Taxonomic and Functional Diversity of Heterotrophic Protists (Cercozoa and Endomyxa) from Biological Soil Crusts
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Site Description
2.2. Sampling Design
2.3. DNA Extraction and Amplification, Illumina Sequencing
2.4. Determination of Biocrust Chemical Properties
2.5. Statistical Analyses
3. Results
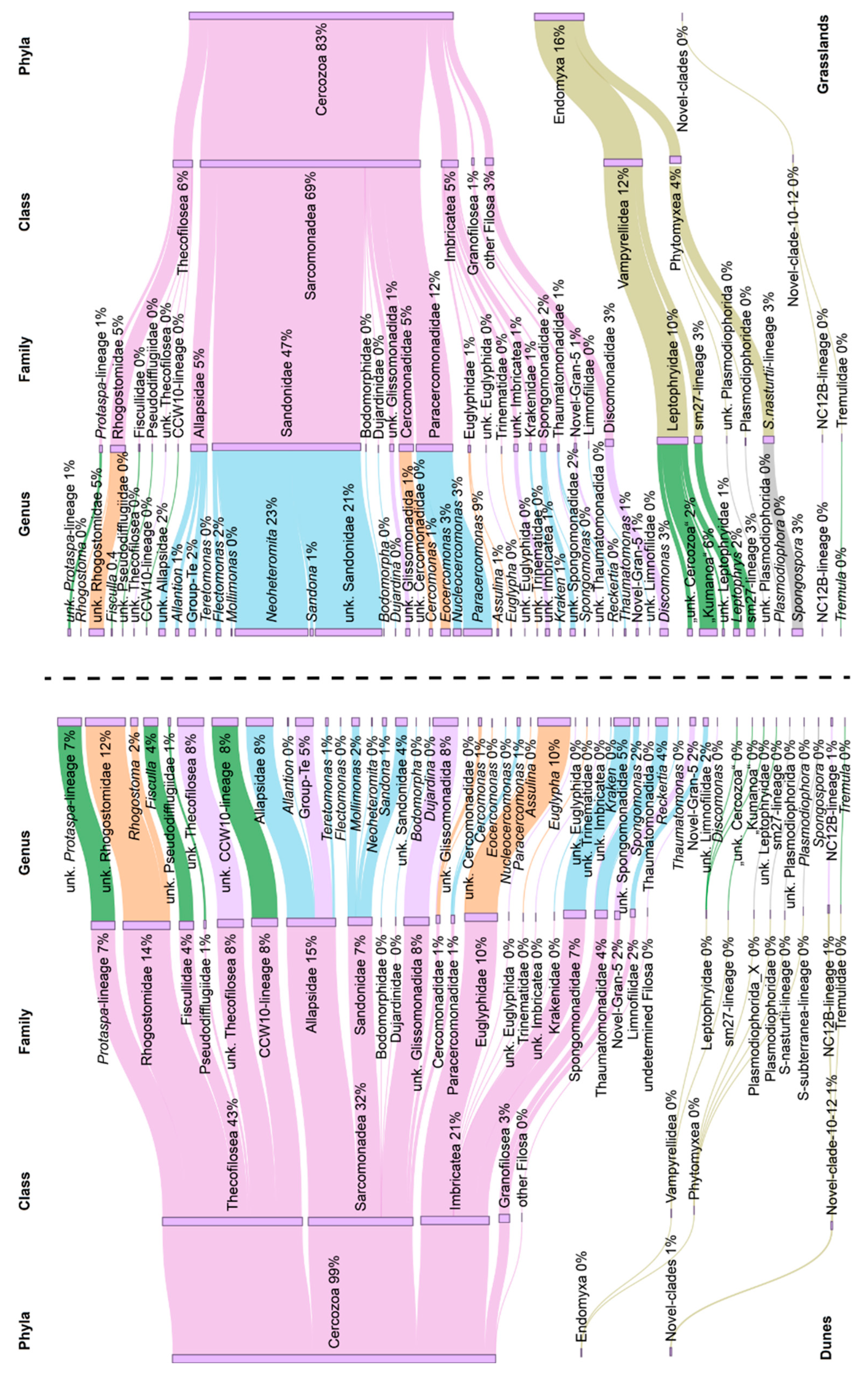
3.1. Cercozoan Community Structure, Alpha, and Beta Diversity
3.2. Feeding Behavior of Cercozoa and Endomyxa
4. Discussion
4.1. Cercozoan and Endomyxan Diversity and Community Composition
4.2. Dunes and Grasslands Accommodate Different Bacterivores and Algivorous Cercozoa and Endomyxa
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belnap, J.; Büdel, B.; Lange, O.L. Biological soil crusts: Characteristics and distribution. In Biological Soil Crusts: Structure, Function and Management, 2nd ed.; Belnap, J., Lange, O.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 3–30. [Google Scholar]
- Darby, B.J.; Neher, D.A. Microfauna within biological soil crusts. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 139–157. [Google Scholar] [CrossRef]
- Campbell, S.E. Soil stabilization by a prokaryotic desert crust: Implications for precamcrian land biota. Orig. Life 1979, 9, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.; Belnap, J.; Reheis, M.; Lamothe, P.; Luiszer, F. Aeolian dust in Colorado Plateau soils: Nutrient inputs and recent change in source. Proc. Natl. Acad. Sci. USA 2001, 98, 7123–7127. [Google Scholar] [CrossRef] [PubMed]
- Elbert, W.; Weber, B.; Burrows, S.; Steinkamp, J.; Büdel, B.; Andreae, M.O.; Pöschl, U. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 2012, 5, 459–462. [Google Scholar] [CrossRef]
- Belnap, J.; Prasse, R.; Harper, K.T. Influence of biological soil crustson soil environments and vascular plants. In Biological Soil Crusts: Structure, Function and Management, 2nd ed.; Belnap, J., Lange, O.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 281–300. [Google Scholar]
- Clarholm, M. Interactions of bacteria, protozoa and plants leading to mineralization of soil nitrogen. Soil Biol. Biochem. 1985, 17, 181–187. [Google Scholar] [CrossRef]
- Ekelund, F.; Rønn, R. Notes on protozoa in agricultural soil with emphasis on heterotrophic flagellates and naked amoebae and their ecology. FEMS Microbiol. Rev. 1994, 15, 321–353. [Google Scholar] [CrossRef]
- Bonkowski, M. Protozoa and plant growth: The microbial loop in soil revisited. New Phytol. 2004, 162, 617–631. [Google Scholar] [CrossRef]
- Bates, S.T.; Clemente, J.C.; Flores, G.E.; Walters, W.A.; Parfrey, L.W.; Knight, R.; Fierer, N. Global biogeography of highly diverse protistan communities in soil. ISME J. 2013, 7, 652–659. [Google Scholar] [CrossRef]
