqg42 | R. quinquecostatus | Serbia | KC703030 |
| CrHo12_650 | H. obsoletus | Austria | KJ469725 |
| N9 | Nettle | France | JQ977729 |
| Aa25 | Grapevine | Italy | HM008614 |
| N3 | Nettle | Slovenia | JQ977723 |
| 149/11 | Grapevine | Italy | KJ145347 |
| B7 | Grapevine | Italy | HM008608 |
| Mp49 | Grapevine | Italy | HM008607 |
| CrH12_721 | H. obsoletus | Austria | KJ469731 |
| 36861_SLO_C | Bindweed | Slovenia | JQ977741 |
| CH1 | Grapevine | Italy | AM992105 |
| PO | H. obsoletus | France | AM992095 |
| Mvercer2 | Grapevine | Italy | HM008612 |
| Samples | Host | Location | Year | Tuf | Stamp | Vmp1 | Lineage |
|---|---|---|---|---|---|---|---|
| Parthenocissus C | Virginia creeper | Italy | 2005 | A | E | I(V3) | I |
| Grapevine FC 10044 | Grapevine | Italy | 2010 | A | E(St19) | I(V3) | I |
| Grapevine BO 9866 | Grapevine | Italy | 2010 | A | E(St19) | I(V3) | I |
| Grapevine FE 9805 | Grapevine | Italy | 2010 | A | E(St8) | I(V3) | I |
| Grapevine RA 9802 | Grapevine | Italy | 2010 | A | E(St19) | I(V3) | I |
| Parthenocissus 1 | Virginia creeper | Italy | 2018 | A | E(st19) | I(V3) | I |
| Grapevine PM1 | Grapevine | Italy | 2019 | A | E(st8) | I(V3) | I |
| Grapevine TB1 | Grapevine | Italy | 2020 | A | E | I(V3) | I |
| Grapevine TB10 | Grapevine | Italy | 2020 | A | E | I(V3) | I |
| Grapevine TB2 | Grapevine | Italy | 2020 | A | E | I(V3) | I |
| Grapevine TB4 | Grapevine | Italy | 2020 | A | E | I(V3) | I |
| Grapevine TB7 | Grapevine | Italy | 2020 | A | E | I(V3) | I |
| Grapevine TC4 | Grapevine | Italy | 2020 | A | E | I(V3) | I |
| Grapevine TC6 | Grapevine | Italy | 2020 | A | E | I(V3) | I |
| Grapevine TB3 | Grapevine | Italy | 2020 | A | E(St9) | I(V3) | I |
| P. tricuspidata S | Boston Ivy | Italy | 2020 | A(mix) | E | I(V3) | I |
| Grapevine I6 | Grapevine | Hungary | 2008 | B | A(St5) | A(V2-TA) | II |
| Tomato 127 | Tomato | Hungary | 2008 | B | A | A(V2-TA) | II |
| Parsley 228/09 | Parsley | Serbia | 2009 | B | A | A(V2-TA) | II |
| Parsley 231/09 | Parsley | Serbia | 2009 | B | A | A(V2-TA) | II |
| Pepper 101/10 | Pepper | Serbia | 2010 | B | A | A(V2-TA) | II |
| Parsnip 153/16 | Parsnip | Serbia | 2016 | B | A | A(V2-TA) | II |
| MOL | Periwinkle | France | * | B | A | B(V4) | III |
| Potato N126a | Potato | Hungary | 2008 | B | A(St5) | B(V4) | III |
| Grapevine 190/09 | Grapevine | Serbia | 2009 | B | A | B(V4) | III |
| Parsley 226/09 | Parsley | Serbia | 2009 | B | A | B(V4) | III |
| Valeriana 262/09 | Valerian | Serbia | 2009 | B | A | B(V4) | III |
| Carrot 89/10 | Carrot | Serbia | 2010 | B | A(St1) | B(V4) | III |
| Grapevine 138/10 | Grapevine | Serbia | 2010 | B | A | B(V4) | III |
