Effects of Durum Wheat Cultivars with Different Degrees of FHB Susceptibility Grown under Different Meteorological Conditions on the Contamination of Regulated, Modified and Emerging Mycotoxins
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Field Experiment and Samples
2.2. FHB Symptoms
2.3. Multi-Mycotoxin LC-MS/MS Analysis
2.3.1. Extraction and Sample Preparation
2.3.2. LC-MS/MS Analysis
2.4. Statistical Analysis
3. Results
3.1. Meteorological Data
3.2. Yield Parameters
3.3. FHB Symptoms
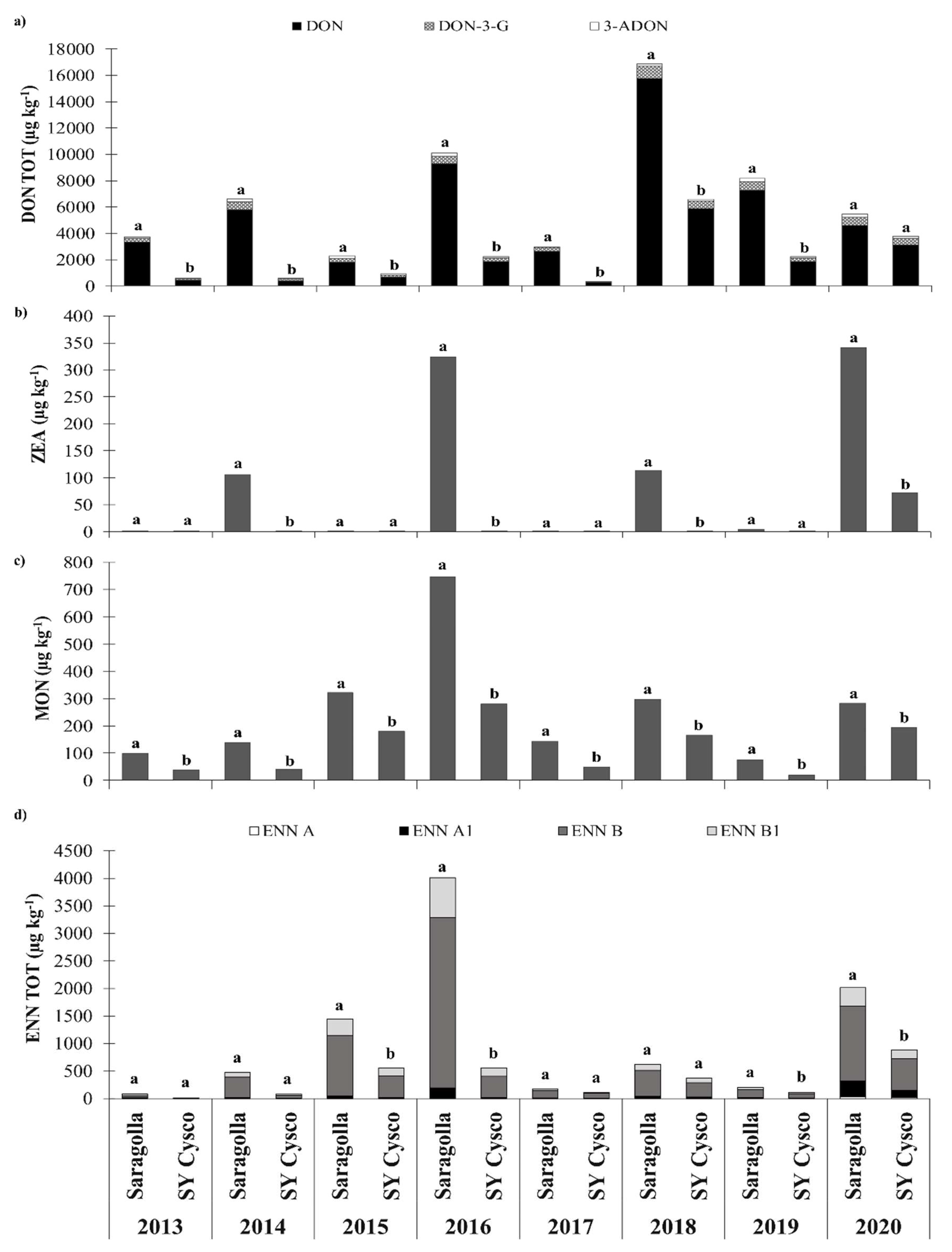
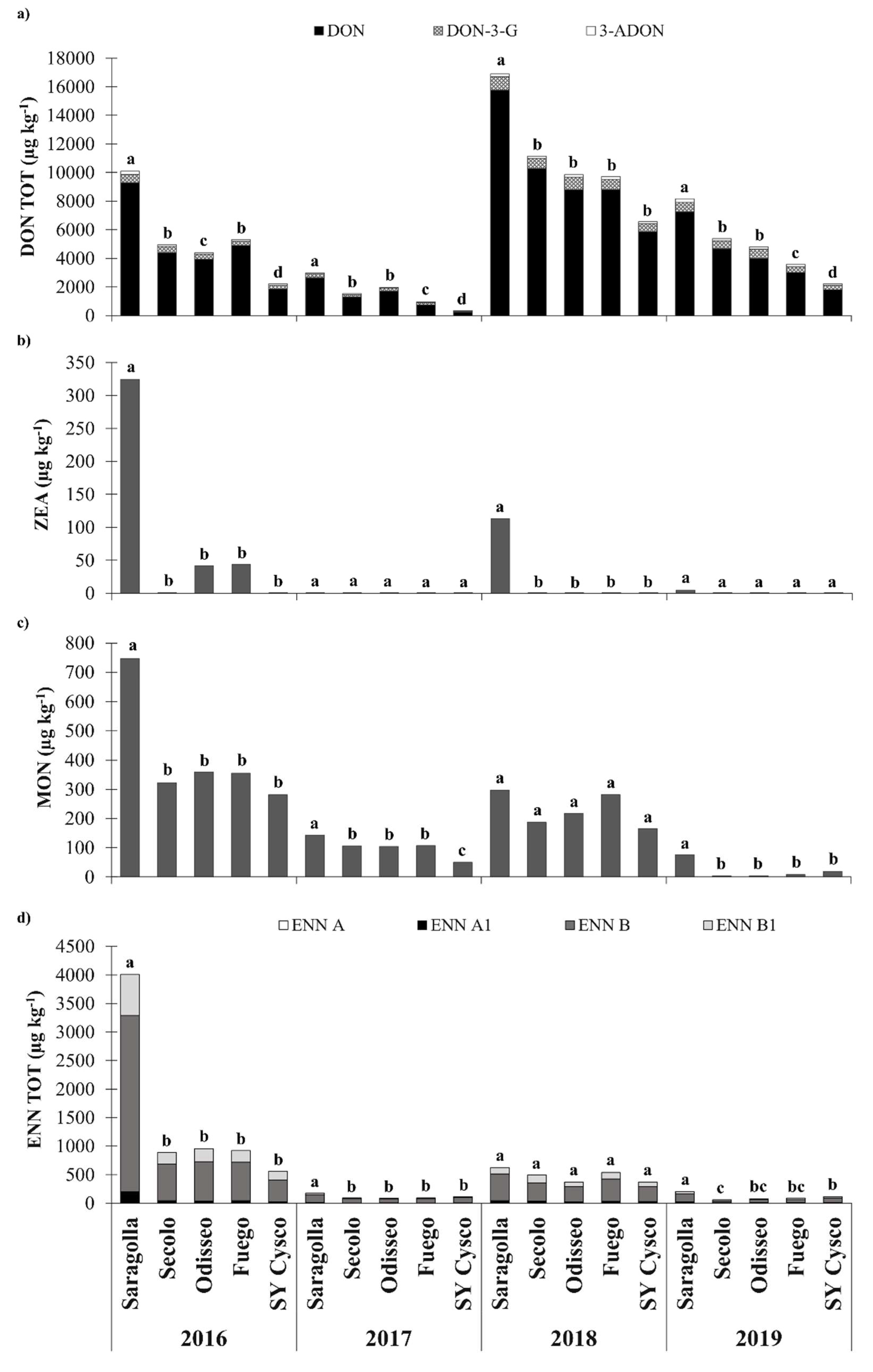
3.4. Mycotoxin Contamination
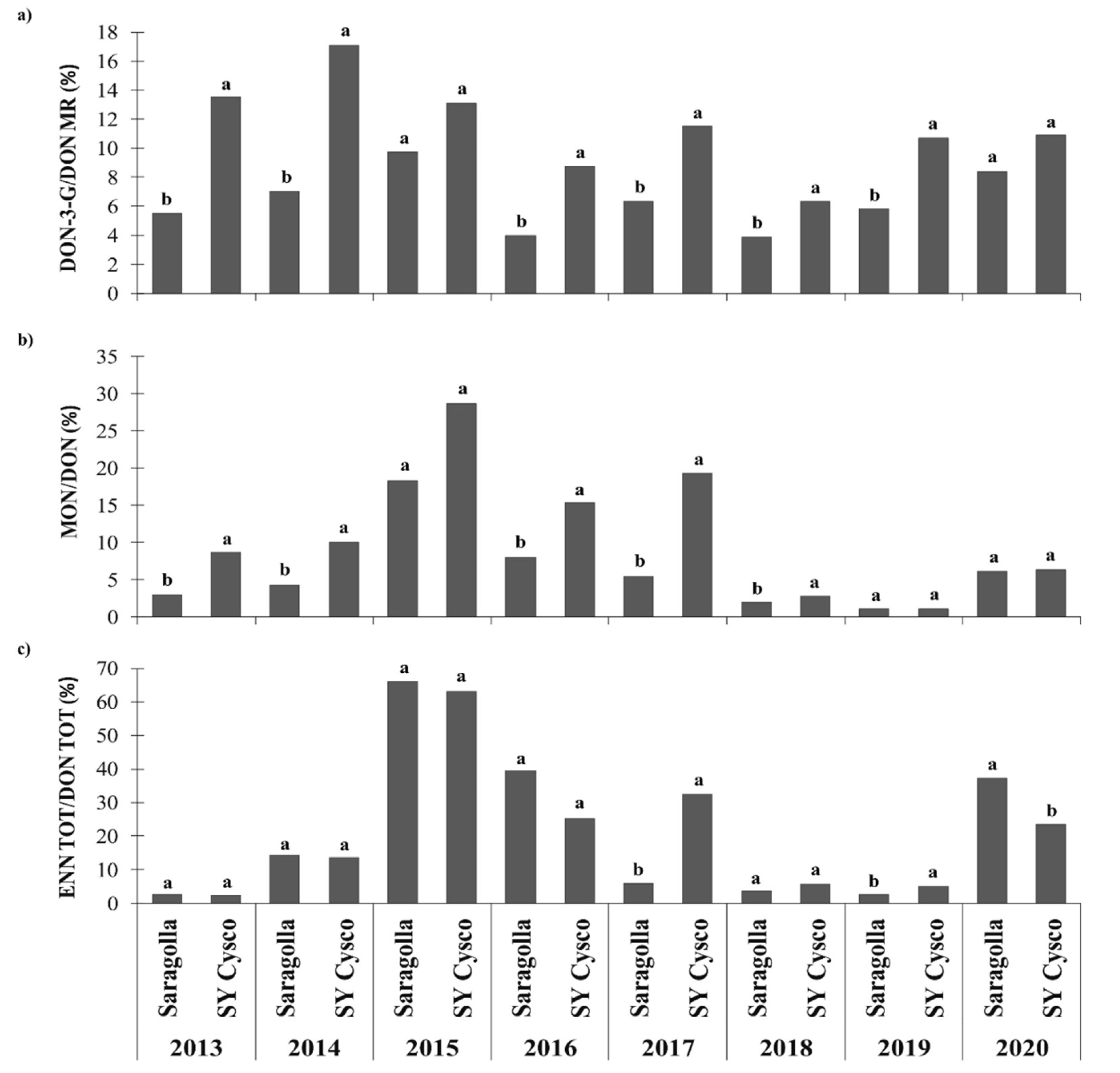
3.5. Ratio between the Modified and Emerging Mycotoxins and DON
3.6. Correlations between Agro-Meteorological Parameters and Mycotoxins
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Handmer, J.; Honda, Y.; Kundzewicz, Z.W.; Arnell, N.; Benito, G.; Hatfield, J.; Mohamed, I.F.; Peduzzi, P.; Wu, S.; Sherstyukov, B.; et al. Changes in impacts of climate extremes: Human systems and ecosystems. In Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation, A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change (IPCC); Field, C.B., Barros, V., Stocker, T.F., Qin, D., Dokken, D.J., Ebi, K.L., Mastrandrea, M.D., Mach, K.J., Plattner, G.-K., Allen, S.K., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; pp. 231–290. [Google Scholar]
- Porter, J.R.; Semenov, M.A. Crop responses to climatic variation. Phil. Trans. R. Soc. B 2005, 360, 2021–2035. [Google Scholar] [CrossRef]
- Medina, A.; González-Jartín, J.M.; Sainz, M.J. Impact of global warming on mycotoxins. Curr. Opin. Food Sci. 2017, 18, 76–81. [Google Scholar] [CrossRef]
- Blandino, M.; Scarpino, V.; Sulyok, M.; Krska, R.; Reyneri, A. Effect of agronomic programmes with different susceptibility to deoxynivalenol risk on emerging contamination in winter wheat. Europ. J. Agron. 2017, 85, 12–24. [Google Scholar] [CrossRef]