- Geisen, S.; Tveit, A.T.; Clark, I.M.; Richter, A.; Svenning, M.M.; Bonkowski, M.; Urich, T. Metatranscriptomic census of active protists in soils. ISME J. 2015, 9, 2178–2190. [Google Scholar] [CrossRef]
- Urich, T.; Lanzén, A.; Qi, J.; Huson, D.H.; Schleper, C.; Schuster, S.C. Simultaneous assessment of soil microbial community structure and function through analysis of the meta-transcriptome. PLoS ONE 2008, 3. [Google Scholar] [CrossRef]
- Grossmann, L.; Jensen, M.; Heider, D.; Jost, S.; Glücksman, E.; Hartikainen, H.; Mahamdallie, S.S.; Gardner, M.; Hoffmann, D.; Bass, D.; et al. Protistan community analysis: Key findings of a large-scale molecular sampling. ISME J. 2016, 10, 2269–2279. [Google Scholar] [CrossRef]
- Fiore-Donno, A.M.; Richter-Heitmann, T.; Degrune, F.; Dumack, K.; Regan, K.; Mahran, S.; Boeddinghaus, R.; Rillig, M.; Friedrich, M.W.; Kandeler, E.; et al. Environmental selection and spatiotemporal structure of a major group of soil protists (rhizaria: Cercozoa) in a temperate grassland. Front. Microbiol. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dumack, K.; Fiore-Donno, A.M.; Bass, D.; Bonkowski, M. Making sense of environmental sequencing data: Ecologically important functional traits of the protistan groups cercozoa and endomyxa (rhizaria). Mol. Ecol. Resour. 2019, 20, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Domonell, A.; Brabender, M.; Nitsche, F.; Bonkowski, M.; Arndt, H. Community structure of cultivable protists in different grassland and forest soils of Thuringia. Pedobiologia 2013, 56, 1–7. [Google Scholar] [CrossRef]
- Bugge Harder, C.; Rønn, R.; Brejnrod, A.; Bass, D.; Al-Soud, W.A.; Ekelund, F. Local diversity of heathland cercozoa explored by in-depth sequencing. ISME J. 2016, 10, 2488–2497. [Google Scholar] [CrossRef] [PubMed]
- Degrune, F.; Dumack, K.; Fiore-Donno, A.M.; Bonkowski, M.; Sosa-Hernández, M.A.; Schloter, M.; Kautz, T.; Fischer, D.; Rillig, M.C. Distinct communities of cercozoa at different soil depths in a temperate agricultural field. FEMS Microbiol. Ecol. 2019, 95, 1–7. [Google Scholar] [CrossRef]
- Fiore-Donno, A.M.; Rixen, C.; Rippin, M.; Glaser, K.; Samolov, E.; Karsten, U.; Becker, B.; Bonkowski, M. New barcoded primers for efficient retrieval of cercozoan sequences in high-throughput environmental diversity surveys, with emphasis on worldwide biological soil crusts. Mol. Ecol. Resour. 2017, 18, 229–239. [Google Scholar] [CrossRef]
- Khanipour Roshan, S.; Dumack, K.; Bonkowski, M.; Karsten, U.; Glaser, K. Stramenopiles and Cercozoa dominate the heterotrophic protist community of biological soil crusts irrespective of edaphic factors. Pedobiologia 2020, 83, 150673. [Google Scholar] [CrossRef]
- Arnalds, O. Icelandic soils. Dev. Quat. Sci. 2005, 5, 309–318. [Google Scholar] [CrossRef]
- Arnalds, O. Soils of Iceland. JÖKULL 2008, 58, 409–421. [Google Scholar]