| Periwinkle 202/10 | Periwinkle | Serbia | 2010 | B | A(St1) | B(V4) | III |
| Periwinkle 80/10 | Periwinkle | Serbia | 2010 | B | A(St1) | B(V4) | III |
| Tobacco 111/10 | Tobacco | Serbia | 2010 | B | A | B(V4) | III |
| Tobacco 159/10 | Tobacco | Serbia | 2010 | B | A | B(V4) | III |
| Bindweed 79/11 | Bindweed | Serbia | 2011 | B | A | B(V4) | III |
| Grapevine 69/11 | Grapevine | Serbia | 2011 | B | A(St1) | B(V4) | III |
| Periwinkle 97/11 | Periwinkle | Serbia | 2011 | B | A(St1) | B(V4) | III |
| Parsnip 161/16 | Parsnip | Serbia | 2016 | B | A | B(V4) | III |
| Bindweed 113/18 | Bindweed | Serbia | 2018 | B | A(St2) | B(V4) | III |
| Potato N126b | Potato | Hungary | 2008 | B | A(St5) | C(V14) | IV |
| Periwinkle 147/09 | Periwinkle | Serbia | 2009 | B | A(St2) | C(V14) | IV |
| Valeriana 224/09 | Valerian | Serbia | 2009 | B | A | C(V14) | IV |
| Grapevine 122/10 | Grapevine | Serbia | 2010 | B | A | C(V14) | IV |
| Grapevine 124/10 | Grapevine | Serbia | 2010 | B | A | C(V14) | IV |
| Grapevine 134/10 | Grapevine | Serbia | 2010 | B | A | C(V14) | IV |
| Parsnip 162/16 | Parsnip | Serbia | 2010 | B | A | C(V14) | IV |
| Pepper 100/10 | Pepper | Serbia | 2010 | B | A | C(V14) | IV |
| Bindweed 81/11 | Bindweed | Serbia | 2011 | B | A | C(V14) | IV |
| Grapevine 66/11 | Grapevine | Serbia | 2011 | B | A(St1) | C(V14) | IV |
| Grapevine 113/12 | Grapevine | Serbia | 2012 | B | A | C(V14) | IV |
| Celery 252/13 | Celery | Serbia | 2013 | B | A | C(V14) | IV |
| Celery 299/13 | Celery | Serbia | 2013 | B | A(St2) | C(V14) | IV |
| Grapevine J1 | Grapevine | Italy | 2016 | B | A(St1) | C(V14) | IV |
| Bindweed 284/17 | Bindweed | Montenegro | 2017 | B | A(St1) | C(V14) | IV |
| Pepper 223/17 | Pepper | Serbia | 2017 | B | A | F(V7-A) | V |
| Tomato Ca a | Tomato | Italy | 2017 | B | A(St5) | G(V15) | VI |
| Tomato Ca 1a | Tomato | Italy | 2017 | B | A | G(V15) | VI |
| Tomato Ca b | Tomato | Italy | 2017 | B | A(St5) | G(V15) | VI |
| Grapevine BO 9867 | Grapevine | Italy | 2010 | B | A(St5) | J(V12) | VII |
| Grapevine TC7 | Grapevine | Italy | 2020 | B | A | J(V12) | VII |
| Grapevine TC1 | Grapevine | Italy | 2020 | B | A(St5) | J(V12) | VII |
| Tomato ORII | Tomato | Italy | 2021 | B | A(St5) | J(V12) | VII |
| H. obsoletus 72D | H. obsoletus | Italy | 2019 | B | A(St1) | N(V17) | VIII |
| Carrot 154/09 | Carrot | Serbia | 2009 | B | B(St4) | A(V2-TA) | IX |
| Corn 121/09 | Corn | Serbia | 2009 | B | B | A(V2-TA) | IX |
| Corn 107/09 | Corn | Serbia | 2009 | B | B(St4) | A(V2-TA) | IX |
| Parsley 149/09 | Parsley | Serbia | 2009 | B | B | A(V2-TA) | IX |
| Grapevine 123/10 | Grapevine | Serbia | 2010 | B | B | A(V2-TA) | IX |