- Trnka, M.; Rötter, R.P.; Ruiz-Ramos, M.; Kersebaum, K.C.; Olesen, J.E.; Žalud, Z.; Semenov, M.A. Adverse weather conditions for European wheat production will become more frequent with climate change. Nat. Clim. Chang. 2014, 4, 637–643. [Google Scholar] [CrossRef]
- Chakraborty, S.; Tiedemann, A.V.; Teng, P.S. Climate change: Potential impact on plant diseases. Environ. Pollut. 2000, 108, 317–326. [Google Scholar] [CrossRef]
- Ramesh, K.; Matloob, A.; Aslam, F.; Florentine, S.K.; Chauhan, B.S. Weeds in a changing climate: Vulnerabilities, consequences, and implications for future weed management. Front. Plant Sci. 2017, 8, 95. [Google Scholar] [CrossRef]
- Jeger, M.J.; Pautasso, M. Plant disease and global change–the importance of long-term data sets. New Phytol. 2008, 177, 2–5. [Google Scholar] [CrossRef]
- Beres, B.L.; Rahmani, E.; Clarke, J.M.; Grassini, P.; Pozniak, C.J.; Geddes, C.M.; Porker, K.D.; May, W.E.; Ransom, J.K. A Systematic Review of Durum Wheat: Enhancing Production Systems by Exploring Genotype, Environment, and Management (G × E × M) Synergies. Front. Plant Sci. 2020, 11, 568657. [Google Scholar] [CrossRef]
- Miraglia, M.; Marvin, H.J.P.; Kleter, G.A.; Battilani, P.; Brera, C.; Coni, E.; Cubadda, F.; Croci, L.; De Santis, B.; Dekkers, S.; et al. Climate change and food safety: An emerging issue with special focus on Europe. Food Chem. Toxicol. 2009, 47, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No. 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Van der Fels-Klerx, H.J.; Liu, C.; Battilani, P. Modelling climate change impacts on mycotoxin contamination. World Mycotoxin J. 2016, 9, 717–726. [Google Scholar] [CrossRef]
