Co-Cultivation of Fusarium, Alternaria, and Pseudomonas on Wheat-Ears Affects Microbial Growth and Mycotoxin Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal and Bacterial Isolates
2.2. Inoculum Production
2.3. Experimental Design
2.4. Quantitative Analyses Via qPCR
2.5. Mycotoxin Analyses Via HPLC-MS/MS
2.5.1. Extraction of Wheat Samples
2.5.2. Analytical Standards and Calibration
2.5.3. HPLC-MS/MS Conditions
2.5.4. Quantification, Performance Limits and Quality Control
2.6. Data Management
2.7. Statistical Analyses
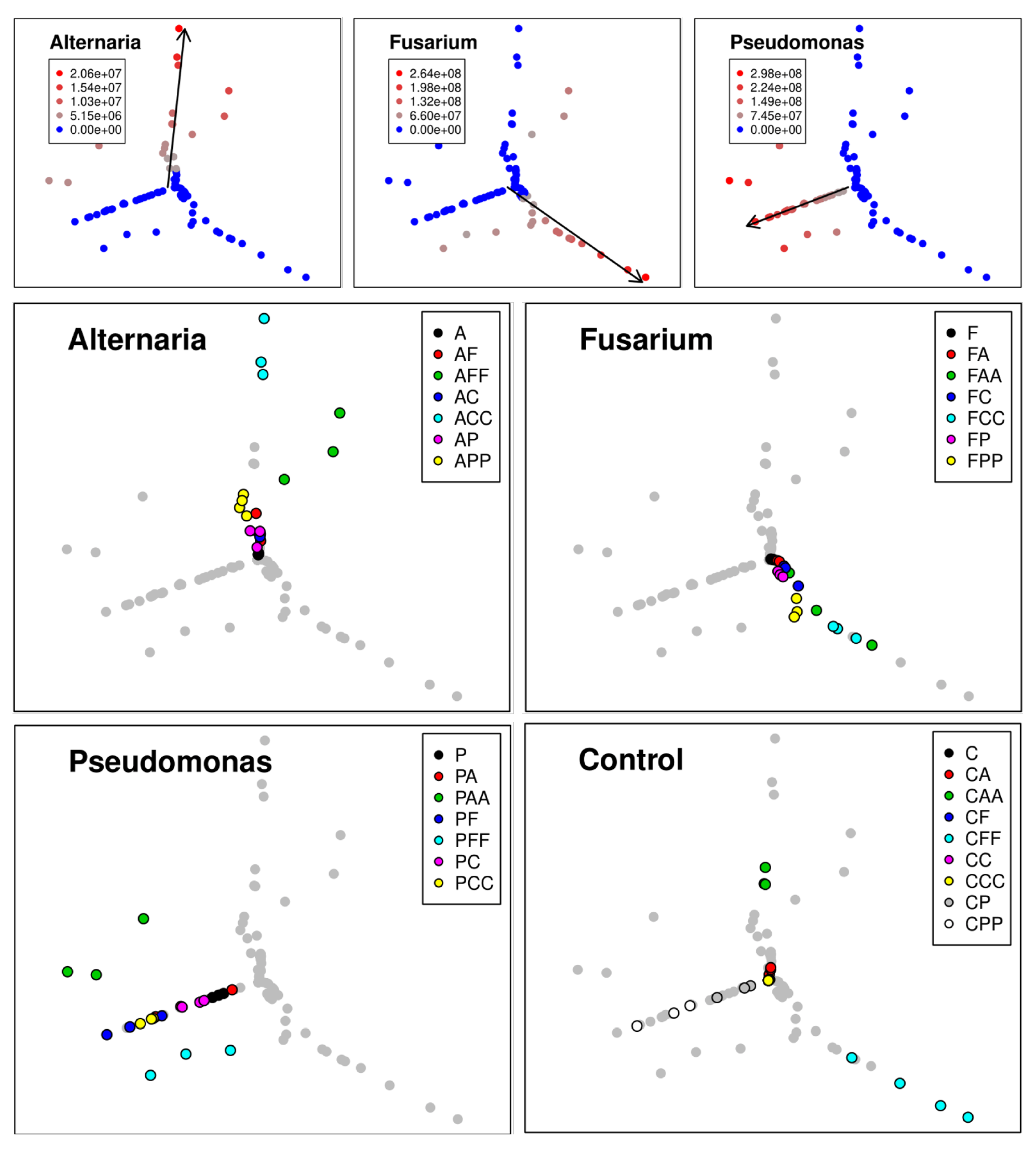
2.7.1. qPCR Data Analysis Via Self-ORGANIZING Map with Sammon Mapping
2.7.2. Mycotoxin Data Analysis Via Dunnett Multiple Comparison Procedure
3. Results
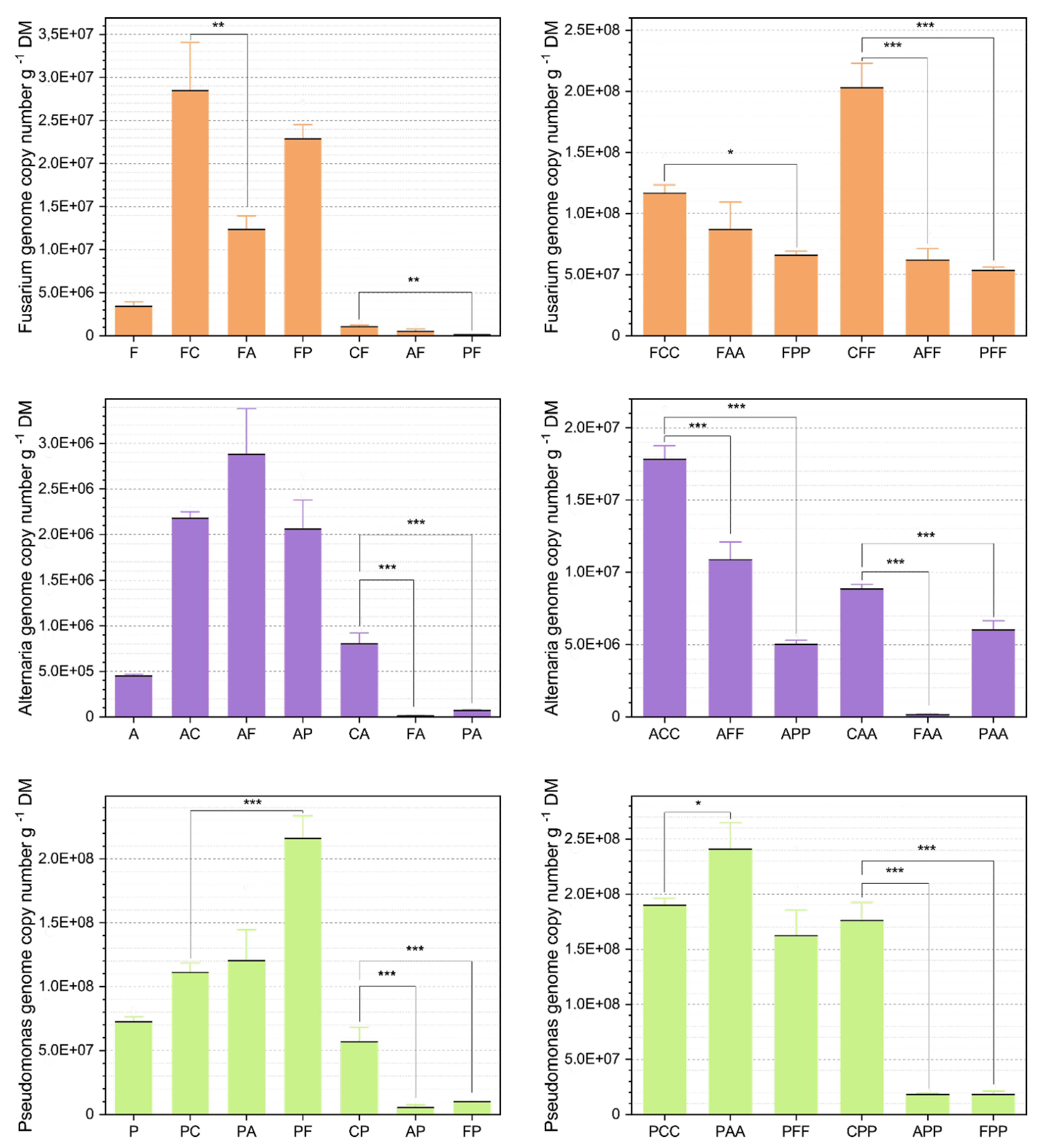
3.1. Abundance Analysis Via qPCR
3.1.1. Comparison of the Average Abundances
Alternaria At625 Abundances
Fusarium Fg23 Abundances
Pseudomonas Ps9 Abundances
3.1.2. Self-Organizing Map with Sammon Mapping
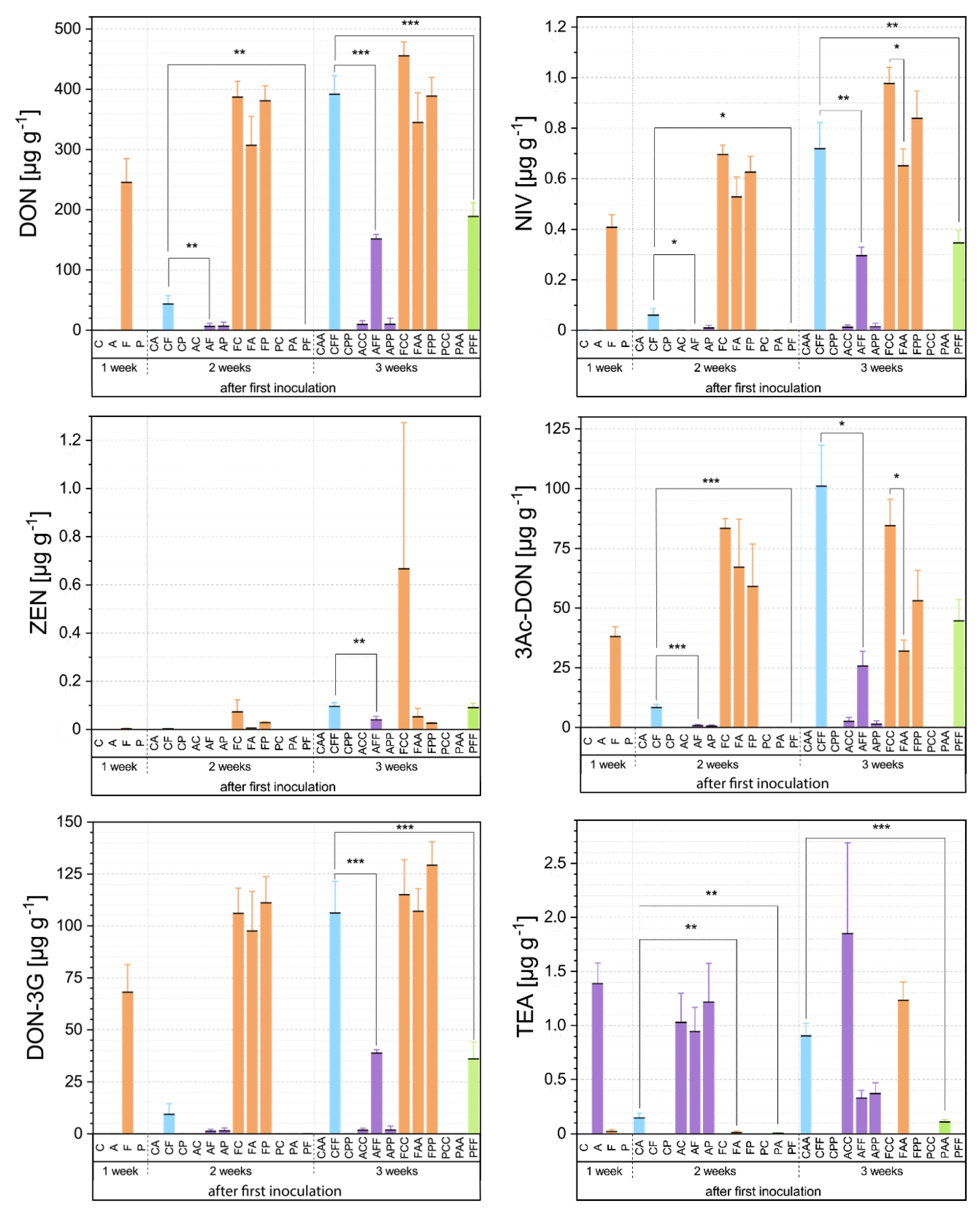
3.2. Mycotoxin Analyses Via HPLC-MS/MS
3.2.1. First Inoculation with Fusarium Fg23
3.2.2. First Inoculation with Alternaria At625
3.2.3. First Inoculation with Pseudomonas Ps9
3.3. Relationship between Fungal Growth and Mycotoxin Production during Competitive Interactions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A. | Alternaria |
| 3-Ac-DON | 3-acetyl deoxynivalenol |
| 15-Ac-DON | 15-acetyl deoxynivalenol |
| ALT | altenuene |
| AME | alternariol monomethyl ether |