- Miller, T.E.; Gornish, E.S.; Buckley, H.L. Climate and coastal dune vegetation: Disturbance, recovery, and succession. Plant Ecol. 2010, 206, 97–104. [Google Scholar] [CrossRef]
- Novo, F.; Díaz, M.; Zunzunegui, M.; Mora, R.; Gallego-Fernández, J. Plant functional types in coastal dune habitats. In Coastal Dunes: Ecology and Conservation; Martínez, M.L., Psuty, N.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 155–169. [Google Scholar] [CrossRef]
- McLachlan, A.; Brown, A.C. The Ecology of Sandy Shores, 2nd ed.; Elsevier: New York, NY, USA, 2006. [Google Scholar]
- Vázquez, G. The role of algal mats on community succession in dunes and dune slacks. In Coastal Dunes: Ecology and Conservation; Martínez, M.L., Psuty, N.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 189–203. [Google Scholar] [CrossRef]
- Deutsche Wetterdienst, DWD. Available online: https://www.dwd.de/ (accessed on 16 October 2020).
- Iceland Met Office. Climatological Data. Available online: https://en.vedur.is/climatology/data/ (accessed on 16 October 2020).
- Pushkareva, E.; Baumann, K.; Van, T.A.; Mikhailyuk, T.; Baum, C.; Hrynkiewicz, K.; Thiem, D.; Köpcke, T.; Karsten, U.; Leinweber, P. Phosphorus turnover and diversity of microbial phototrophs and heterotrophs in Icelandic biocrusts. Geoderma 2020, 386, 114905. [Google Scholar] [CrossRef]
- Fiore-Donno, A.M.; Richter-Heitmann, T.; Bonkowski, M. Contrasting responses of protistan plant parasites and phagotrophs to ecosystems, land management and soil properties. Front. Microbiol. 2020, 11, 1823. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guillou, L.; Bachar, D.; Audic, S.; Bass, D.; Berney, C.; Bittner, L.; Boutte, C.; Burgaud, G.; De Vargas, C.; Decelle, J.; et al. The protist ribosomal reference database (PR2): A catalog of unicellular eukaryote small sub-unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 2013, 41, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Blume, H.P.; Stahr, K.; Leinweber, P. Bodenkundliches Praktikum; Spektrum Akademischer Verlag: Heidelberg, Germany, 2010; p. 255. [Google Scholar]
- Baumann, K.; Glaser, K.; Mutz, J.E.; Karsten, U.; Maclennan, A.; Hu, Y.; Michalik, D.; Kruse, J.; Eckhardt, K.U.; Schall, P. Biological soil crusts of temperate forests: Their role in P cycling. Soil. Biol. Biochem. 2017, 109, 156–166. [Google Scholar] [CrossRef]
- Berthold, M.; Zimmer, D.; Schumann, R. A simplified method for total phosphorus digestion with potassium persulphate at sub-boiling temperatures in different environmental samples. Rostocker Meeresbiol. Beitr. 2015, 25, 7–25. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Liaw, W.H.A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; Schwartz, M. Gplots: Various R Programming Tools for Plotting Data. R Package 2009. Volume 2. Available online: https://CRAN.R-project.org/web/packages/gplots (accessed on 2 January 2020).