- Blandino, M.; Badeck, F.W.; Giordano, D.; Marti, A.; Rizza, F.; Scarpino, V.; Vaccino, P. Elevated CO2 Impact on Common Wheat (Triticum aestivum L.) Yield, Wholemeal Quality, and Sanitary Risk. J. Agric. Food Chem. 2020, 68, 10574–10585. [Google Scholar] [CrossRef]
- Van der Fels-Klerx, H.; Van Asselt, E.D.; Madsen, M.S.; Olesen, J.E. Impact of climate change effects on contamination of cereal grains with deoxynivalenol. PLoS ONE 2013, 8, e73602. [Google Scholar]
- Blandino, M.; Haidukowski, M.; Pascale, M.; Plizzari, L.; Scudellari, D.; Reyneri, A. Integrated strategies for the control of Fusarium head blight and deoxynivalenol contamination in winter wheat. Field Crop. Res. 2012, 133, 139–149. [Google Scholar] [CrossRef]
- Haile, J.K.; N’Diaye, A.; Walkowiak, S.; Nilsen, K.T.; Clarke, J.M.; Kutcher, H.R.; Steiner, B.; Buerstmayr, H.; Pozniak, C.J. Fusarium Head Blight in Durum Wheat: Recent Status, Breeding Directions, and Future Research Prospects. Phytopathology 2019, 109, 1664–1675. [Google Scholar] [CrossRef]
- Juan, C.; Covarelli, L.; Beccari, G.; Colasante, V.; Mañes, J. Simultaneous analysis of twenty-six mycotoxins in durum wheat grain from Italy. Food Control 2016, 62, 322–329. [Google Scholar] [CrossRef]
- Jestoi, M. Emerging Fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin—A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef]
- Rychlik, M.; Humpf, H.; Marko, D.; Dänicke, S.; Mally, A.; Berthiller, F.; Klaffke, H.; Lorenz, N. Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res. 2014, 30, 197–205. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Martins, L.M.; von Hertwig, A.M.; Bertoldo, R.; Sant’Ana, A.S. Deoxynivalenol and its masked forms: Characteristics, incidence, control and fate during wheat and wheat based products processing-A review. Trends Food Sci. Technol. 2018, 71, 13–24. [Google Scholar] [CrossRef]