| Grapevine 120/12 | Grapevine | Serbia | 2012 | B | B | A(V2-TA) | IX |
| Celery 251/13 | Celery | Serbia | 2013 | B | B | A(V2-TA) | IX |
| Corn 241/13 | Corn | Bulgaria | 2013 | B | B | A(V2-TA) | IX |
| Corn 244/13 | Corn | Bulgaria | 2013 | B | B | A(V2-TA) | IX |
| Corn 263/13 | Corn | Serbia | 2013 | B | B | A(V2-TA) | IX |
| Corn 242/13 | Corn | Bulgaria | 2013 | B | B(St4) | A(V2-TA) | IX |
| Grapevine 189/09 | Grapevine | Serbia | 2009 | B | B(St3) | C(V14) | X |
| Parsley 150/09 | Parsley | Serbia | 2009 | B | B | C(V14) | X |
| Grapevine 144/10 | Grapevine | Serbia | 2010 | B | B | D(b und1) | XI |
| Grapevine 165/12 | Grapevine | Bulgaria | 2012 | B | B | E(V18) | XII |
| Tomato P2 | Tomato | Portugal | 1998 | B | B | G(V15) | XIII |
| Grapevine 3S | Grapevine | Spain | 2018 | B | B | K(b und3) | XIV |
| Parsnip 152/16 | Parsnip | Serbia | 2016 | B | D | A(V2-TA) | XIX |
| Grapevine TC5 | Grapevine | Italy | 2020 | B | B(St10) | M(V11) | XV |
| STOL | Periwinkle | Serbia | * | B | B(St4) | A(V2-TA) | XVI |
| Tomato Herd | Tomato | Portugal | 1998 | B | C | B(V4) | XVII |
| Tomato P3 | Tomato | Portugal | 1998 | B | C | B(V4) | XVII |
| Tomato Herd 4 | Tomato | Portugal | 1998 | B | C | G(V15) | XVIII |
| Grapevine TB11 | Grapevine | Italy | 2020 | B | D | J(V12) | XX |
| Grapevine CC2 | Grapevine | Italy | 2019 | B | D(St18) | J(V12) | XX |
| Tomato P | Tomato | Portugal | 1998 | B | E | B(V4) | XXI |
| Grapevine I9 | Grapevine | Hungary | 2008 | B | E | E(V18) | XXII |
| Grapevine I10 | Grapevine | Hungary | 2008 | B | E(St11) | E(V18) | XXII |
| Grapevine I8 | Grapevine | Hungary | 2008 | B | E(St11) | E(V18) | XXII |
| ASLO | Periwinkle | Slovenia | * | B | E | H(bund2) | XXIII |
| Grapevine FE 9806 | Grapevine | Italy | 2010 | B | E | I(V3) | XXIV |
| P-TV | Periwinkle | Italy | * | B | A | O(V7) | XXV |
| STOF | Periwinkle | France | * | B | A | - | n.d. |
| Tomato P3 | Tomato | Portugal | 1997 | B | B | - | n.d. |
| Tomato P4 | Tomato | Portugal | 1997 | B | B(St10) | - | n.d. |
| Grapevine CHCA1 | Grapevine | Italy | 2015 | - | E(St8) | - | n.d. |
| Grapevine J2 | Grapevine | Italy | 2016 | - | A(St1) | - | n.d. |
| Grapevine C1 | Grapevine | Italy | 2016 | B | A(St1) | - | n.d. |
| Grapevine GY5 | Grapevine | Italy | 2018 | B | A(St1) | - | n.d. |
| Grapevine TC3 | Grapevine | Italy | 2020 | B | - | - | n.d. |
| Potato N128a | Potato | Hungary | 2008 | B | - | A(V2-TA) | n.d. |
| Potato N128b | Potato | Hungary | 2008 | B | - | A(V2-TA) | n.d. |
| Grapevine RA 9827 | Grapevine | Italy | 2010 | B | A | a mix | n.d. |
| Tobacco 150/10 | Tobacco | Serbia | 2010 | B | B(St3) | a mix | n.d. |
| Tomato N130 | Tomato | Hungary | 2008 | B | - | B(V4) | n.d. |