- Oksanen, J.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; Stevens, M.; Wagner, H. Vegan: Community Ecology. R Package 2015. Available online: http://CRAN.R-project.org/package=vegan (accessed on 2 January 2020).
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Bass, D.; Richards, T.A.; Matthai, L.; Marsh, V.; Cavalier-Smith, T. DNA evidence for global dispersal and probable endemicity of protozoa. BMC. Evol. Biol. 2007, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.; Lado, C. Ecological niche models reveal the importance of climate variability for the biogeography of protosteloid amoebae. ISME J. 2012, 6, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Howe, A.T.; Bass, D.; Vickerman, K.; Chao, E.E.; Cavalier-Smith, T. Phylogeny, taxonomy, and astounding genetic diversity of glissomonadida ord. nov., the dominant gliding zooflagellates in soil (protozoa: Cercozoa). Protist 2009, 160, 159–189. [Google Scholar] [CrossRef] [PubMed]
- Lentendu, G.; Wubet, T.; Chatzinotas, A.; Wilhelm, C.; Buscot, F.; Schlegel, M. Effects of long-term differential fertilization on eukaryotic microbial communities in an arable soil: A multiple barcoding approach. Mol. Ecol. 2014, 23, 3341–3355. [Google Scholar] [CrossRef]
- Griffiths, B.; Spilles, A.; Bonkowski, M. C:N:P stoichiometry and nutrient limitation of the soil microbial biomass in a grazed grassland site under experimental P limitation or excess. Ecol. Process. 2012, 1, 6. [Google Scholar] [CrossRef]
- Griffiths, R.I.; Thomson, B.C.; James, P.; Bell, T.; Bailey, M.; Whiteley, A.S. The bacterial biogeography of British soils. Environ. Microbiol. 2011, 13, 1642–1654. [Google Scholar] [CrossRef]
- Trap, J.; Bonkowski, M.; Plassard, C.; Villenave, C.; Blanchart, E. Ecological importance of soil bacterivores for ecosystem functions. Plant. Soil. 2016, 398, 1–24. [Google Scholar] [CrossRef]
- Fierer, N.; Strickland, M.S.; Liptzin, D.; Bradford, M.A.; Cleveland, C.C. Global patterns in belowground communities. Ecol. Lett. 2009, 12, 1–12. [Google Scholar] [CrossRef]
- Dequiedt, S.; Saby, N.P.; Lelievre, M.; Jolivet, C.; Thioulouse, J.; Toutain, B.; Arrouays, D.; Bispo, A.; Lemanceau, P.; Ranjard, L. Biogeographical patterns of soil molecular microbial biomass as influenced by soil characteristics and management. Glob. Ecol. Biogeogr. 2011, 20, 641–652. [Google Scholar] [CrossRef]
- Büdel, B.; Colesie, C.; Green, T.G.A.; Grube, M.; Lázaro Suau, R.; Loewen-Schneider, K.; Maier, S.; Peer, T.; Pintado, A.; Raggio, J.; et al. Improved appreciation of the functioning and importance of biological soil crusts in Europe: The soil crust international project (SCIN). Biodivers. Conserv. 2014, 23, 1639–1658. [Google Scholar] [CrossRef]
- Dojani, S.; Kauff, F.; Weber, B.; Büdel, B. Genotypic and phenotypic diversity of cyanobacteria in biological soil crusts of the succulent karoo and nama karoo of southern Africa. Microb. Ecol. 2014, 67, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Patzelt, D.J.; Hodač, L.; Friedl, T.; Pietrasiak, N.; Johansen, J.R. Biodiversity of soil cyanobacteria in the hyper-arid Atacama Desert, Chile. J. Phycol. 2014, 50, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.; Mikhailyuk, T.; Dreßler, M.; Leinweber, P.; Karsten, U. Biological soil crusts from coastal dunes at the Baltic Sea: Cyanobacterial and algal biodiversity and related soil properties. Microb. Ecol. 2016, 71, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Glaser, K.; Baumann, K.; Leinweber, P.; Mikhailyuk, T.; Karsten, U. Algal richness in BSCs in forests under different management intensity with some implications for P cycling. Biogeosciences 2018, 15, 4181–4192. [Google Scholar] [CrossRef]