| AOH | alternariol |
| DM | dry matter |
| DON | deoxynivalenol |
| DON-3G | deoxynivalenol-3-glucoside |
| F. | Fusarium |
| FHB | Fusarium head blight |
| gnc | genome copy number |
| IS-A | internal standard solution for Alternaria toxins |
| IS-F | internal standard solution for Fusarium toxins |
| LOD | limits of detection |
| LOQ | limits of quantification |
| NIV | nivalenol |
| P. | Pseudomonas |
| PCA | Potato Carrot Agar |
| SNA | Synthetic Nutrient Agar |
| SM | Sammon Mapping |
| SOM | Self-Organizing Map |
| TeA | tenuazonic acid |
| TEN | tentoxin |
| ZEN | zearalenone |
References
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Oláh, J.; Popp, J. Losses in the grain supply chain: Causes and solutions. Sustainability 2020, 12, 2342. [Google Scholar] [CrossRef]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (scab) in small grain cereals—a review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Tillmann, M.; von Tiedemann, A.; Winter, M. Crop rotation effects on incidence and diversity of Fusarium species colonizing stem bases and grains of winter wheat. J. Plant Dis. Prot. 2017, 124, 121–130. [Google Scholar] [CrossRef]
- Legrand, F.; Picot, A.; Cobo-Díaz, J.F.; Chen, W.; Le Floch, G. Challenges facing the biological control strategies for the management of Fusarium Head Blight of cereals caused by F. graminearum. Biol. Control 2017, 113, 26–38. [Google Scholar] [CrossRef]