| Valeriana 222/09 | Valerian | Serbia | 2009 | - | A | B(V4) | n.d. |
| Grapevine RA 9709 | Grapevine | Italy | 2010 | A | - | I(V3) | n.d. |
| Grapevine CHSM2 | Grapevine | Italy | 2015 | - | E(St19) | I(V3) | n.d. |
| Grapevine CS2 | Grapevine | Italy | 2019 | - | - | I(V3) | n.d. |
| Grapevine GM3 | Grapevine | Italy | 2019 | - | - | I(V3) | n.d. |
| H. obsoletus 67C | H. obsoletus | Italy | 2019 | - | E | I(V3) | n.d. |
| H. obsoletus 67F | H. obsoletus | Italy | 2019 | - | E(St19) | I(V3) | n.d. |
| Grapevine PM2 | Grapevine | Italy | 2019 | - | E(St8) | I(V3) | n.d. |
| Grapevine TB12 | Grapevine | Italy | 2020 | - | E | I(V3) | n.d. |
| Grapevine TB5 | Grapevine | Italy | 2020 | - | E | I(V3) | n.d. |
| Grapevine TC2 | Grapevine | Italy | 2020 | A | - | I(V3) | n.d. |
| Grapevine TB8 | Grapevine | Italy | 2020 | - | B | J(V12) | n.d. |
| Samples | Origin | Tuf Variant | GenBank Acc. No | Stamp Profile | Stamp Variant | GenBank Acc. No. | Vmp1 Profile | GenBank Acc. No. |
|---|---|---|---|---|---|---|---|---|
| Grapevine 138/10 | Serbia | b1 | / | A | / | / | B(V4) | + |
| Pepper 223/17 | Serbia | b1 | / | A | / | / | F(V7-A) | MW791438 |
| P-TV | Italy | b1 | / | A | / | / | O(V7) | MW791437 |
| Grapevine 3S | Spain | b1 | MZ970611 | B | / | MW759856 | K(und3) | MW791432 |
| Celery 251/13 | Serbia | b1 | / | B | / | / | A(V2-TA) | + |
| Tomato Herd 4 | Portugal | b1 | / | C | / | + | G(V15) | MW791434 |
| Tomato P3 | Portugal | b1 | / | C | / | MW759855 | B(V4) | / |
| Grapevine J2 | Italy | - | - | A | St1 | + | - | - |
| Bindweed 284/17 | Montenegro | b1 | / | A | St1 | + | C(V14) | + |
| Carrot 89/10 | Serbia | b1 | / | A | St1 | + | B(V4) | + |
| H. obsoletus 72D | Italy | b1 | / | A | St1 | MW759854 | N(V17) | MW791433 |
| Periwinkle 202/10 | Serbia | b1 | / | A | St1 | + | B(V4) | + |
| Periwinkle 80/10 | Serbia | b1 | / | A | St1 | + | B(V4) | MW791442 |
| Grapevine C1 | Italy | b1 | / | A | St1 | + | - | - |
| Grapevine GY5 | Italy | b1 | MZ970610 | A | St1 | + | - | - |
| Grapevine J1 | Italy | b1 | / | A | St1 | + | C(V14) | / |
| Grapevine 69/11 | Serbia | b1 | / | A | St1 | + | B(V4) | / |
| Grapevine 66/11 | Serbia | b1 | / | A | St1 | + | C(V14) | / |
| Grapevine TC5 | Italy | b1 | / | B | St10 | MW759860 | M(V11) | / |
| Tomato P4 | Portugal | b1 | / | B | St10 | + | - | - |
| Grapevine I10 | Hungary | b2 | MZ970609 | E | St11 | MW759861 | E(V18) | MW791439 |
| Grapevine I8 | Hungary | b2 | MZ970607 | E | St11 | + | E(V18) | + |
| Grapevine CC2 | Italy | b1 | MZ970606 | D | St18 | + | J(V12) | + |
| Grapevine TB11 | Italy | b1 | MW755980 | D | St18 | MW759859 | J(V12) | + |
