A Murine Skin Infection Model Capable of Differentiating the Dermatopathology of Community-Associated MRSA Strain USA300 from Other MRSA Strains
Abstract
1. Introduction
2. Materials and Methods
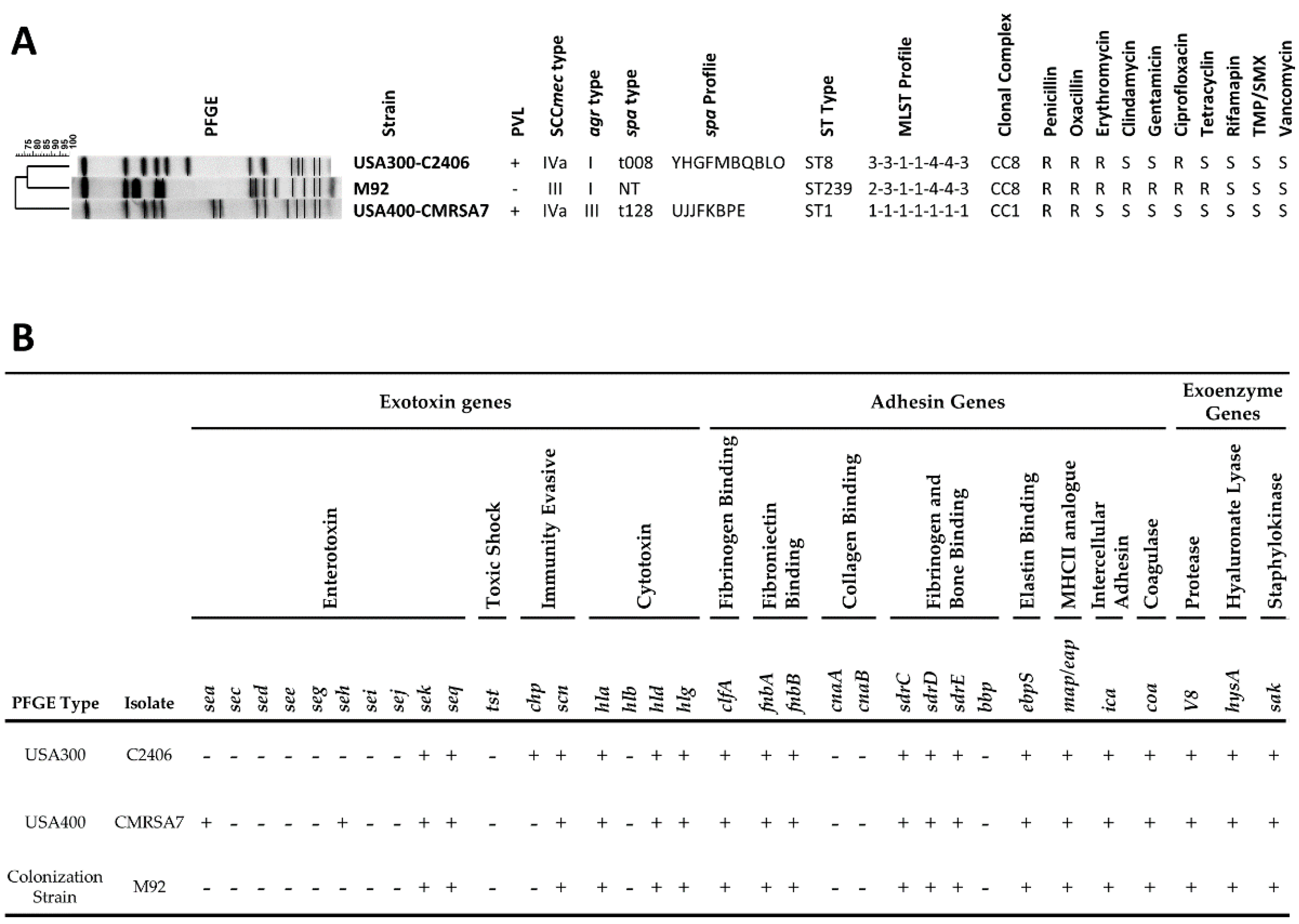
2.1. MRSA Strains and Their Phenotypic and Genotypic Characterization
2.2. Murine Skin Infection Model
2.3. Histology
2.4. Myeloperoxidase (MPO) Assay
2.5. Spinning Disk Confocal Microscopy
2.6. In Vivo Neutrophil Depletion
2.7. Mouse Antibacterial Response PCR Array
2.8. Cytokine and Chemokine Assay
2.9. Statistical Analysis
3. Results
3.1. Genotypic and Phenotypic Characterization and Virulence Gene Profiles of MRSA Strains
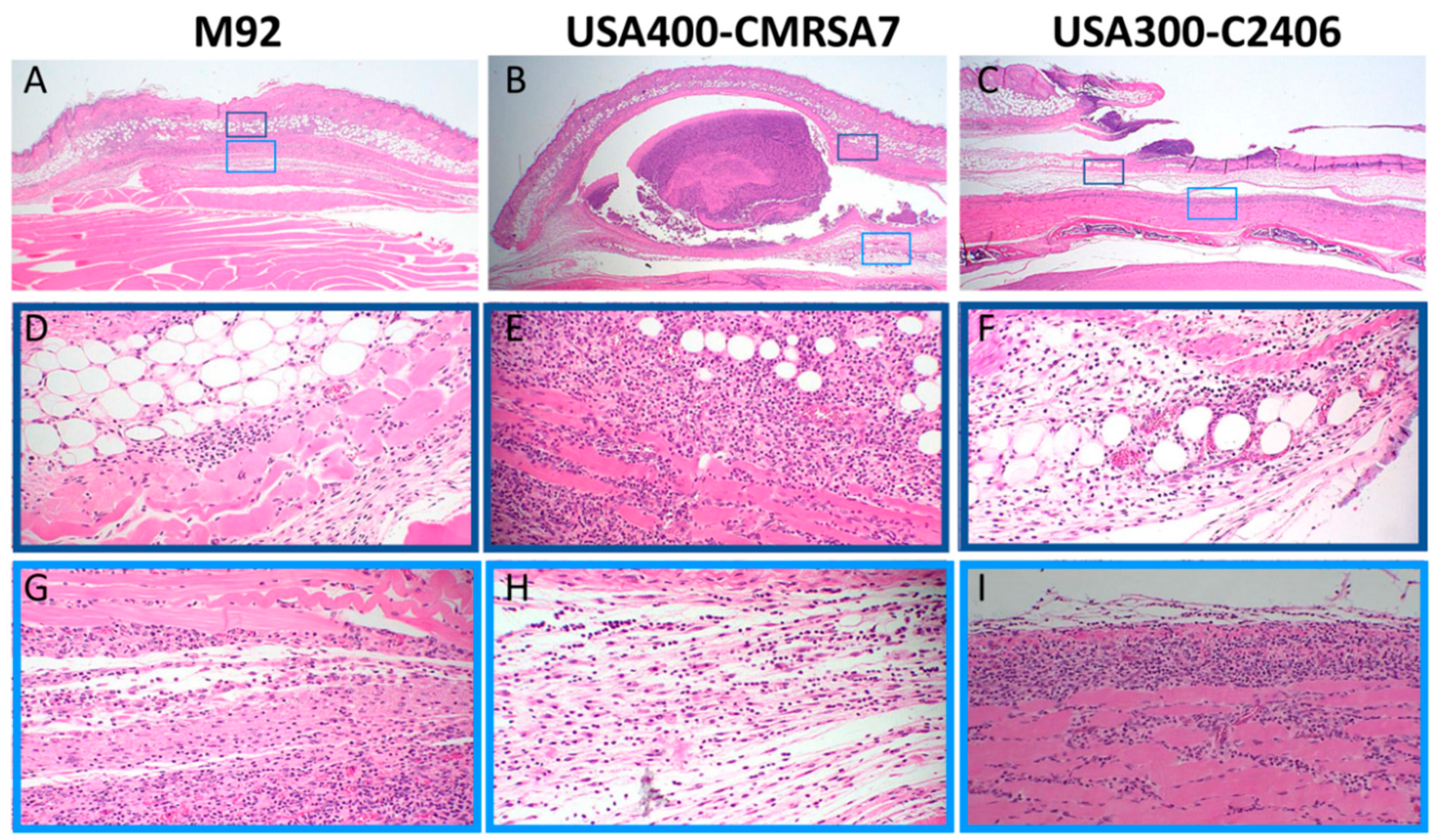
3.2. USA300 Induced Extensive Open Lesions (Dermonecrosis) while M92 and USA400 Caused Localized Infection
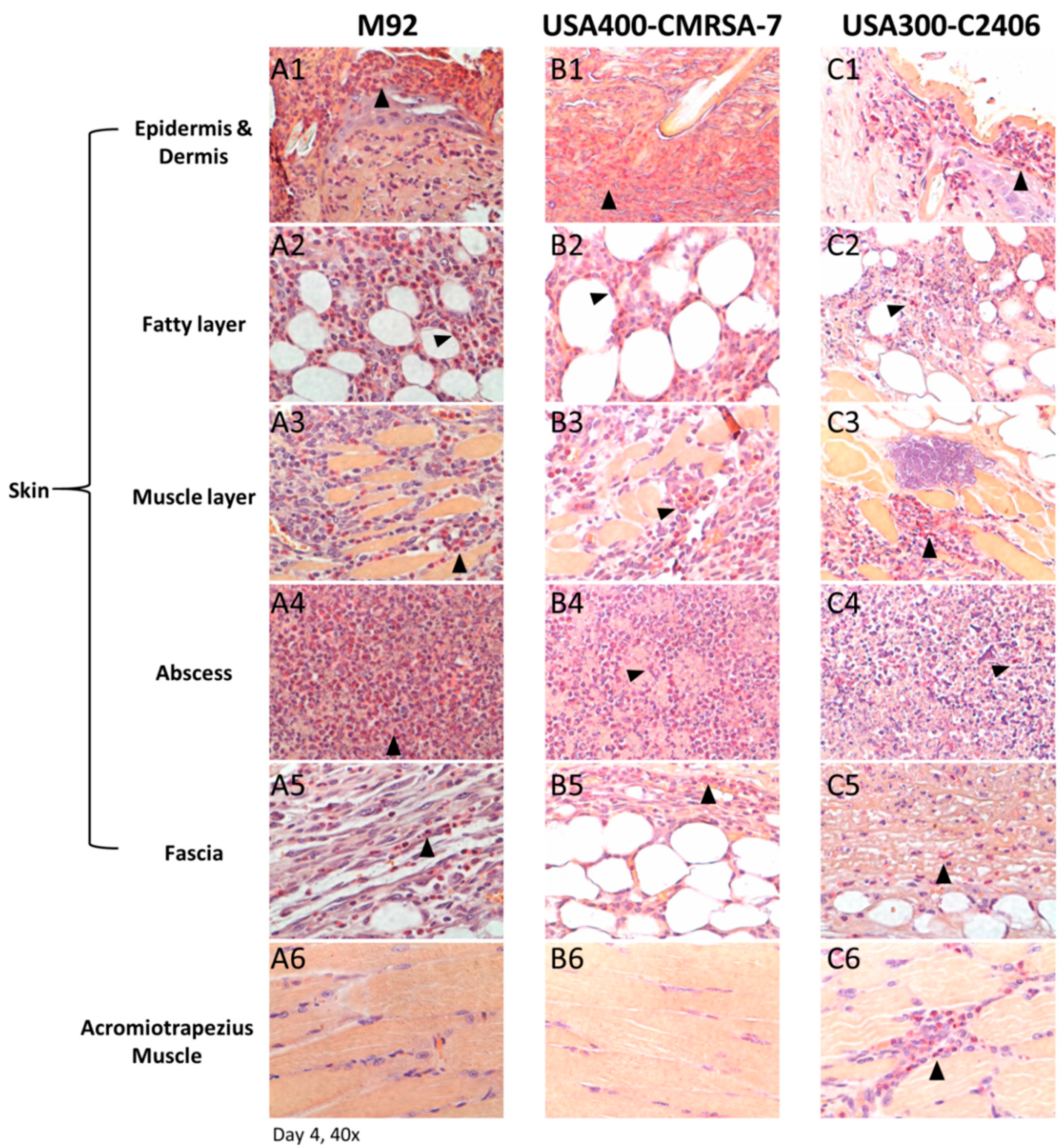
3.3. Profound Inflammatory Cell Infiltration by USA300
3.4. Primary Infiltrating Inflammatory Cell Type Was the Neutrophil
3.5. USA300 Induced Prolonged Periods of Excessive Neutrophil Infiltration
3.6. Significantly Greater Neutrophil (But Not CD4 T Cell) Adhesion and Emigration Triggered by USA300
3.7. Co-localization of Higher Bacterial Load with Neutrophil Infiltration in USA300 Infection
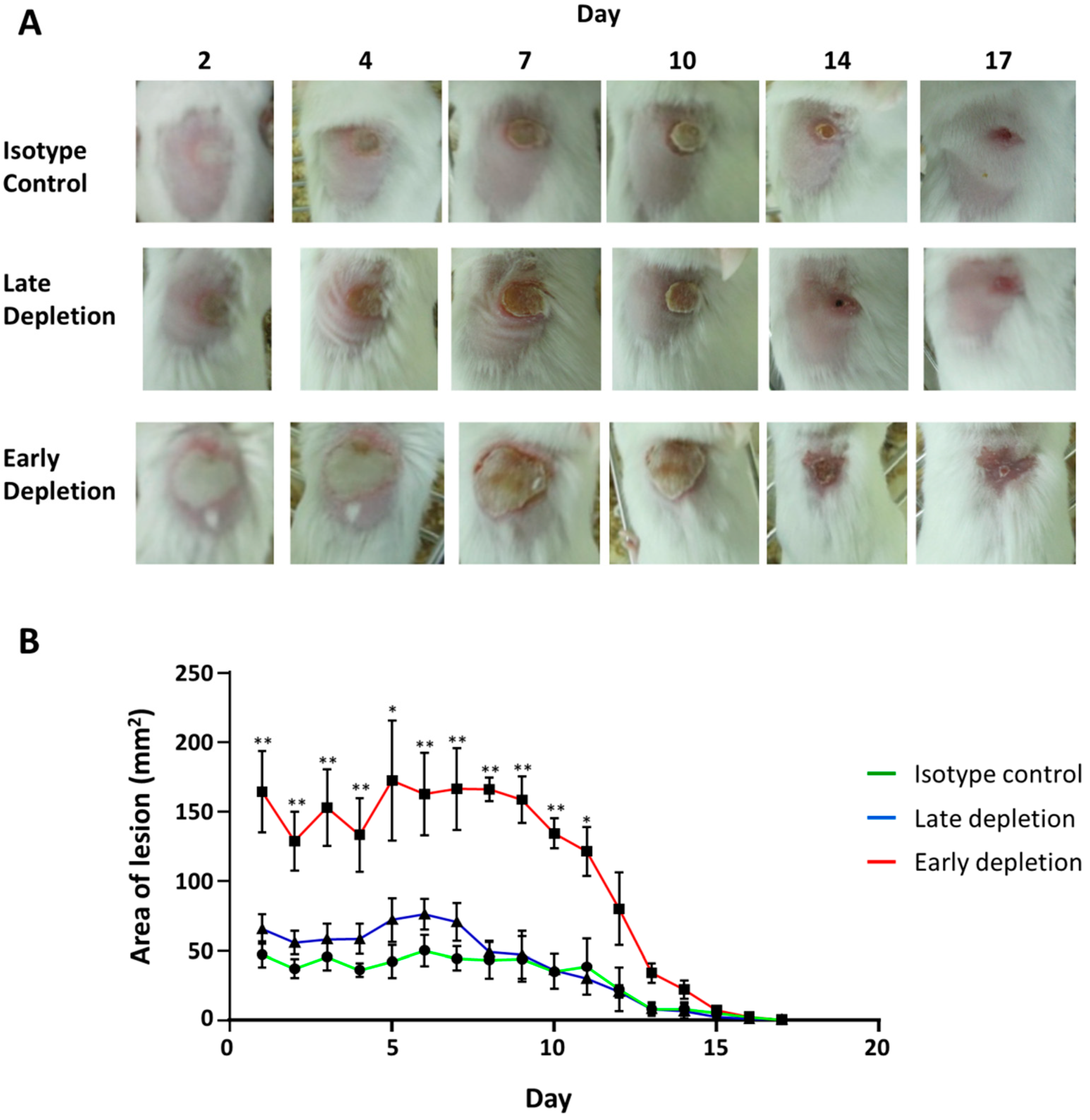
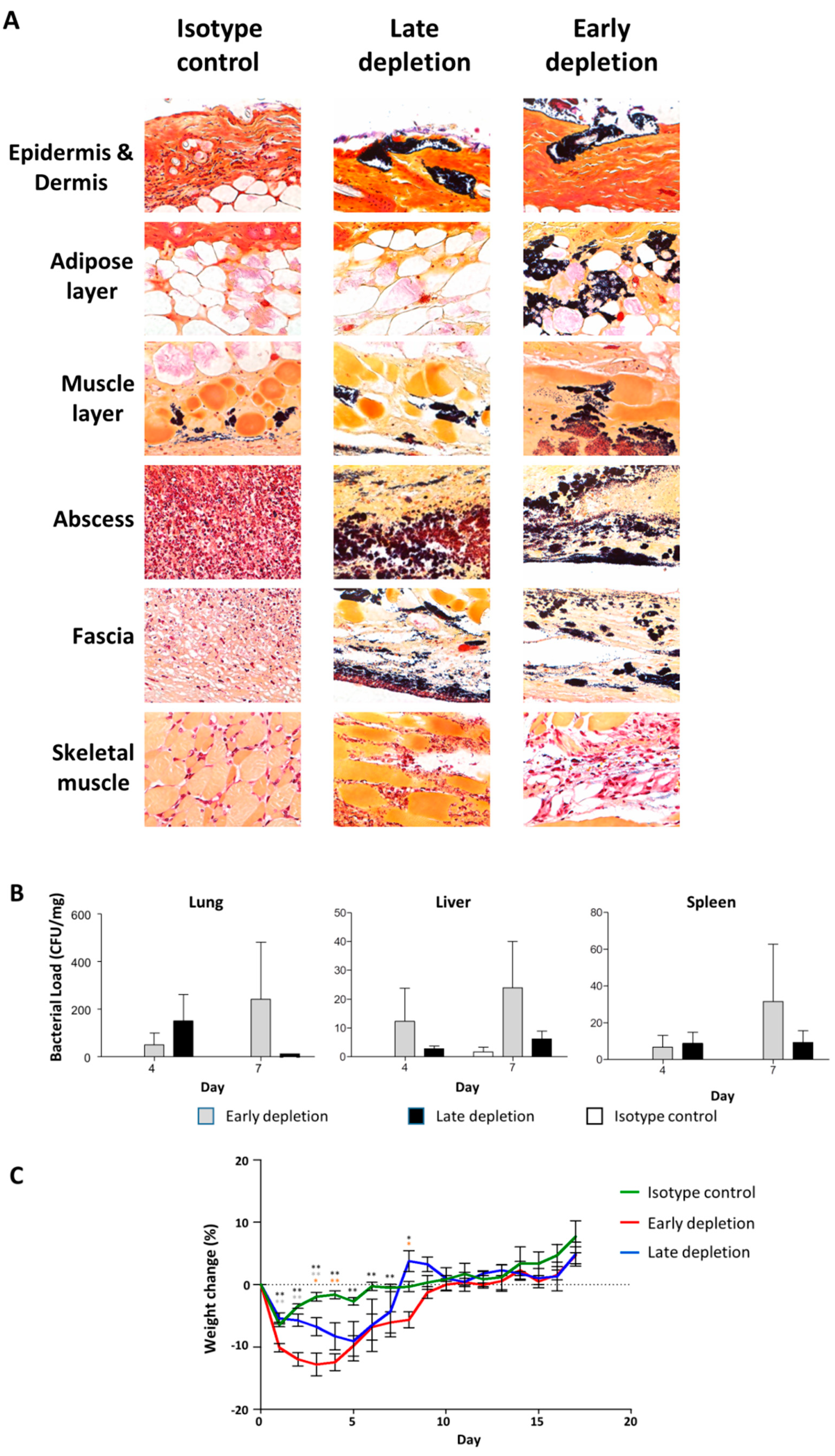
3.8. Neutrophil Depletion Resulted in a More Severe Infection
3.9. Neutrophil Depletion Resulted in Bacterial Dissemination and Invasive Infection
3.10. Unique Pattern of Molecular Transcription Results from Infection with USA300
3.11. Unique Cytokine and Chemokine Profiles in USA300-Infected Lesions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- David, M.Z.; Cadilla, A.; Boyle-Vavra, S.; Daum, R.S. Replacement of HA-MRSA by CA-MRSA infections at an academic medical center in the midwestern United States, 2004–2005 to 2008. PLoS ONE 2014, 9, e92760. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Flynn, N.M.; King, J.H.; Monchaud, C.; Morita, M.; Cohen, S.H. Comparisons of community-associated methicillin-resistant Staphylococcus aureus (MRSA) and hospital-associated MSRA infections in Sacramento, California. J. Clin. Microbiol. 2006, 44, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Baud, O.; Giron, S.; Aumeran, C.; Mouly, D.; Bardon, G.; Besson, M.; Delmas, J.; Coignard, B.; Tristan, A.; Vandenesch, F.; et al. First outbreak of community-acquired MRSA USA300 in France: Failure to suppress prolonged MRSA carriage despite decontamination procedures. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1757–1762. [Google Scholar] [CrossRef]