- Howe, A.T.; Bass, D.; Scoble, J.M.; Lewis, R.; Vickerman, K.; Arndt, H.; Cavalier-Smith, T. Novel cultured protists identify deep-branching environmental DNA clades of cercozoa: New genera tremula, micrometopion, minimassisteria, nudifila, peregrinia. Protist 2011, 162, 332–372. [Google Scholar] [CrossRef]
- Bonkowski, M.; Villenave, C.; Griffiths, B. Rhizosphere fauna: The functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil. 2009, 321, 213–233. [Google Scholar] [CrossRef]
- Dumack, K.; Müller, M.E.H.; Bonkowski, M. Description of lecythium terrestris sp. nov. (chlamydophryidae, cercozoa), a soil dwelling protist feeding on fungi and algae. Protist 2016, 167, 93–105. [Google Scholar] [CrossRef]
- Dumack, K.; Flues, S.; Hermanns, K.; Bonkowski, M. Rhogostomidae (cercozoa) from soils, roots and plant leaves (arabidopsis thaliana): Description of rhogostoma epiphylla sp. nov. and R. cylindrica sp. nov. Eur. J. Protistol. 2017, 60, 76–86. [Google Scholar] [CrossRef]
- Dumack, K.; Mausbach, P.; Hegmann, M.; Bonkowski, M. Polyphyly in the thecate amoeba genus lecythium (chlamydophryidae, tectofilosida, cercozoa), redescription of its type species L. hyalinum, description of L. jennyae sp. nov. and the establishment of fisculla gen. nov. and fiscullidae fam. nov. Protist 2017, 168, 294–310. [Google Scholar] [CrossRef]
- Dumack, K.; Baumann, C.; Bonkowski, M. A bowl with marbles: Revision of the thecate amoeba genus lecythium (chlamydophryidae, tectofilosida, cercozoa, rhizaria) including a description of four new species and an identification key. Protist 2016, 167, 440–459. [Google Scholar] [CrossRef]
- Dumack, K.; Öztoprak, H.; Rüger, L.; Bonkowski, M. Shedding light on the polyphyletic thecate amoeba genus plagiophrys: Transition of some of its species to rhizaspis (tectofilosida, thecofilosea, cercozoa) and the establishment of sacciforma gen. nov. and rhogostomidae fam. nov. (cryomonadida, thecofilos. Protist 2017, 168, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Dumack, K.; Pundt, J.; Bonkowski, M. Food choice experiments indicate selective fungivorous predation in fisculla terrestris (thecofilosea, cercozoa). J. Eukaryot. Microbiol. 2018, 66, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Seppey, C.V.W.; Singer, D.; Dumack, K.; Fournier, B.; Belbahri, L.; Mitchell, E.A.D.; Lara, E. Distribution patterns of soil microbial eukaryotes suggests widespread algivory by phagotrophic protists as an alternative pathway for nutrient cycling. Soil Biol. Biochem. 2017, 112, 68–75. [Google Scholar] [CrossRef]
- Öztoprak, H.; Walden, S.; Heger, T.; Bonkowski, M.; Dumack, K. What drives the diversity of the most abundant terrestrial cercozoan family (rhogostomidae, cercozoa, rhizaria)? Microorganisms 2020, 8, 1123. [Google Scholar] [CrossRef] [PubMed]
- Jauss, R.T.; Walden, S.; Fiore-Donno, A.M.; Dumack, K.; Schaffer, S.; Wolf, R.; Schlegel, M.; Bonkowski, M. From forest soil to the canopy: Increased habitat diversity does not increase species richness of cercozoa and oomycota in tree canopies. Front. Microbiol. 2020, 11, 3364. [Google Scholar] [CrossRef] [PubMed]