- Xu, X.; Nicholson, P. Community Ecology of Fungal Pathogens Causing Wheat Head Blight. Annu. Rev. Phytopathol. 2009, 47, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Logrieco, A.; Moretti, A.; Solfrizzo, M. Alternaria toxins and plant diseases: An overview of origin, occurrence and risks. World Mycotoxin J. 2009, 2, 129–140. [Google Scholar] [CrossRef]
- Magan, N.; Cayley, G.R.; Lacey, J. Effect of water activity and temperature on mycotoxin production by Alternaria alternata in culture and on wheat grain. Appl. Environ. Microbiol. 1984, 47, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C.B.; Mitchell, T.K.; Craven, K.D.; Cho, Y.; Cramer, R.A., Jr. At Death’s Door: Alternaria Pathogenicity Mechanisms. Plant Pathol. J. 2008, 24, 101–111. [Google Scholar] [CrossRef]
- Vučković, J.N.; Brkljača, J.S.; Bodroža-Solarov, M.I.; Bagi, F.F.; Stojšin, V.B.; Ćulafić, J.N.; Aćimović, M.G. Alternaria spp. on small grains. Food Feed Res. 2012, 39, 79–88. [Google Scholar]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Korn, U.; Müller, T.; Ulrich, A.; Müller, M.E.H. Impact of aggressiveness of Fusarium graminearum and F. culmorum isolates on yield parameters and mycotoxin production in wheat. Mycotoxin Res. 2011, 27, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminantsin Foodstuffs. 2006. Available online: https://www.ecolex.org/details/legislation/commission-regulation-ec-no-18812006-setting-maximum-levels-for-certain-contaminants-in-foodstuffs-lex-faoc068134/ (accessed on 19 December 2006).