- Lee, H.; Kim, E.S.; Choi, C.; Seo, H.; Shin, M.; Bok, J.H.; Cho, J.E.; Kim, C.J.; Shin, J.W.; Kim, T.S.; et al. Outbreak among healthy newborns due to a new variant of USA300-related meticillin-resistant Staphylococcus aureus. J. Hosp. Infect. 2014, 87, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Vindel, A.; Trincado, P.; Cuevas, O.; Ballesteros, C.; Bouza, E.; Cercenado, E. Molecular epidemiology of community-associated methicillin-resistant Staphylococcus aureus in Spain: 2004–12. J. Antimicrob. Chemother. 2014, 69, 2913–2919. [Google Scholar] [CrossRef][Green Version]
- Suaya, J.A.; Mera, R.M.; Cassidy, A.; O’Hara, P.; Amrine-Madsen, H.; Burstin, S.; Miller, L.G. Incidence and cost of hospitalizations associated with Staphylococcus aureus skin and soft tissue infections in the United States from 2001 through 2009. BMC Infect. Dis. 2014, 14, 296. [Google Scholar] [CrossRef]
- Thom, H.; Thompson, J.C.; Scott, D.A.; Halfpenny, N.; Sulham, K.; Corey, G.R. Comparative efficacy of antibiotics for the treatment of acute bacterial skin and skin structure infections (ABSSSI): A systematic review and network meta-analysis. Curr. Med. Res. Opin. 2015, 31, 1539–1551. [Google Scholar] [CrossRef]
- Moran, G.J.; Krishnadasan, A.; Gorwitz, R.J.; Fosheim, G.E.; McDougal, L.K.; Carey, R.B.; Talan, D.A. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 2006, 355, 666–674. [Google Scholar] [CrossRef]
- Carrillo-Marquez, M.A.; Hulten, K.G.; Hammerman, W.; Mason, E.O.; Kaplan, S.L. USA300 is the predominant genotype causing Staphylococcus aureus septic arthritis in children. Pediatr. Infect. Dis. J. 2009, 28, 1076–1080. [Google Scholar] [CrossRef]
- Kempker, R.R.; Farley, M.M.; Ladson, J.L.; Satola, S.; Ray, S.M. Association of methicillin-resistant Staphylococcus aureus (MRSA) USA300 genotype with mortality in MRSA bacteremia. J. Infect. 2010, 61, 372–381. [Google Scholar] [CrossRef]
- Kreisel, K.M.; Stine, O.C.; Johnson, J.K.; Perencevich, E.N.; Shardell, M.D.; Lesse, A.J.; Gordin, F.M.; Climo, M.W.; Roghmann, M.C. USA300 methicillin-resistant Staphylococcus aureus bacteremia and the risk of severe sepsis: Is USA300 methicillin-resistant Staphylococcus aureus associated with more severe infections? Diagn. Microbiol. Infect. Dis. 2011, 70, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Seybold, U.; Kourbatova, E.V.; Johnson, J.G.; Halvosa, S.J.; Wang, Y.F.; King, M.D.; Ray, S.M.; Blumberg, H.M. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin. Infect. Dis. 2006, 42, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.; Park, M.; Robben, P.; Whitman, T.; Ellis, M.W. USA300 methicillin-resistant Staphylococcus aureus emerging as a cause of bloodstream infections at military medical centers. Infect. Control Hosp. Epidemiol. 2013, 34, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Nakaminami, H.; Ohtani, H.; Yamada, K.; Nasu, Y.; Takadama, S.; Noguchi, N.; Fujii, T.; Matsumoto, T. An outbreak of severe infectious diseases caused by methicillin-resistant Staphylococcus aureus USA300 clone among hospitalized patients and nursing staff in a tertiary care university hospital. J. Infect. Chemother. 2020, 26, 76–81. [Google Scholar] [CrossRef]