- Cirlini, M.; Generotti, S.; Dall’Erta, A.; Lancioni, P.; Ferrazzano, G.; Massi, A.; Galaverna, G.; Dall’Asta, C. Durum Wheat (Triticum Durum Desf.) Lines Show Different Abilities to Form Masked Mycotoxins under Greenhouse Conditions. Toxins 2014, 6, 81–95. [Google Scholar] [CrossRef]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging Fusarium and Alternaria mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (scab) in small grain cereal - A Review. Plant Path. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Scarpino, V.; Reyneri, A.; Blandino, M. Development and Comparison of Two Multiresidue Methods for the Determination of 17 Aspergillus and Fusarium Mycotoxins in Cereals Using HPLC-ESI-TQ-MS/MS. Front. Microbiol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Beccari, G.; Prodi, A.; Senatore, M.T.; Balmas, V.; Tini, F.; Onofri, A.; Pedini, L.; Sulyok, M.; Brocca, L.; Covarelli, L. Cultivation Area Affects the Presence of Fungal Communities and Secondary Metabolites in Italian Durum Wheat Grains. Toxins 2020, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Orlando, B.; Grignon, G.; Vitry, C.; Kashefifard, K.; Valade, R. Fusarium species and enniatin mycotoxins in wheat, durum wheat, triticale and barley harvested in France. Mycotoxin Res. 2019, 35, 369–380. [Google Scholar] [CrossRef]
- Gorczyca, A.; Oleksy, A.; Gala-Czekaj, D.; Urbaniak, M.; Laskowska, M.; Waśkiewicz, A.; Stępień, L. Fusarium head blight incidence and mycotoxin accumulation in three durum wheat cultivars in relation to sowing date and density. Sci. Nat. 2018, 105, 2. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Blagden, R.; Chan, J.; Gaba, D.; Mckendry, T.; Pleskach, K.; Roscoe, M. Fusarium and Alternaria mycotoxins present in Canadian wheat and durum harvest samples. Can. J. Plant Pathol. 2019, 41, 403–414. [Google Scholar] [CrossRef]