- Marasas, W.F.O.; Nelson, P.E.; Toussoun, T.A. Toxigenic Fusarium Species. Identity and Mycotoxicology; Pennsylvania State University: State College, PA, USA, 1984. [Google Scholar]
- Pestka, J.J. Toxicological Mechanisms and Potential Health Effects of Deoxynivalenol and Nivalenol. World Mycotoxin J. 2010, 4, 323–347. [Google Scholar] [CrossRef]
- Reddy, K.; Song, J.; Lee, H.J.; Kim, M.; Kim, D.W.; Jung, H.; Kim, B.; Lee, Y.; Yu, D.; Kim, D.W.; et al. Effects of High Levels of Deoxynivalenol and Zearalenone on Growth Performance, and Hematological and Immunological Parameters in Pigs. Toxins 2018, 10, 114. [Google Scholar] [CrossRef]
- Zhou, H.; Guog, T.; Dai, H.; Yu, Y.; Zhang, Y.; Ma, L. Deoxynivalenol: Toxicological profiles and perspective views for future research. World Mycotoxin J. 2020, 13, 179–188. [Google Scholar] [CrossRef]
- Aichinger, G.; Beisl, J.; Marko, D. Genistein and delphinidin antagonize the genotoxic effects of the mycotoxin alternariol in human colon carcinoma cells. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Aichinger, G.; Puntscher, H.; Beisl, J.; Kütt, M.L.; Warth, B.; Marko, D. Delphinidin protects colon carcinoma cells against the genotoxic effects of the mycotoxin altertoxin II. Toxicol. Lett. 2018, 284, 136–142. [Google Scholar] [CrossRef]
- Kollarova, J.; Cenk, E.; Schmutz, C.; Marko, D. The mycotoxin alternariol suppresses lipopolysaccharide-induced inflammation in THP-1 derived macrophages targeting the NF-κB signalling pathway. Arch. Toxicol. 2018, 92, 3347–3358. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Patriarca, A.; Magan, N. Alternaria in food: Ecophysiology, mycotoxin production and toxicology. Mycobiology 2015, 43, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Zwickel, T.; Kahl, S.; Klaffke, H.; Rychlik, M.; Müller, M. Spotlight on the Underdogs—An Analysis of Underrepresented Alternaria Mycotoxins Formed Depending on Varying Substrate, Time and Temperature Conditions. Toxins 2016, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Fehr, M.; Pahlke, G.; Fritz, J.; Christensen, M.O.; Boege, F.; Altemöller, M.; Podlech, J.; Marko, D. Alternariol acts as a topoisomerase poison, preferentially affecting the IIα isoform. Mol. Nutr. Food Res. 2009, 53, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, L.; Wagner, J.; Metzler, M. Estrogenic and clastogenic potential of the mycotoxin alternariol in cultured mammalian cells. Food Chem. Toxicol. 2006, 44, 398–408. [Google Scholar] [CrossRef]
- Liu, G.T.; Qian, Y.Z.; Zhang, P.; Dong, Z.M.; Shi, Z.Y.; Zhen, Y.Z.; Miao, J.; Xu, Y.M. Relationships between Alternaria alternata and oesophageal cancer. IARC Sci. Publ. 1991, 105, 258–262. [Google Scholar]
- Solhaug, A.; Torgersen, M.L.; Holme, J.A.; Lagadic-Gossmann, D.; Eriksen, G.S. Autophagy and senescence, stress responses induced by the DNA-damaging mycotoxin alternariol. Toxicology 2014, 326, 119–129. [Google Scholar] [CrossRef]
- Tiessen, C.; Fehr, M.; Schwarz, C.; Baechler, S.; Domnanich, K.; Böttler, U.; Pahlke, G.; Marko, D. Modulation of the cellular redox status by the Alternaria toxins alternariol and alternariol monomethyl ether. Toxicol. Lett. 2013, 216, 23–30. [Google Scholar] [CrossRef]
- Audenaert, K.; Landschoot, S.; Vanheule, A.; Waegeman, W.; De Baets, B.; Haesaert, G. Impact of fungicide timing on the composition of the Fusarium head blight disease complex and the presence of deoxynivalenol (DON) in wheat. In Fungicides—Beneficial and Harmful Aspects; Thajuddin, N., Ed.; InTech: Rijeka, Croatia, 2011; pp. 79–98. [Google Scholar]
- Siou, D.; Gélisse, S.; Laval, V.; Suffert, F.; Lannou, C. Mutual Exclusion between Fungal Species of the Fusarium Head Blight Complex in a Wheat Spike. Appl. Environ. Microbiol. 2015, 81, 4682–4689. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.; Solan, M.; Taylor, A.F.S.; Alexander, I.J.; Johnson, D. Intraspecific Diversity Regulates Fungal Productivity and Respiration. PLoS ONE 2010, 5, e12604. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Nicholson, P.; Ritieni, A. Effects of fungal interactions among Fusarium head blight pathogens on disease development and mycotoxin accumulation. Int. J. Food Microbiol. 2007, 119, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.E.; Steier, I.; Köppen, R.; Siegel, D.; Proske, M.; Korn, U.; Koch, M. Cocultivation of phytopathogenic Fusarium and Alternaria strains affects fungal growth and mycotoxin production. J. Appl. Microbiol. 2012, 113, 874–887. [Google Scholar] [CrossRef]
- Müller, M.; Urban, K.; Köppen, R.; Siegel, D.; Korn, U.; Koch, M. Mycotoxins as antagonistic or supporting agents in the interaction between phytopathogenic Fusarium and Alternaria fungi. World Mycotoxin J. 2015, 8, 311–321. [Google Scholar] [CrossRef]
- Simpson, D.R.; Thomsett, M.A.; Nicholson, P. Competitive interactions between Microdochium nivale var. majus, M. nivale var. nivale and Fusarium culmorum in planta and in vitro. Environ. Microbiol. 2003, 6, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Gannibal, P.B. Factors affecting alternaria appearance in grains in European Russia. Sel’skokhozyaistvennaya Biologiya 2018, 53, 605–615. [Google Scholar] [CrossRef]
- Saß, V.; Milles, J.; Krämer, J.; Prange, A. Competitive interactions of Fusarium graminearum and Alternaria alternata in vitro in relation to deoxynivalenol and zearalenone production. J. Food Agric. Environ. 2007, 5, 257–261. [Google Scholar]
- Andersen, B.; Thrane, U.; Svendsen, A.; Rasmussen, I.A. Associated field mycobiota on malt barley. Can. J. Bot. 1996, 74, 854–858. [Google Scholar] [CrossRef]
- Kosiak, B.; Torp, M.; Skjerve, E.; Andersen, B. Alternaria and Fusarium in Norwegian grains of reduced quality—A matched pair sample study. Int. J. Food Microbiol. 2004, 93, 51–62. [Google Scholar] [CrossRef]
- Xu, X.M.; Parry, D.W.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Edwards, S.G.; Cooke, B.M.; Doohan, F.M.; Monaghan, S.; Moretti, A.; et al. Within-field variability of Fusarium head blight pathogens and their associated mycotoxins. Eur. J. Plant Pathol. 2008, 120, 21–34. [Google Scholar] [CrossRef]