- McRipley, R.J.; Whitney, R.R. Characterization and quantitation of experimental surgical-wound infections used to evaluate topical antibacterial agents. Antimicrob. Agents Chemother. 1976, 10, 38–44. [Google Scholar] [CrossRef]
- Espersen, F.; Frimodt-Moller, N.; Corneliussen, L.; Riber, U.; Rosdahl, V.T.; Skinhoj, P. Effect of treatment with methicillin and gentamicin in a new experimental mouse model of foreign body infection. Antimicrob. Agents Chemother. 1994, 38, 2047–2053. [Google Scholar] [CrossRef]
- Fallon, M.T.; Shafer, W.; Jacob, E. Use of cefazolin microspheres to treat localized methicillin-resistant Staphylococcus aureus infections in rats. J. Surg. Res. 1999, 86, 97–102. [Google Scholar] [CrossRef]
- Abtin, A.; Jain, R.; Mitchell, A.J.; Roediger, B.; Brzoska, A.J.; Tikoo, S.; Cheng, Q.; Ng, L.G.; Cavanagh, L.L.; von Andrian, U.H.; et al. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat. Immunol. 2014, 15, 45–53. [Google Scholar] [CrossRef]
- Tseng, C.W.; Sanchez-Martinez, M.; Arruda, A.; Liu, G.Y. Subcutaneous infection of methicillin resistant Staphylococcus aureus (MRSA). J. Vis. Exp. 2011, 48, 2528. [Google Scholar] [CrossRef]
- Kinoshita, M.; Miyazaki, H.; Ono, S.; Inatsu, A.; Nakashima, H.; Tsujimoto, H.; Shinomiya, N.; Saitoh, D.; Seki, S. Enhancement of neutrophil function by interleukin-18 therapy protects burn-injured mice from methicillin-resistant Staphylococcus aureus. Infect. Immun. 2011, 79, 2670–2680. [Google Scholar] [CrossRef]
- Gilbert, M.; MacDonald, J.; Gregson, D.; Siushansian, J.; Zhang, K.; Elsayed, S.; Laupland, K.; Louie, T.; Hope, K.; Mulvey, M.; et al. Outbreak in Alberta of community-acquired (USA300) methicillin-resistant Staphylococcus aureus in people with a history of drug use, homelessness or incarceration. CMAJ 2006, 175, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Christianson, S.; Golding, G.R.; Campbell, J.; Mulvey, M.R. Comparative genomics of Canadian epidemic lineages of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 2007, 45, 1904–1911. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, K.; Conly, J.; McClure, J.A.; Elsayed, S.; Louie, T.; Zhang, K. Caenorhabditis elegans as a host model for community-associated methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2010, 16, 245–254. [Google Scholar] [CrossRef] [PubMed]