- Palacios, S.A.; Erazo, J.G.; Ciasca, B.; Lattanzio, V.M.T.; Reynoso, M.M.; Farnochi, M.C.; Torres, A.M. Occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in durum wheat from Argentina. Food Chem. 2017, 230, 728–734. [Google Scholar] [CrossRef]
- Steiner, B.; Michel, S.; Maccaferri, M.; Lemmens, M.; Tuberosa, R.; Buerstmayr, H. Exploring and exploiting the genetic variation of Fusarium head blight resistance for genomic-assisted breeding in the elite durum wheat gene pool. Theor. Appl. Genet. 2019, 132, 969–988. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef] [PubMed]
- Juroszek, P.; von Tiedemann, A. Linking plant disease models to climate change scenarios to project future risks of crop diseases: A review. J. Plant Dis. Prot. 2015, 122, 3–15. [Google Scholar] [CrossRef]
- Desjardins, A.E. Fusarium mycotoxins. Chemistry, Genetics, and Biology; APS Press: St. Paul, MN, USA, 2006; p. 268. [Google Scholar]
- Van der Fels-Klerx, H.J.; Klemsdal, S.; Hietaniemi, V.; Lindblad, M.; Ioannou-Kakouri, E.; Van Asselt, E.D. Mycotoxin contamination of cereal grain commodities in relation to climate in North West Europe. Food Addit. Contam. Part A 2012, 29, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Krska, R.; Domig, K.J.; Kneifel, W.; Juge, N.; Schuhmacher, R.; Adam, G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 2011, 206, 264–267. [Google Scholar] [CrossRef] [PubMed]
- FAO. Summary and Conclusions. In Joint Food and Agriculture Organization/World health Organization Expert Committee on Food Additives, Proceedings of Joint FAO/WHO Expert Committee on Food Additives Seventy-Second Meeting, Rome, Italy, 16–25 February 2010; FAO: Rome, Italy, 2010; Available online: http://www.who.int/foodsafety/chem/summary72_rev.pdf (accessed on 16 March 2010).