- Müller, T.; Ruppel, S.; Behrendt, U.; Lentzsch, P.; Müller, M.E. Antagonistic potential of fluorescent pseudomonads colonizing wheat heads against mycotoxin producing alternaria and fusaria. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Trivedi, P.; Pandey, A.; Palni, L.M.S. In vitro evaluation of antagonistic properties of Pseudomonas corrugata. Microbiol. Res. 2008, 163, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.; Défago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef]
- Alimi, M.; Soleimani, M.J.; Darzi, M.T. Characterization and application of microbial antagonists for control of Fusarium head blight of wheat caused by Fusarium graminearum using single and mixture strain of antagonistic bacteria on resistance and susceptible cultivars. Afr. J. Microbiol. Res. 2012, 6, 326–334. [Google Scholar] [CrossRef]
- Yoshida, S.; Ohba, A.; Liang, Y.M.; Koitabashi, M.; Tsushima, S. Specificity of Pseudomonas Isolates on Healthy and Fusarium Head Blight-Infected Spikelets of Wheat Heads. Microb. Ecol. 2012, 64, 214–225. [Google Scholar] [CrossRef]
- Khan, M.R.; Doohan, F.M. Bacterium-mediated control of Fusarium head blight disease of wheat and barley and associated mycotoxin contamination of grain. Biol. Control 2009, 48, 42–47. [Google Scholar] [CrossRef]
- Müller, T.; Lentzsch, P.; Behrendt, U.; Barkusky, D.; Müller, M.E. Pseudomonas simiae effects on the mycotoxin formation by fusaria and alternaria in vitro and in a wheat field. Mycotoxin Res. 2020, 36, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Rojas, E.C.; Sapkota, R.; Jensen, B.; Jørgensen, H.J.; Henriksson, T.; Jørgensen, L.N.; Nicolaisen, M.; Collinge, D.B. Fusarium Head Blight Modifies Fungal Endophytic Communities During Infection of Wheat Spikes. Microb. Ecol. 2020, 79, 397–408. [Google Scholar] [CrossRef]
- Marín, S.; Sanchis, V.; Arnau, F.; Ramos, A.J.; Magan, N. Colonisation and competitiveness of Aspergillus and Penicillium species on maize grain in the presence of Fusarium moniliforme and Fusarium proliferatum. Int. J. Food Microbiol. 1998, 45, 107–117. [Google Scholar] [CrossRef]
- Lacey, J.; Bateman’, G.L.; Mirocha’, C.J. Effects of infection time and moisture on development of ear blight and deoxynivalenol production by Fusarium spp. in wheat. Ann. Appl. Biol. 1999, 134, 277–283. [Google Scholar] [CrossRef]
- Ellis, S.A.; Gooding, M.J.; Thompson, A.J. Factors influencing the relative susceptibility of wheat cultivars (Triticum aestivum L.) to blackpoint. Crop Prot. 1996, 15, 69–76. [Google Scholar] [CrossRef]
- Thomma, B.P. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.; Schenk, P.M. Inner plant values: Diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 2017, 8, 2552. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, G.; Kumar, A.M. Pseudomonas fluorescens, a potential bacterial antagonist to control plant diseases. J. Plant Interact. 2005, 1, 123–134. [Google Scholar] [CrossRef]
- Hirano, S.S.; Upper, C.D. Dynamics, spread, and persistence of a single genotype of Pseudomonas syringae relative to those of its conspecifics on populations of snap bean leaflets. Appl. Environ. Microbiol. 1993, 59, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Lilley, A.K.; Hails, R.S.; Cory, J.S.; Bailey, M.J. The dispersal and establishment of pseudomonad populations in the phyllosphere of sugar beet by phytophagous caterpillars. FEMS Microbiol. Ecol. 1997, 24, 151–157. [Google Scholar] [CrossRef]
- Müller, T.; Behrendt, U.; Ruppel, S.; von der Waydbrink, G.; Müller, M.E. Fluorescent Pseudomonads in the Phyllosphere of Wheat: Potential Antagonists Against Fungal Phytopathogens. Curr. Microbiol. 2016, 72, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Nierenberg, H. Untersuchungen über die Morphologische und Biologische Differenzierung in der Fusarium-Sektion Liseola; Kommissionsverlag Paul Parey: Berlin, Germany, 1976. [Google Scholar]
- Simmons, G.E. Alternaria taxonomy: Current status, viewpoint, challenge. Alternaria Biology, Plant Diseases and Metabolites; Elsevier: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Knop, W. Über die Ernährung der Pflanzen durch wässerige Lösungen unter Ausschluß des Bodens. Landw. Versuchsstat. 1860, 2, 65–99. [Google Scholar]
- Bergmark, L.; Poulsen, P.H.B.; Al-Soud, W.A.; Norman, A.; Hansen, L.H.; Sørensen, S.J. Assessment of the specificity of Burkholderia and Pseudomonas qPCR assays for detection of these genera in soil using 454 pyrosequencing. FEMS Microbiol. Lett. 2012, 333, 77–84. [Google Scholar] [CrossRef]
- Sammon, J.W. A Nonlinear Mapping for Data Structure Analysis. IEEE Trans. Comput. 1969, C-18, 401–409. [Google Scholar] [CrossRef]
- Kohonen, T. Self-organized formation of topologically correct feature maps. Biol. Cybern. 1982, 43, 59–69. [Google Scholar] [CrossRef]
- Huang, T.S.; Kohonen, T.; Schroeder, M.R. (Eds.) Self-Organizing Maps, 3rd ed.; Springer Series in Information Sciences; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar] [CrossRef]
- Weber, M.; Teeling, H.; Huang, S.; Waldmann, J.; Kassabgy, M.; Fuchs, B.M.; Klindworth, A.; Klockow, C.; Wichels, A.; Gerdts, G.; et al. Practical application of self-organizing maps to interrelate biodiversity and functional data in NGS-based metagenomics. ISME J. 2011, 5, 918–928. [Google Scholar] [CrossRef]
- Bowman, J.S.; Amaral-Zettler, L.A.; Rich, J.J.; Luria, C.M.; Ducklow, H.W. Bacterial community segmentation facilitates the prediction of ecosystem function along the coast of the western Antarctic Peninsula. ISME J. 2017, 11, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Spanoghe, M.C.; Marique, T.; Rivière, J.; Moulin, M.; Dekuijper, C.; Nirsha, A.; Bonnave, M.; Lanterbecq, D. Genetic patterns recognition in crop species using self-organizing map: The example of the highly heterozygous autotetraploid potato (Solanum tuberosum L.). Genet. Resour. Crop Evol. 2020, 67, 947–966. [Google Scholar] [CrossRef]
- Yan, J. Package ’Som’ Self-Organizing Map (with Application in Gene Clustering). 2016. Available online: https://cran.r-project.org/web/packages/som/som.pdf (accessed on 6 July 2016).