- McClure, J.A.; Zhang, K. Complete Genome Sequence of a Community-Associated Methicillin-Resistant Staphylococcus aureus Hypervirulent Strain, USA300-C2406, Isolated from a Patient with a Lethal Case of Necrotizing Pneumonia. Genome Announc. 2017, 5, e00461-17. [Google Scholar] [CrossRef]
- McClure, J.A.; Zhang, K. Complete Genome Sequence of the Methicillin-Resistant Staphylococcus aureus Colonizing Strain M92. Genome Announc. 2017, 5, e00478-17. [Google Scholar] [CrossRef]
- Houle, S.; Papez, M.D.; Ferazzini, M.; Hollenberg, M.D.; Vergnolle, N. Neutrophils and the kallikrein-kinin system in proteinase-activated receptor 4-mediated inflammation in rodents. Br. J. Pharmacol. 2005, 146, 670–678. [Google Scholar] [CrossRef]
- Hickey, M.J.; Kanwar, S.; McCafferty, D.M.; Granger, D.N.; Eppihimer, M.J.; Kubes, P. Varying roles of E-selectin and P-selectin in different microvascular beds in response to antigen. J. Immunol. 1999, 162, 1137–1143. [Google Scholar]
- Turabelidze, A.; Guo, S.; DiPietro, L.A. Importance of housekeeping gene selection for accurate reverse transcription-quantitative polymerase chain reaction in a wound healing model. Wound Repair Regen. 2010, 18, 460–466. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cho, J.S.; Guo, Y.; Ramos, R.I.; Hebroni, F.; Plaisier, S.B.; Xuan, C.; Granick, J.L.; Matsushima, H.; Takashima, A.; Iwakura, Y.; et al. Neutrophil-derived IL-1beta is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS Pathog. 2012, 8, e1003047. [Google Scholar] [CrossRef]
- Cho, J.S.; Pietras, E.M.; Garcia, N.C.; Ramos, R.I.; Farzam, D.M.; Monroe, H.R.; Magorien, J.E.; Blauvelt, A.; Kolls, J.K.; Cheung, A.L.; et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Investig. 2010, 120, 1762–1773. [Google Scholar] [CrossRef] [PubMed]
- Agalar, C.; Eroglu, E.; Sari, M.; Sari, A.; Daphan, C.; Agalar, F. The effect of G-CSF in an experimental MRSA graft infection in mice. J. Investig. Surg. 2005, 18, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Hume, E.B.; Cole, N.; Khan, S.; Garthwaite, L.L.; Aliwarga, Y.; Schubert, T.L.; Willcox, M.D. A Staphylococcus aureus mouse keratitis topical infection model: Cytokine balance in different strains of mice. Immunol. Cell Biol. 2005, 83, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Kielian, T.; Barry, B.; Hickey, W.F. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. J. Immunol. 2001, 166, 4634–4643. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Koyama, A. Methicillin-resistant Staphylococcus aureus-related glomerulonephritis. Nephrol. Dial. Transplant. 1999, 14 (Suppl. 1), 17–18. [Google Scholar] [CrossRef][Green Version]
- McNicholas, S.; Talento, A.F.; O’Gorman, J.; Hannan, M.M.; Lynch, M.; Greene, C.M.; Humphreys, H.; Fitzgerald-Hughes, D. Cytokine responses to Staphylococcus aureus bloodstream infection differ between patient cohorts that have different clinical courses of infection. BMC Infect. Dis. 2014, 14, 580. [Google Scholar] [CrossRef]