- Codex Alimentarius Commission. Report of the Fifth Session of the Codex Committee on Contaminants in Foods; Codex Alimentarius Commission: The Hague, The Netherlands, 2011. [Google Scholar]
- Lorenz, N.; Dänicke, S.; Edler, L.; Gottschalk, C.; Lassek, E.; Marko, D.; Rychlik, M.; Mally, A. A critical evaluation of health risk assessment of modified mycotoxins with a special focus on zearalenone. Mycotoxin Res. 2019, 35, 27–46. [Google Scholar] [CrossRef]
- Jonsson, M.; Atosuo, J.; Jestoi, M.; Nathanail, A.V.; Kokkonen, U.-M.; Anttila, M.; Koivisto, P.; Lilius, E.-M.; Peltonen, K. Repeated dose 28-day oral toxicity study of moniliformin in rats. Toxicol. Lett. 2015, 233, 38–44. [Google Scholar] [CrossRef]
- Prosperini, A.; Berrada, H.; Ruiz, M.J.; Caloni, F.; Coccini, T.; Spicer, L.J.; Perego, M.C.; Lafranconi, A. A Review of the Mycotoxin Enniatin B. Front. Public Health 2017, 5, 304. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Dall’asta, C.; Corradini, R.; Marchelli, R.; Sulyok, M.; Krska, R.; Adam, G.; Schuhmacher, R. Occurrence of deoxynivalenol and its 3-β-D-glucoside in wheat and maize. Food Addit. Contam. Part A 2009, 26, 507–511. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Dall’Erta, A.; Mantovani, P.; Massi, A.; Galaverna, G. Occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in durum wheat. World Mycotoxin J. 2013, 6, 83–91. [Google Scholar] [CrossRef]
- Lemmens, M.; Steiner, B.; Sulyok, M.; Nicholson, P.; Mesterhazy, A.; Buerstmayr, H. Masked mycotoxins: Does breeding for enhanced Fusarium head blight resistance result in more deoxynivalenol-3-glucoside in new wheat varieties? World Mycotoxin J. 2016, 9, 741–754. [Google Scholar] [CrossRef]
- Spaggiari, M.; Righetti, L.; Galaverna, G.; Giordano, D.; Scarpino, V.; Blandino, M.; Dall’Asta, C. HR-MS profiling and distribution of native and modified Fusarium mycotoxins in tritordeum, wheat and barley whole grains and corresponding pearled fractions. J. Cereal Sci. 2019, 87, 178–184. [Google Scholar] [CrossRef]
- Ovando-Martínez, M.; Ozsisli, B.; Anderson, J.; Whitney, K.; Ohm, J.-B.; Simsek, S. Analysis of deoxynivalenol and deoxynivalenol-3-glucoside in hard red spring wheat inoculated with Fusarium graminearum. Toxins 2013, 5, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Audenaert, K.; De Boevre, M.; Vanheule, A.; Callewaert, J.; Bekaert, B.; Höfte, M.; De Saeger, S.; Haesaert, G. Mycotoxin glucosylation in commercial wheat varieties: Impact on resistance to Fusarium graminearum under laboratory and field conditions. Food Control 2013, 34, 756–762. [Google Scholar] [CrossRef]
- Schweiger, W.; Steiner, B.; Ametz, C.; Siegwart, G.; Wiesenberger, G.; Berthiller, F.; Lemmens, M.; Jia, H.; Adam, G.; Muehlbauer, G.J.; et al. Transcriptomic characterization of two major Fusarium resistance quantitative trait loci (QTLs), Fhb1 and Qfhs.ifa-5A, identifies novel candidate genes. Mol. Plant. Pathol. 2013, 14, 772–785. [Google Scholar] [CrossRef]
- Scarpino, V.; Reyneri, A.; Sulyok, M.; Krska, R.; Blandino, M. Impact of the insecticide application to maize cultivated in different environmental conditions on emerging mycotoxins. Field Crops Res. 2018, 217, 188–198. [Google Scholar] [CrossRef]

| Year | Sowing Date | N Fertilization | Anthesis | Harvest Date | |||
|---|---|---|---|---|---|---|---|
| GS 23 1 | GS 51 1 | GS 65 1 | |||||
| 2013 | 6 November 2012 | 11 March 2013 | 9 May 2013 | 17 May 2013 | 10 July 2013 | ||