| H. obsoletus 67F | Italy | - | - | E | St19 | + | I(V3) | + |
| Grapevine CHSM2 | Italy | - | - | E | St19 | + | I(V3) | + |
| Grapevine RA 9802 | Italy | a | / | E | St19 | + | I(V3) | + |
| Grapevine BO 9866 | Italy | a | / | E | St19 | + | I(V3) | + |
| Grapevine FC 10044 | Italy | a | / | E | St19 | + | I(V3) | + |
| Parthenocissus 1 | Italy | a | MZ970608 | E | St19 | OL412284 | I(V3) | + |
| Bindweed 113/18 | Serbia | b1 | / | A | St2 | OL412285 | B(V4) | + |
| Celery 299/13 | Serbia | b1 | / | A | St2 | + | C(V14) | + |
| Periwinkle 147/09 | Serbia | b1 | / | A | St2 | + | C(V14) | MW791440 |
| Grapevine 189/09 | Serbia | b1 | / | B | St3 | MW759852 | C(V14) | / |
| Tobacco 150/10 | Serbia | b1 | / | B | St3 | + | mix | / |
| Carrot 154/09 | Serbia | b1 | / | B | St4 | + | A(V2-TA) | + |
| Corn 242/13 | Bulgaria | b1 | / | B | St4 | MW759851 | A(V2-TA) | MW791441 |
| Corn 107/09 | Serbia | b1 | / | B | St4 | + | A(V2-TA) | / |
| Grapevine BO 9867 | Italy | b1 | / | A | St5 | MW759857 | J(V12) | MW791436 |
| Tomato ORII | Italy | b1 | + | A | St5 | + | J(V12) | + |
| Grapevine I6 | Hungary | b1 | / | A | St5 | + | A(V2-TA) | / |
| Grapevine TC1 | Italy | b1 | / | A | St5 | + | J(V12) | / |
| Potato N126a | Hungary | b1 | / | A | St5 | + | B(V4) | / |
| Potato N126b | Hungary | b1 | / | A | St5 | + | C(V14) | / |
| Tomato Ca a | Italy | b1 | / | A | St5 | + | G(V15) | / |
| Tomato Ca b | Italy | b1 | / | A | St5 | + | G(V15) | / |
| Grapevine PM2 | Italy | - | - | E | St8 | + | I(V3) | + |
| Grapevine CHCA1 | Italy | - | - | E | St8 | + | - | - |
| Grapevine PM1 | Italy | a | / | E | St8 | MW759853 | I(V3) | + |
| Grapevine FE 9805 | Italy | a | / | E | St8 | + | I(V3) | + |
| Grapevine TB3 | Italy | a | MW755979 | E | St9 | MW759858 | I(V3) | MW791435 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contaldo, N.; Stepanović, J.; Pacini, F.; Bertaccini, A.; Duduk, B. Molecular Variability and Host Distribution of ‘Candidatus Phytoplasma solani’ Strains from Different Geographic Origins. Microorganisms 2021, 9, 2530. https://doi.org/10.3390/microorganisms9122530
Contaldo N, Stepanović J, Pacini F, Bertaccini A, Duduk B. Molecular Variability and Host Distribution of ‘Candidatus Phytoplasma solani’ Strains from Different Geographic Origins. Microorganisms. 2021; 9(12):2530. https://doi.org/10.3390/microorganisms9122530
Chicago/Turabian StyleContaldo, Nicoletta, Jelena Stepanović, Francesco Pacini, Assunta Bertaccini, and Bojan Duduk. 2021. "Molecular Variability and Host Distribution of ‘Candidatus Phytoplasma solani’ Strains from Different Geographic Origins" Microorganisms 9, no. 12: 2530. https://doi.org/10.3390/microorganisms9122530