- Tseng, C.W.; Kyme, P.; Low, J.; Rocha, M.A.; Alsabeh, R.; Miller, L.G.; Otto, M.; Arditi, M.; Diep, B.A.; Nizet, V.; et al. Staphylococcus aureus Panton-Valentine leukocidin contributes to inflammation and muscle tissue injury. PLoS ONE 2009, 4, e6387. [Google Scholar] [CrossRef]
- Yoshii, T.; Magara, S.; Miyai, D.; Nishimura, H.; Kuroki, E.; Furudoi, S.; Komori, T.; Ohbayashi, C. Local levels of interleukin-1beta, -4, -6 and tumor necrosis factor alpha in an experimental model of murine osteomyelitis due to Staphylococcus aureus. Cytokine 2002, 19, 59–65. [Google Scholar] [CrossRef]
- Cohen, P.R. Community-acquired methicillin-resistant Staphylococcus aureus skin infections: Implications for patients and practitioners. Am. J. Clin. Dermatol. 2007, 8, 259–270. [Google Scholar] [CrossRef]
- Gorwitz, R.J. A review of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr. Infect. Dis. J. 2008, 27, 1–7. [Google Scholar] [CrossRef]
- Kim, M.H.; Granick, J.L.; Kwok, C.; Walker, N.J.; Borjesson, D.L.; Curry, F.R.; Miller, L.S.; Simon, S.I. Neutrophil survival and c-kit(+)-progenitor proliferation in Staphylococcus aureus-infected skin wounds promote resolution. Blood 2011, 117, 3343–3352. [Google Scholar] [CrossRef] [PubMed]
- Molne, L.; Verdrengh, M.; Tarkowski, A. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect. Immun. 2000, 68, 6162–6167. [Google Scholar] [CrossRef] [PubMed]
- Rigby, K.M.; DeLeo, F.R. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin. Immunopathol. 2012, 34, 237–259. [Google Scholar] [CrossRef]
- de Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil migration in infection and wound repair: Going forward in reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Furuya, Y.; Roberts, S.; Metzger, D.W. Role of Interleukin-12 in Protection against Pulmonary Infection with Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 6308–6316. [Google Scholar] [CrossRef]
- Zabieglo, K.; Majewski, P.; Majchrzak-Gorecka, M.; Wlodarczyk, A.; Grygier, B.; Zegar, A.; Kapinska-Mrowiecka, M.; Naskalska, A.; Pyrc, K.; Dubin, A.; et al. The inhibitory effect of secretory leukocyte protease inhibitor (SLPI) on formation of neutrophil extracellular traps. J. Leukoc. Biol. 2015, 98, 99–106. [Google Scholar] [CrossRef]
- Hruz, P.; Zinkernagel, A.S.; Jenikova, G.; Botwin, G.J.; Hugot, J.P.; Karin, M.; Nizet, V.; Eckmann, L. NOD2 contributes to cutaneous defense against Staphylococcus aureus through alpha-toxin-dependent innate immune activation. Proc. Natl. Acad. Sci. USA 2009, 106, 12873–12878. [Google Scholar] [CrossRef]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Takeda, K.; Akira, S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999, 11, 443–451. [Google Scholar] [CrossRef]