| 2014 | 27 October 2013 | 7 March 2014 | 23 April 2014 | 5 May 2014 | 10 July 2014 | ||
| 2015 | 8 November 2014 | 12 March 2015 | 4 May 2015 | 10 May 2015 | 29 June 2015 | ||
| 2016 | 6 November 2015 | 23 February 2016 | 3 May 2016 | 10 May 2016 | 1 July 2016 | ||
| 2017 | 4 November 2016 | 7 March 2017 | 28 April 2017 | 12 May 2017 | 27 June 2017 | ||
| 2018 | 31 October 2017 | 23 February 2018 | 7 May 2018 | 11 May 2018 | 3 July 2018 | ||
| 2019 | 16 November 2018 | 6 March 2019 | 30 April 2019 | 16 May 2019 | 1 July 2019 | ||
| 2020 | 6 November 2019 | 5 March 2020 | 23 April 2020 | 12 May 2020 | 29 June 2020 | ||
| Parameters | Month | Average 1990–2010 | 2012–13 | 2013–14 | 2014–15 | 2015–16 | 2016–17 | 2017–18 | 2018–19 | 2019–20 |
|---|---|---|---|---|---|---|---|---|---|---|
| Rainfall | November | 85 | 182 | 68 | 438 | 5 | 158 | 48 | 124 | 314 |
| (mm) | December | 48 | 11 | 139 | 89 | 4 | 45 | 33 | 11 | 132 |
| January | 38 | 17 | 117 | 36 | 14 | 4 | 107 | 6 | 5 | |
| February | 35 | 40 | 129 | 75 | 120 | 45 | 60 | 43 | 1 | |
| March | 38 | 118 | 71 | 88 | 52 | 69 | 109 | 17 | 62 | |
| April | 88 | 165 | 138 | 75 | 37 | 34 | 93 | 116 | 81 | |
| May | 105 | 101 | 84 | 96 | 171 | 79 | 188 | 178 | 122 | |
| June | 66 | 16 | 144 | 86 | 83 | 149 | 35 | 40 | 113 | |
| Nov—June | 503 | 650 | 890 | 982 | 486 | 582 | 673 | 535 | 830 | |
| GDD 1 | November | 205 | 272 | 251 | 303 | 270 | 238 | 224 | 292 | 249 |
| (Σ °C d−1) | December | 110 | 113 | 169 | 184 | 164 | 144 | 113 | 151 | 193 |
| January | 99 | 142 | 149 | 159 | 119 | 97 | 178 | 141 | 168 | |
| February | 142 | 110 | 179 | 143 | 158 | 152 | 113 | 195 | 229 | |
| March | 240 | 208 | 335 | 304 | 252 | 349 | 223 | 314 | 285 | |
| April | 337 | 398 | 428 | 409 | 405 | 415 | 456 | 393 | 414 | |
| May | 510 | 480 | 515 | 569 | 490 | 554 | 583 | 478 | 579 | |
| June | 594 | 620 | 637 | 659 | 624 | 673 | 665 | 667 | 624 | |
| Nov—June | 2239 | 2343 | 2662 | 2728 | 2481 | 2622 | 2554 | 2632 | 2741 |
| Factor | Source of Variation | Grain Yield | TW | FHB Incidence 1 | FHB Severity 2 |
|---|---|---|---|---|---|
| (t ha−1) | (kg hL−1) | (%) | (%) | ||
| Year | 2013 | 5.9 cd | 80.8 a | 47.5 cd | 4.6 cd |
| 2014 | 7.3 a | 77.8 b | 28.6 e | 2.0 d | |
| 2015 | 4.3 f | 80.5 a | 39.3 de | 1.4 d | |
| 2016 | 6.5 bc | 76.4 c | 73.6 a | 20.9 a | |
| 2017 | 6.1 bc | 77.8 b | 46.2 cd | 3.8 cd | |
| 2018 | 5.0 e | 74.8 d | 44.7 cd | 10.2 b | |
| 2019 | 5.3 de | 79.7 a | 53.6 bc | 6.7 bc | |
| 2020 | 6.7 ab | 79.3 ab | 63.9 ab | 6.7 bc | |
| p-value 3 | <0.001 | <0.001 | <0.001 | <0.001 | |
| sem 4 | 2.6 | 5.5 | 36.3 | 16.6 | |
| Cultivar (cv) | Saragolla | 5.6 b | 76.2 b | 71.3 a | 12.4 a |
| SY Cysco | 6.0 a | 80.6 a | 27.2 b | 1.7 b | |
| p-value 3 | 0.015 | <0.001 | <0.001 | <0.001 | |
| sem 4 | 0.2 | 3.0 | 30.9 | 7.5 | |
| Year × cv | p-value 3 | 0.006 | <0.001 | 0.071 | <0.001 |
| sem 4 | 1.3 | 3.9 | 18.1 | 18.9 |
| Factor | Source of Variation | Grain Yield | TW | FHB Incidence 1 | FHB Severity 2 |
|---|---|---|---|---|---|
| (t ha−1) | (kg hL−1) | (%) | (%) | ||
| Year | 2016 | 6.3 a | 76.3 c | 70.7 a | 26.4 a |
| 2017 | 5.8 b | 77.3 b | 40.2 b | 4.3 c | |
| 2018 | 5.0 c | 76.3 c | 41.7 b | 8.4 b | |
| 2019 | 5.2 c | 80.3 a | 42.5 b | 4.6 c | |
| p-value 3 | <0.001 | <0.001 | <0.001 | <0.001 | |
| sem 4 | 1.0 | 3.5 | 26.4 | 18.3 | |
| Cultivar (cv) | Saragolla | 5.3 b | 74.5 d | 77.9 a | 17.9 b |