- Venables, W.; Ripley, B. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002. [Google Scholar]
- Dunnett, C.W. A Multiple Comparison Procedure for Comparing Several Treatments with a Control. J. Am. Stat. Assoc. 1955, 50, 1096–1121. [Google Scholar] [CrossRef]
- Fakhrunnisa, H.; Hashmi, M.H.; Ghaffar, A. In vitro interaction of fusarium spp., with other fungi. Pak. J. Bot. 2006, 38, 1317–1322. [Google Scholar]
- Riungu, G.; Muthomi, J.; Narla, R. Effect of antagonistic microorganisms on severity of Fusarium head blight of wheat and grain yield. Afr. Crop Sci. Conf. Proc. 2007, 8, 827–832. [Google Scholar]
- Müller, T.; Behrendt, U. Exploiting the biocontrol potential of plant-associated pseudomonads—A step towards pesticide-free agriculture? Biol. Control 2021, 155, 104538. [Google Scholar] [CrossRef]
- Palazzini, J.; Roncallo, P.; Cantoro, R.; Chiotta, M.; Yerkovich, N.; Palacios, S.; Echenique, V.; Torres, A.; Ramirez, M.; Karlovsky, P.; et al. Biocontrol of Fusarium graminearum sensu stricto, Reduction of Deoxynivalenol Accumulation and Phytohormone Induction by Two Selected Antagonists. Toxins 2018, 10, 88. [Google Scholar] [CrossRef]
- Shi, C.; Yan, P.; Li, J.; Wu, H.; Li, Q.; Guan, S. Biocontrol of Fusarium graminearum Growth and Deoxynivalenol Production in Wheat Kernels with Bacterial Antagonists. Int. J. Environ. Res. Public Health 2014, 11, 1094–1105. [Google Scholar] [CrossRef]
- Dawson, W.A.J.M.; Jestoi, M.; Rizzo, A.; Nicholson, P.; Bateman, G.L. Field Evaluation of Fungal Competitors of Fusarium culmorum and F. graminearum, Causal Agents of Ear Blight of Winter Wheat, for the Control of Mycotoxin Production in Grain. Biocontrol Sci. Technol. 2004, 14, 783–799. [Google Scholar] [CrossRef]
- He, J.; Boland, G.J.; Zhou, T. Concurrent selection for microbial suppression of Fusarium graminearum, Fusarium head blight and deoxynivalenol in wheat. J. Appl. Microbiol. 2009, 106, 1805–1817. [Google Scholar] [CrossRef]
- Ridout, M.E.; Godfrey, B.; Newcombe, G. Effects of Antagonists on Mycotoxins of Seedborne Fusarium spp. in Sweet Corn. Toxins 2019, 11, 438. [Google Scholar] [CrossRef]
- González, H.H.; Martínez, E.J.; Pacin, A.; Resnik, S.L. Relationship between Fusarium graminearum and Alternaria alternata contamination and deoxynivalenol occurrence on Argentinian durum wheat. Mycopathologia 1999, 144, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, J.D.; O’Keeffe, T.L.; Abbas, H.K. Microbial Interactions with Mycotoxigenic Fungi and Mycotoxins. Toxin Rev. 2008, 27, 261–285. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yang, N.; Wen, Z.; Sun, X.; Chai, Y.; Ma, Z. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Utermark, J.; Karlovsky, P. Role of zearalenone lactonase in protection of Gliocladium roseum from fungitoxic effects of the mycotoxin zearalenone. Appl. Environ. Microbiol. 2007, 73, 637–642. [Google Scholar] [CrossRef]
- Poppenberger, B.; Sieberer, T. Detoxification of the Fusarium Mycotoxin Deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. Artic. J. Biol. Chem. 2003. [Google Scholar] [CrossRef]
- Warth, B.; Fruhmann, P.; Wiesenberger, G.; Kluger, B.; Sarkanj, B.; Lemmens, M.; Hametner, C.; Fröhlich, J.; Adam, G.; Krska, R.; et al. Deoxynivalenol-sulfates: Identification and quantification of novel conjugated (masked) mycotoxins in wheat. Anal. Bioanal. Chem. 2015, 407, 1033–1039. [Google Scholar] [CrossRef]
- Schneweis, I.; Meyer, K.; Engelhardt, G.; Bauer, J. Occurrence of zearalenone-4-β-D-glucopyranoside in wheat. J. Agric. Food Chem. 2002, 50, 1736–1738. [Google Scholar] [CrossRef]