- Patot, S.; Imbert, P.R.; Baude, J.; Martins Simoes, P.; Campergue, J.B.; Louche, A.; Nijland, R.; Bes, M.; Tristan, A.; Laurent, F.; et al. The TIR homologue lies near resistance genes in Staphylococcus aureus, coupling modulation of virulence and antimicrobial susceptibility. PLoS Pathog. 2017, 13, e1006092. [Google Scholar] [CrossRef]
- Bridenbaugh, L.D. The 1990 John J. Bonica lecture. Acute pain therapy--whose responsibility? Reg. Anesth. 1990, 15, 219–222. [Google Scholar]
- Koymans, K.J.; Feitsma, L.J.; Brondijk, T.H.; Aerts, P.C.; Lukkien, E.; Lossl, P.; van Kessel, K.P.; de Haas, C.J.; van Strijp, J.A.; Huizinga, E.G. Structural basis for inhibition of TLR2 by staphylococcal superantigen-like protein 3 (SSL3). Proc. Natl. Acad. Sci. USA 2015, 112, 11018–11023. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, R.; Itoh, S.; Kamoshida, G.; Takii, T.; Fujii, S.; Tsuji, T.; Onozaki, K. Staphylococcal superantigen-like protein 3 binds to the Toll-like receptor 2 extracellular domain and inhibits cytokine production induced by Staphylococcus aureus, cell wall component, or lipopeptides in murine macrophages. Infect. Immun. 2012, 80, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.S.; Cho, J.S. Immunity against Staphylococcus aureus cutaneous infections. Nat. Rev. Immunol. 2011, 11, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Greenfeder, S.; Umland, S.P.; Cuss, F.M.; Chapman, R.W.; Egan, R.W. Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respir. Res. 2001, 2, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Fukuda, K.; Pan, J.; Makino, S.; Baba, A.; Hori, S.; Ogawa, S. Leukemia inhibitory factor, a potent cardiac hypertrophic cytokine, activates the JAK/STAT pathway in rat cardiomyocytes. Circ. Res. 1997, 81, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Jennes, W.; Vereecken, C.; Fransen, K.; de Roo, A.; Kestens, L. Disturbed secretory capacity for macrophage inflammatory protein (MIP)-1 alpha and MIP-1 beta in progressive HIV infection. AIDS Res. Hum. Retrovir. 2004, 20, 1087–1091. [Google Scholar] [CrossRef]
- Asai, A.; Tsuda, Y.; Kobayashi, M.; Hanafusa, T.; Herndon, D.N.; Suzuki, F. Pathogenic role of macrophages in intradermal infection of methicillin-resistant Staphylococcus aureus in thermally injured mice. Infect. Immun. 2010, 78, 4311–4319. [Google Scholar] [CrossRef][Green Version]




| USA300-C2406 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Role | Cytokine/Chemokine | Mock Infected conc. | M92 conc. | USA400- CMRSA7 conc. | USA300- C2406 conc. | Vs. Mock Infected | Vs. M92 | Vs. USA400- CMRSA7 |
