Competitive Exclusion of Intra-Genus Salmonella in Neonatal Broilers
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Birds
2.2. Bacterial Strains and Growth Conditions
2.3. Experimental Design and Sample Collection
2.4. Bacteriological Analysis
2.5. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Statistical Analysis
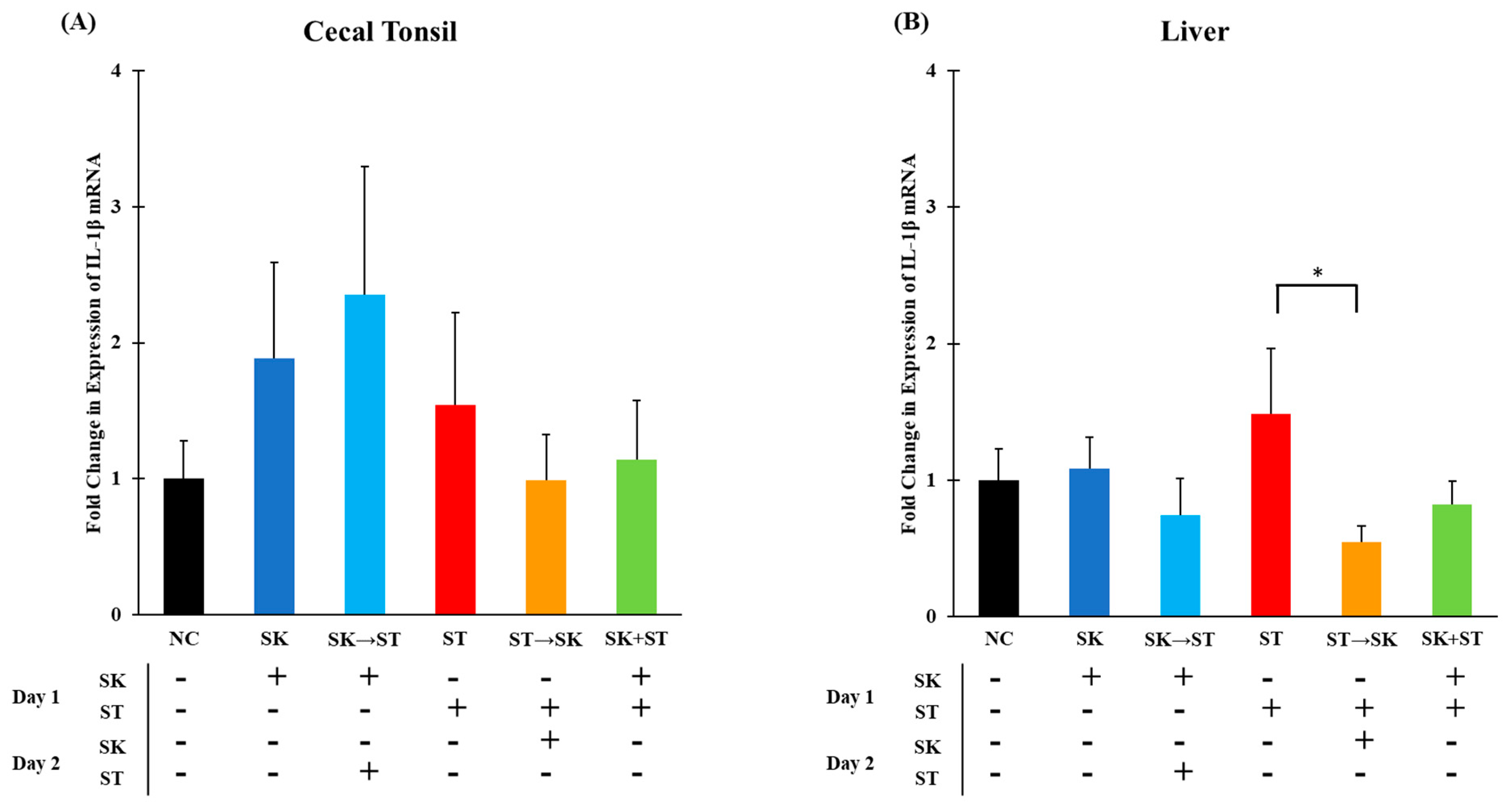