| Fuego | 5.3 b | 78.1 bc | 38.9 cd | 3.8 cd | |
| Odisseo | 5.5 b | 77.4 c | 42.4 c | 6.4 c | |
| Secolo | 5.6 b | 78.1 bc | 52.1 b | 24.9 a | |
| SY Cysco | 6.1 a | 80.3 a | 31.1 d | 2.4 d | |
| p-value 3 | <0.001 | <0.001 | <0.001 | <0.001 | |
| sem 4 | 0.6 | 4.3 | 37.8 | 18.3 | |
| Year × cv | p-value 3 | 0.029 | <0.001 | 0.001 | <0.001 |
| sem 4 | 1.3 | 3.3 | 35.4 | 47.3 |
| Correlation | Rainfall May | Rainfall June | GDD Nov–June | GDD May | GDD June | Grain Yield | DON | DON-3-G | DON TOT | ZEA | MON | ENN TOT | DON-3-G/ DON MR | MON/ DON R | ENN TOT/ DON TOT R |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rainfall Nov–June | −0.453 ** | 0.193 | 0.501 ** | 0.530 ** | −0.021 | −0.061 | -0.094 | −0.027 | −0.088 | 0.056 | −0.061 | 0.018 | 0.375 ** | 0.390 ** | 0.485 ** |
| Rainfall May | −0.740 ** | −0.244 * | −0.203 | −0.006 | −0.258 * | 0.612 ** | 0.620 ** | 0.619 ** | 0.066 | 0.244 * | 0.167 | −0.480 ** | −0.519 ** | −0.275 * | |
| Rainfall June | 0.435 ** | 0.259 * | 0.057 | 0.424 ** | −0.435 ** | −0.451 ** | −0.440 ** | 0.114 | 0.032 | 0.048 | 0.278 * | 0.449 ** | 0.288 ** | ||
| GDD Nov–June | 0.384 ** | 0.516 ** | −0.135 | −0.163 | 0.020 | −0.147 | 0.030 | −0.247 * | −0.063 | 0.322 ** | 0.180 | 0.317 ** | |||
| GDD May | 0.384 ** | −0.262 * | 0.232 * | 0.186 | 0.228 * | 0.041 | 0.134 | 0.027 | −0.041 | 0.238 * | 0.219 * | ||||
| GDD June | −0.537 ** | 0.059 | 0.108 | 0.061 | −0.381 ** | −0.450 ** | −0.427 ** | 0.038 | −0.115 | −0.230 * | |||||
| Grain Yield | −0.231 * | −0.192 | −0.230 * | 0.282 ** | 0.063 | 0.130 | 0.206 | −0.011 | −0.035 | ||||||
| DON | 0.870 ** | 0.999 ** | 0.378 ** | 0.414 ** | 0.295 ** | −0.646 ** | −0.483 ** | −0.245 * | |||||||
| DON-3-G | 0.889 ** | 0.298 ** | 0.223 * | 0.177 | −0.457 ** | −0.569 ** | −0.294 ** | ||||||||
| DON TOT | 0.380 ** | 0.406 ** | 0.292 ** | −0.637 ** | −0.493 ** | −0.247 * | |||||||||
| ZEA | 0.589 ** | 0.728 ** | −0.245 * | −0.048 | 0.265 * | ||||||||||
| MON | 0.884 ** | −0.468 ** | 0.204 | 0.485 ** | |||||||||||
| ENN TOT | −0.307 ** | 0.160 | 0.560 ** | ||||||||||||
| DON-3-G/DON MR | 0.427 ** | 0.171 | |||||||||||||
| MON/DON R | 0.800 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarpino, V.; Blandino, M. Effects of Durum Wheat Cultivars with Different Degrees of FHB Susceptibility Grown under Different Meteorological Conditions on the Contamination of Regulated, Modified and Emerging Mycotoxins. Microorganisms 2021, 9, 408. https://doi.org/10.3390/microorganisms9020408
Scarpino V, Blandino M. Effects of Durum Wheat Cultivars with Different Degrees of FHB Susceptibility Grown under Different Meteorological Conditions on the Contamination of Regulated, Modified and Emerging Mycotoxins. Microorganisms. 2021; 9(2):408. https://doi.org/10.3390/microorganisms9020408
Chicago/Turabian StyleScarpino, Valentina, and Massimo Blandino. 2021. "Effects of Durum Wheat Cultivars with Different Degrees of FHB Susceptibility Grown under Different Meteorological Conditions on the Contamination of Regulated, Modified and Emerging Mycotoxins" Microorganisms 9, no. 2: 408. https://doi.org/10.3390/microorganisms9020408
APA StyleScarpino, V., & Blandino, M. (2021). Effects of Durum Wheat Cultivars with Different Degrees of FHB Susceptibility Grown under Different Meteorological Conditions on the Contamination of Regulated, Modified and Emerging Mycotoxins. Microorganisms, 9(2), 408. https://doi.org/10.3390/microorganisms9020408