- Nakagawa, H.; Ohmichi, K.; Sakamoto, S.; Sago, Y.; Kushiro, M.; Nagashima, H.; Yoshida, M.; Nakajima, T. Detection of a new Fusarium masked mycotoxin in wheat grain by high-resolution LC-Orbitrap™ MS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 1447–1456. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Dall’Erta, A.; Mantovani, P.; Massi, A.; Galaverna, G. Occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in durum wheat. World Mycotoxin J. 2013, 6, 83–91. [Google Scholar] [CrossRef]
- Rasmussen, P.H.; Nielsen, K.F.; Ghorbani, F.; Spliid, N.H.; Nielsen, G.C.; Jørgensen, L.N. Occurrence of different trichothecenes and deoxynivalenol- 3-β-D-glucoside in naturally and artificially contaminated Danish cereal grains and whole maize plants. Mycotoxin Res. 2012, 28, 181–190. [Google Scholar] [CrossRef]
- Lemmens, M.; Steiner, B.; Sulyok, M.; Nicholson, P.; Mesterhazy, A.; Buerstmayr, H. Masked mycotoxins: Does breeding for enhanced Fusarium head blight resistance result in more deoxynivalenol-3-glucoside in new wheat varieties? World Mycotoxin J. 2016, 9, 741–754. [Google Scholar] [CrossRef]
- Diamond, J.M. Assembly of Species Communities. In Ecology and Evolution of Communities; Harvard University Press: Boston, MA, USA, 1975. [Google Scholar]
- Connell, J.H.; Slatyer, R.O. Mechanisms of Succession in Natural Communities and Their Role in Community Stability and Organization Joseph. Am. Nat. 1977, 111, 1119–1144. [Google Scholar] [CrossRef]
- Weidlich, E.W.; von Gillhaussen, P.; Delory, B.M.; Blossfeld, S.; Poorter, H.; Temperton, V.M. The importance of being first: Exploring priority and diversity effects in a grassland field experiment. Front. Plant Sci. 2017, 7. [Google Scholar] [CrossRef]
- Slatkin, M. Competition and Regional Coexistence. Ecology 1974, 55, 128–134. [Google Scholar] [CrossRef]
- Eriksson, O.; Eriksson, Å. Effects of arrival order and seed size on germination of grassland plants: Are there assembly rules during recruitment? Ecol. Res. 1998, 13, 223–239. [Google Scholar] [CrossRef]
- Fukami, T. Historical Contingency in Community Assembly: Integrating Niches, Species Pools, and Priority Effects. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 1–23. [Google Scholar] [CrossRef]
- Vaughn, K.J.; Young, T.P. Short-term priority over exotic annuals increases the initial density and longer-term cover of native perennial grasses. Ecol. Appl. 2015, 25, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Tsolis, R.M.; Bäumler, A.J. Gastrointestinal host-pathogen interaction in the age of microbiome research. Curr. Opin. Microbiol. 2020, 53, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Shaani, Y.; Zehavi, T.; Eyal, S.; Miron, J.; Mizrahi, I. Microbiome niche modification drives diurnal rumen community assembly, overpowering individual variability and diet effects. ISME J. 2018, 12, 2446–2457. [Google Scholar] [CrossRef]
- Nicolaisen, M.; Justesen, A.F.; Knorr, K.; Wang, J.; Pinnschmidt, H.O. Fungal communities in wheat grain show significant co-existence patterns among species. Fungal Ecol. 2014, 11, 145–153. [Google Scholar] [CrossRef]
- Schiro, G.; Colangeli, P.; Müller, M.E. A Metabarcoding Analysis of the Mycobiome of Wheat Ears Across a Topographically Heterogeneous Field. Front. Microbiol. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hiscox, J.; Savoury, M.; Müller, C.T.; Lindahl, B.D.; Rogers, H.J.; Boddy, L. Priority effects during fungal community establishment in beech wood. ISME J. 2015, 9, 2246–2260. [Google Scholar] [CrossRef] [PubMed]