- Hess, S.; Sausen, N.; Melkonian, M. Shedding light on vampires: The phylogeny of vampyrellid amoebae revisited. PLoS ONE 2012, 7, e31165. [Google Scholar] [CrossRef]
- Hess, S. Hunting for agile prey: Trophic specialisation in leptophryid amoebae (vampyrellida, rhizaria) revealed by two novel predators of planktonic algae. FEMS Microbiol. Ecol. 2017, 93, 1–12. [Google Scholar] [CrossRef][Green Version]
- Old, K.M.; Darbyshire, J.F. Soil fungi as food for giant amoebae. Soil Biol. Biochem. 1978, 10, 93–100. [Google Scholar] [CrossRef]
- Sayre, R.M.; Wergin, W.P. Morphology and fine structure of the trophozoites of theratromyxa weberi (protozoa: Vampyrellidae) predacious on soil nematodes. Can. J. Microbiol. 1989, 35, 589–602. [Google Scholar] [CrossRef]
- Mikhailyuk, T.; Glaser, K.; Tsarenko, P.; Demchenko, E.; Karsten, U. Composition of biological soil crusts from sand dunes of Baltic Sea coast in the context of an integrative approach to the taxonomy of microalgae and cyanobacteria. Eur. J. Phycol. 2019, 54, 263–290. [Google Scholar] [CrossRef]
- Arnalds, O. The Soils of Iceland; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Lara, E.; Heger, T.J.; Mitchell, E.A.D.; Meisterfeld, R.; Ekelund, F. SSU rRNA reveals a sequential increase in shell complexity among the euglyphid testate amoebae (rhizaria: Euglyphida). Protist 2007, 158, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Flues, S.; Bass, D.; Bonkowski, M. Grazing of leaf-associated cercomonads (protists: Rhizaria: Cercozoa) structures bacterial community composition and function. Environ. Microbiol. 2017, 19, 3297–3309. [Google Scholar] [CrossRef] [PubMed]


| Country | Locations | Geographic Coordinates | |
|---|---|---|---|
| Germany, Baltic Sea (coastline) | Riedensee | 54° 09.179 N | 11° 41.431 E |
| Heiligendamm | 54° 10.816 N | 11° 51.346 E | |
| Warnemünde | 54° 10.816 N | 12° 04.827 E | |
| Baabe | 54° 21.267 N | 13° 43.050 E | |
| Karlshagen | 54° 08.216 N | 13° 49.716 E | |
| Iceland | Litla Skardt | 64°43′28.884″ N | 21°36′49.392″ W |
| Fiflholt | 64°42′4.968″ N | 22°8′24.936″ W | |
| Krákunes | 64°39′21.672″ N | 22°20′50.352″ W | |
| Giljar | 64°40′0.804″ N | 21°4′8.148″ W | |
| Borgarfjarðarbrau | 64°39′4.82″ N | 21°23′40.056″ W | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanipour Roshan, S.; Dumack, K.; Bonkowski, M.; Leinweber, P.; Karsten, U.; Glaser, K. Taxonomic and Functional Diversity of Heterotrophic Protists (Cercozoa and Endomyxa) from Biological Soil Crusts. Microorganisms 2021, 9, 205. https://doi.org/10.3390/microorganisms9020205
Khanipour Roshan S, Dumack K, Bonkowski M, Leinweber P, Karsten U, Glaser K. Taxonomic and Functional Diversity of Heterotrophic Protists (Cercozoa and Endomyxa) from Biological Soil Crusts. Microorganisms. 2021; 9(2):205. https://doi.org/10.3390/microorganisms9020205
Chicago/Turabian StyleKhanipour Roshan, Samira, Kenneth Dumack, Michael Bonkowski, Peter Leinweber, Ulf Karsten, and Karin Glaser. 2021. "Taxonomic and Functional Diversity of Heterotrophic Protists (Cercozoa and Endomyxa) from Biological Soil Crusts" Microorganisms 9, no. 2: 205. https://doi.org/10.3390/microorganisms9020205
APA StyleKhanipour Roshan, S., Dumack, K., Bonkowski, M., Leinweber, P., Karsten, U., & Glaser, K. (2021). Taxonomic and Functional Diversity of Heterotrophic Protists (Cercozoa and Endomyxa) from Biological Soil Crusts. Microorganisms, 9(2), 205. https://doi.org/10.3390/microorganisms9020205