| Protection | IL-17A | 0.0185 (0.0017–0.0416) | 1.072 † (0.0768–2.2690) | 0.1850 (0.0454–0.3744) | 0.8985 (0.4565–1.4555) | |||
| IL-1a | 37.9831 (25.0558–45.3151) | 29.6027 (10.8897–54.1207) | 20.2297 (6.1071–33.1123) | 30.2987 (20.9430–39.5406) | ||||
| IL-1b | 0.5015 (0.2672–0.8363) | 9.356 † (2.06583–18.1579) | 1.3075 (0.1432–2.9846) | 7.3872 (3.9964–11.6633) | ||||
| Severity | IL-4 | 0.0129 (0.0097–0.0183) | 0.0273 (0.0122–0.0553) | 0.0215 (0.0119–0.0339) | 0.0918 (0.0680–0.1042) | ** | ** | ** |
| IL-6 | 0.0270 (0.0000–0.049) | 1.418 (0.1269–4.8495) | 1.1344 (0.1020–2.7832) | 16.3018 (5.1485–37.0610) | * | * | * | |
| IFNγ | 0.0000 (0.0000–0.0000) | 0.0666 (0.0000–0.2207) | 0.0150 (0.0000–0.0748) | 0.1793 (0.0664–0.3274) | ** | ** | ||
| TNFa | 0.0684 (0.000–0.1614) | 0.7811 (0.2561–2.0464) | 0.2644 (0.2373–0.5366) | 1.6897 (0.9215–2.6344) | ** | ** | ||
| GM-CSF | 0.1779 (0.0000–0.5163) | 0.6738 (0.0000–1.2190) | 0.5376 (0.0000–0.9983) | 1.8187 (0.6250–2.9474) | ** | * | ** | |
| G-CSF | 0.1111 (0.0000–0.4109) | 81.6 † (19.2325–115.1702) | 8.7264 (3.864253–22.4210) | 175.9966 (102.2350–224.3600) | ** | * | ** | |
| M-CSF | 0.0775 (0.0492–0.1206) | 0.3669 (0.2111–0.6281) | 0.4481 (0.1236–0.8643) | 14.6826 (2.4079–40.4880) | * | * | * | |
| KC | 2.3430 (1.7789–3.2617) | 19.8096 (2.0290–47.4260) | 5.7795 (2.2214–12.5455) | 70.1897 (37.6055–135.1504) | ** | * | ** | |
| MIP-2 | 1.0161 (0.3052–1.7494) | 223.4 † (154.0767–271.0803) | 230.60† (219.8402–241.4560) | 357.2018 (246.9371–480.9945) | ** | * | ||
| IL-12(p70) | 0.0702 (0.0169–0.1461) | 0.1498 (0.0987–0.2221) | 0.1746 (0.0593–0.1578) | 0.3607 (0.0000–0.6981) | * | |||
| IL-12(p40) | 0.0086 (0.0000–0.0305) | 0.0160 (0.0000–0.0392) | 0.01974 (0.0000–0.0765) | 0.0736 (0.0000–0.2041) | ||||
| Rantes | 0.0190 (0.0000–0.0335) | 0.4183 † (0.2961–0.6175) | 0.4276 † (0.2293–0.7087) | 0.3085 (0.1040–0.4368) | * | |||
| IL-15 | 1.8561 (0.7573–3.7306) | 0.5533 † (0.0000–1.5679) | 0.7991 (0.0496–1.1612) | 0.9902 (0.5022–1.3107) | ||||
| IL–10 | 0.1976 (0.0878–0.3272) | 0.2875 (0.1620–0.3606) | 0.2817 (0.1698–0.4221) | 0.3899 (0.1375–0.5556) | ||||
| Growth factor | VEGF | 0.0621 (0.0425–0.0883) | 1.1501 (0.2439–2.8221) | 0.2536 (0.0873–0.6286) | 3.1011 (1.6067–4.7437) | ** | * | ** |
| Remaining Th1 and Th2 cytokines | IL-2 | 0.5800 (0.3685–0.7409) | 0.3426 (0.1942–0.5813) | 0.4792 (0.3380–0.7139) | 0.4275 (0.2350–0.6595) | |||
| IL-9 | 3.3111 (2.3307–4.6925) | 2.5649 (1.7161–4.4603) | 2.8168 (1.7129–4.9587) | 2.6974 (1.6700–3.9570) | ||||
| IL-13 | 0.0000 (0.0000–0.0000) | 0.0794 (0.0000–0.2386) | 0.0327 (0.0000–0.1635) | 0.1067 (0.0000–0.2262) | ||||
| Other cytokines (tissue injury, infection, allergic diseases) | IL-5 | 0.0033 (0.0000–0.0131) | 0.0142 (0.0000–0.0195) | 0.0173 (0.0139–0.0203) | 0.2271 (0.1089–0.5159) | ** | ** | ** |