- Hougen-Eitzman, D.; Karban, R. Mechanisms of interspecific competition that result in successful control of Pacific mites following inoculations of Willamette mites on grapevines. Oecologia 1995, 103, 157–161. [Google Scholar] [CrossRef] [PubMed]

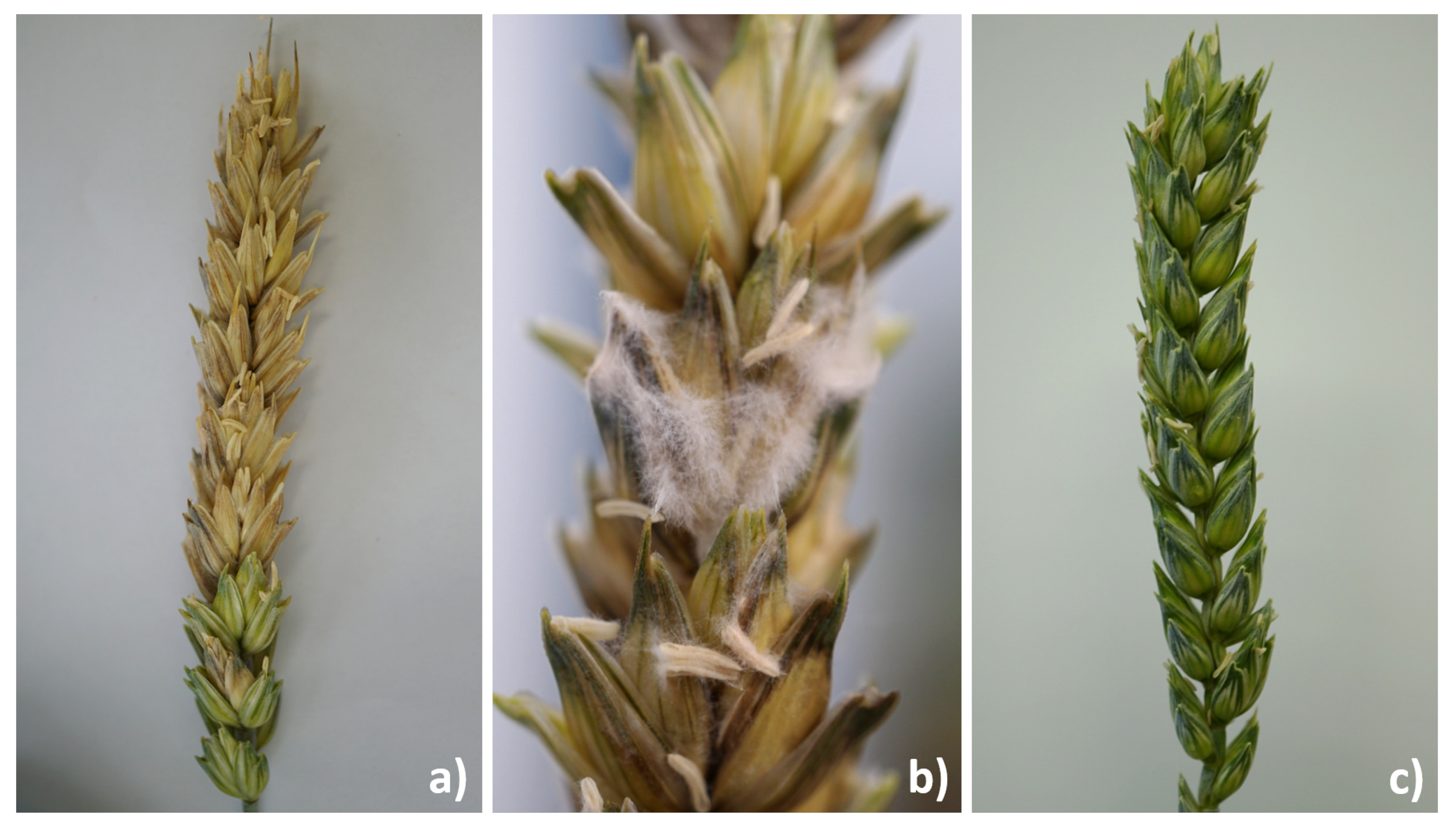
| Time [Day] | 0–6 | 7–15 | 16–24 | 25–48 | 49–59 | 60–66 | 67–End | |
|---|---|---|---|---|---|---|---|---|
| day: | temperature [C] | - | 6 | 6 | 8 | 10 | 12 | 16 |
| humidity [%] | - | 85 | 85 | 95 | 95 | 95 | 95 | |
| duration of exposure [h] | - | 12 | 12 | 14 | 14 | 14 | 14 | |
| photosynthetic active | - | |||||||
| radiation [E/(ms)] | - | 276 | 326 | 415 | 413 | 413 | 420 | |
| night: | temperature [C] | 15 | 4 | 4 | 6 | 8 | 10 | 12 |
| humidity [%] | 80 | 80 | 80 | 80 | 80 | 80 | 80 | |
| duration [h] | 24 | 12 | 12 | 10 | 10 | 10 | 10 |
| Pse449 | probe | 5’- Fam-ACAGAATAAGCACCGGCTAAC-BHQ -3’ |
| Pse435F | forward | 5’- ACTTTAAGTTGGGAGGAAGGG -3’ |
| Pse686R | reverse | 5’- ACACAGGAAATTCCACCACCC -3’ |
| Analyte | Precursor Ion [m/z] | Product Ion [m/z] | DP a [V] | CE b [V] | CXP c [V] |
|---|---|---|---|---|---|
| NIV | 371.1 | 59.1 | −45 | −42 | −7 |
| 371.1 | 281.1 | −45 | −22 | −15 | |
| -NIV | 386.1 | 58.9 | −45 | −42 | −7 |
| DON | 355.1 | 59.2 | −40 | −40 | −8 |
| 355.1 | 265.2 | −40 | −22 | −13 | |
| -DON | 370.1 | 279.1 | −45 | −24 | −7 |
| DON-3G | 457.1 | 427.1 | −55 | −16 | −1 |
| 457.1 | 247.1 | −65 | −25 | −11 | |
| 15-Ac-DON | 397.1 | 337.1 | −40 | −10 | −9 |
| 397.1 | 59.1 | −40 | −38 | −8 | |
| 3-Ac-DON | 397.1 | 307.1 | −40 | −20 | −7 |
| 397.1 | 59.1 | −40 | −38 | −8 | |
| -3-Ac-DON | 414.2 | 323.3 | −30 | −24 | −7 |
| ZEN | 317.1 | 131.1 | −80 | −42 | −8 |
| 317.1 | 175.0 | −80 | −40 | −18 | |
| -ZEN | 335.2 | 140.2 | −80 | −34 | −5 |
| AOH | 257.0 | 215.0 | −70 | −36 | −7 |
| 257.0 | 147.0 | −70 | −37 | −8 | |
| D3-AOH | 260.1 | 216.0 | −80 | −40 | −8 |
| 260.1 | 150.0 | −80 | −45 | −8 | |
| ALT | 291.0 | 203.0 | −75 | −44 | −17 |
| 291.0 | 248.0 | −75 | −34 | −7 | |
| D3-ALT | 294.0 | 248.0 | −65 | −35 | −10 |
| 294.0 | 203.0 | −60 | −40 | −10 | |
| AME | 271.0 | 256.0 | −40 | −32 | −13 |
| 271.0 | 213.0 | −40 | −52 | −16 | |
| D3-AME | 274.0 | 256.0 | −40 | −32 | −13 |
| 274.0 | 228.0 | −40 | −52 | −16 | |
| TeA | 196.2 | 139.1 | −70 | −28 | −7 |
| 196.2 | 112.0 | −70 | −30 | −9 | |
| TEN | 413.2 | 141.0 | −50 | −30 | −7 |
| 413.2 | 271.0 | −50 | −24 | −7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, A.; Lischeid, G.; Koch, M.; Lentzsch, P.; Sommerfeld, T.; Müller, M.E.H. Co-Cultivation of Fusarium, Alternaria, and Pseudomonas on Wheat-Ears Affects Microbial Growth and Mycotoxin Production. Microorganisms 2021, 9, 443. https://doi.org/10.3390/microorganisms9020443
Hoffmann A, Lischeid G, Koch M, Lentzsch P, Sommerfeld T, Müller MEH. Co-Cultivation of Fusarium, Alternaria, and Pseudomonas on Wheat-Ears Affects Microbial Growth and Mycotoxin Production. Microorganisms. 2021; 9(2):443. https://doi.org/10.3390/microorganisms9020443
Chicago/Turabian StyleHoffmann, Annika, Gunnar Lischeid, Matthias Koch, Peter Lentzsch, Thomas Sommerfeld, and Marina E. H. Müller. 2021. "Co-Cultivation of Fusarium, Alternaria, and Pseudomonas on Wheat-Ears Affects Microbial Growth and Mycotoxin Production" Microorganisms 9, no. 2: 443. https://doi.org/10.3390/microorganisms9020443
APA StyleHoffmann, A., Lischeid, G., Koch, M., Lentzsch, P., Sommerfeld, T., & Müller, M. E. H. (2021). Co-Cultivation of Fusarium, Alternaria, and Pseudomonas on Wheat-Ears Affects Microbial Growth and Mycotoxin Production. Microorganisms, 9(2), 443. https://doi.org/10.3390/microorganisms9020443