| LIF | 0.0241 (0.0072–0.0560) | 0.2109 (0.0948–0.3703) | 0.0890 (0.0356–0.1318) | 3.9190 (2.0239–7.280) | ** | ** | ** | |
| LIX | 0.0086 (0.0000–0.0431) | 0.0000 (0.0000–0.0000) | 0.000 (0.0000–0.0000) | 1.8256 (0.0690–3.9411) | * | * | * | |
| MCP-1 | 0.3565 (0.2727–0.5333) | 3.2274 (1.2843–4.9421) | 1.5886 (0.9464–1.8880) | 12.1896 (6.0745–21.0912) | ** | ** | ** | |
| MIP-1a | 0.6512 (0.4869–0.8522) | 5.2930 (0.6879–12.8468) | 2.9084 (0.5998–6.7366) | 37.4366 (12.7200–85.4010) | ** | * | * | |
| MIP-1b | 0.0000 (0.0000–0.0000) | 2.058 (0.4973–5.3016) | 1.3585 (0.6601–3.5958) | 13.7665 (6.1825–25.9812) | ** | ** | ** | |
| MIG | 3.3655 (1.4177–5.2275) | 8.3413 (2.6754–17.4490) | 11.3525 (3.9570–34.2732) | 5.4001 (3.3130–8.5667) | ||||
| Other cytokines (immune cell development, virus infection, aging) | IP-10 | 0.0963 (0.0529–0.1781) | 0.8319 (0.0987–2.0276) | 0.4538 (0.15725–0.7421) | 1.7451 (0.7332–3.9248) | * | ||
| Eotaxin | 0.6111 (0.2139–1.2606) | 3.5192 † (0.9022–5.4013) | 1.9032 (1.1310–2.8953) | 4.3125 (1.0695–7.3215) | ** | |||
| IL-3 | 0.0015 (0.0000–0.0075) | 0.0681 (0.0183–0.1806) | 0.1669 (0.0475–0.3370) | 0.1577 (0.0395–0.2731) | * | |||
| IL-7 | 0.1743 (0.0000–0.7500) | 0.1810 (0.0817–0.2839) | 0.0696 (0.0057–0.2083) | 0.2900 (0.1815–0.3531) | * | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Conly, J.; McClure, J.; Wu, K.; Petri, B.; Barber, D.; Elsayed, S.; Armstrong, G.; Zhang, K. A Murine Skin Infection Model Capable of Differentiating the Dermatopathology of Community-Associated MRSA Strain USA300 from Other MRSA Strains. Microorganisms 2021, 9, 287. https://doi.org/10.3390/microorganisms9020287
Zhang J, Conly J, McClure J, Wu K, Petri B, Barber D, Elsayed S, Armstrong G, Zhang K. A Murine Skin Infection Model Capable of Differentiating the Dermatopathology of Community-Associated MRSA Strain USA300 from Other MRSA Strains. Microorganisms. 2021; 9(2):287. https://doi.org/10.3390/microorganisms9020287
Chicago/Turabian StyleZhang, Jack, John Conly, JoAnn McClure, Kaiyu Wu, Bjӧrn Petri, Duane Barber, Sameer Elsayed, Glen Armstrong, and Kunyan Zhang. 2021. "A Murine Skin Infection Model Capable of Differentiating the Dermatopathology of Community-Associated MRSA Strain USA300 from Other MRSA Strains" Microorganisms 9, no. 2: 287. https://doi.org/10.3390/microorganisms9020287
APA StyleZhang, J., Conly, J., McClure, J., Wu, K., Petri, B., Barber, D., Elsayed, S., Armstrong, G., & Zhang, K. (2021). A Murine Skin Infection Model Capable of Differentiating the Dermatopathology of Community-Associated MRSA Strain USA300 from Other MRSA Strains. Microorganisms, 9(2), 287. https://doi.org/10.3390/microorganisms9020287