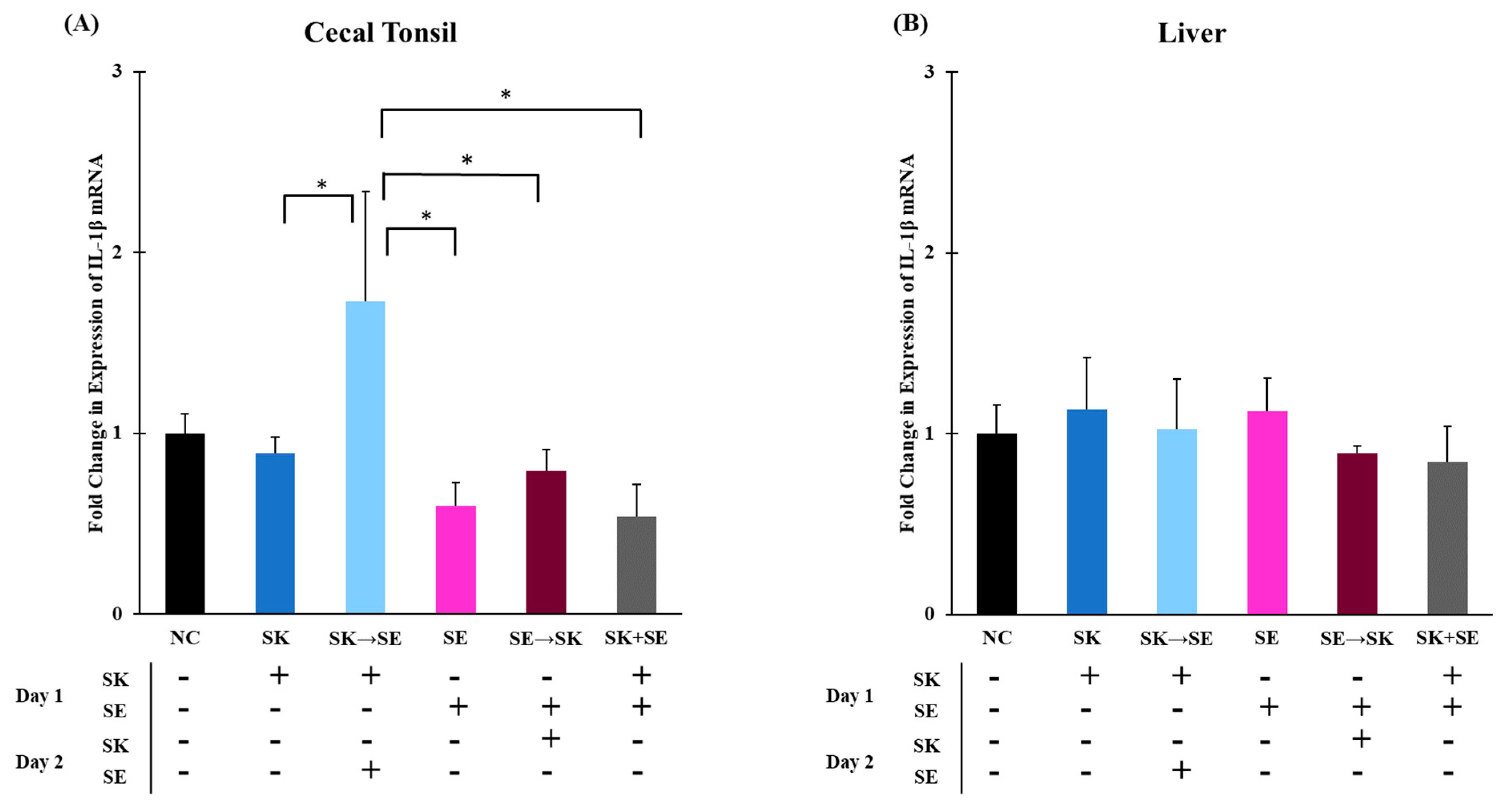
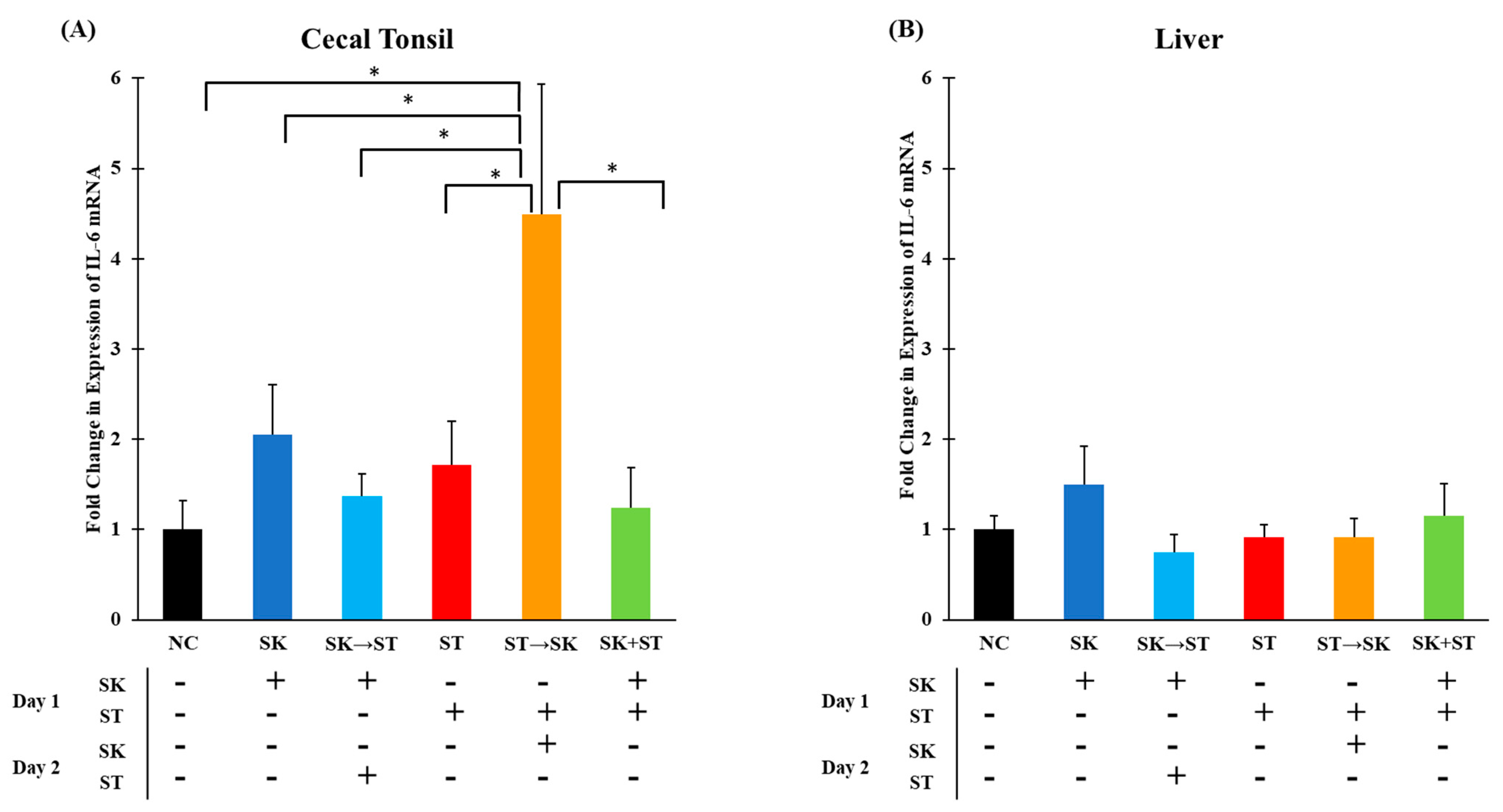
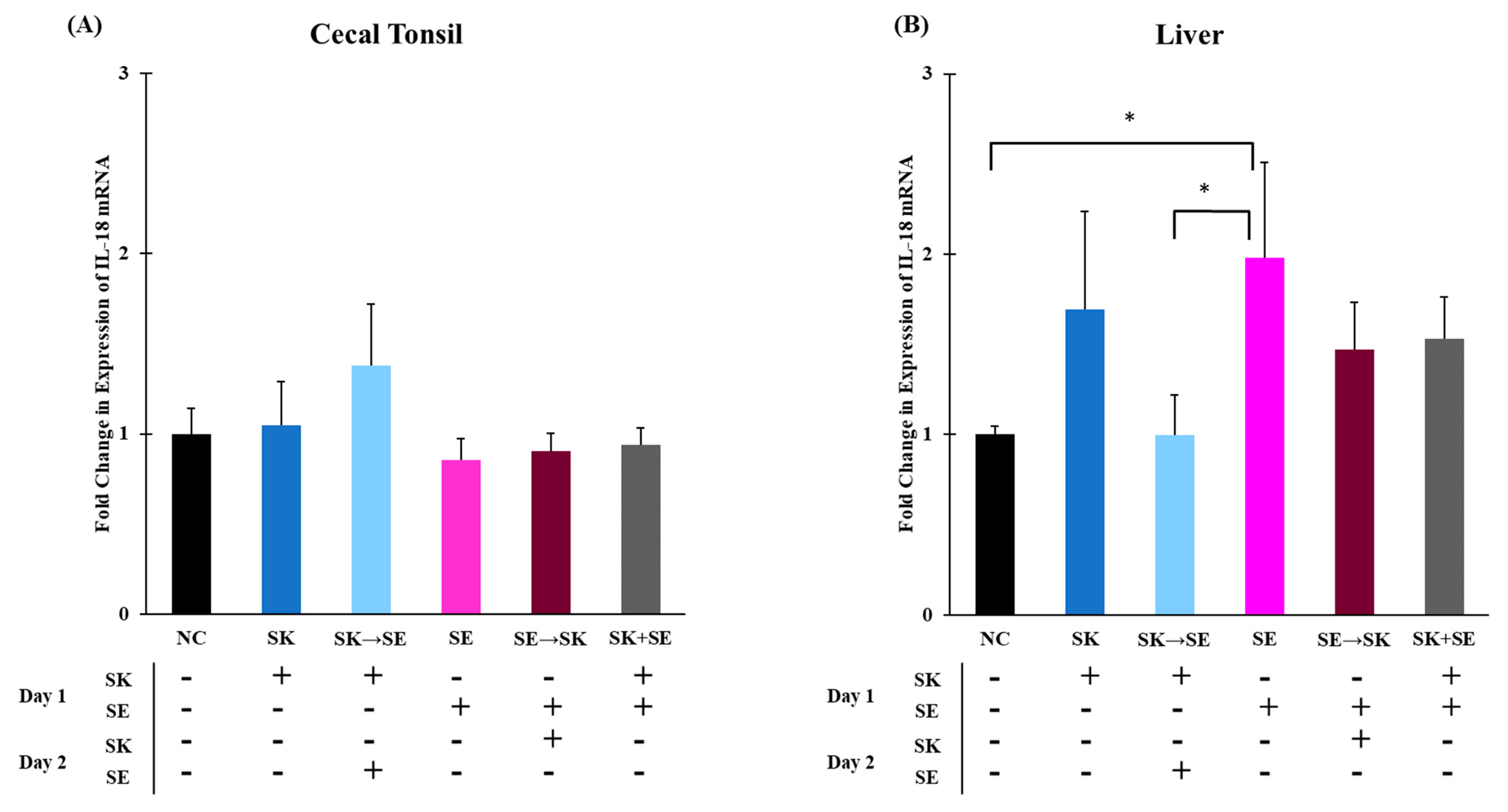
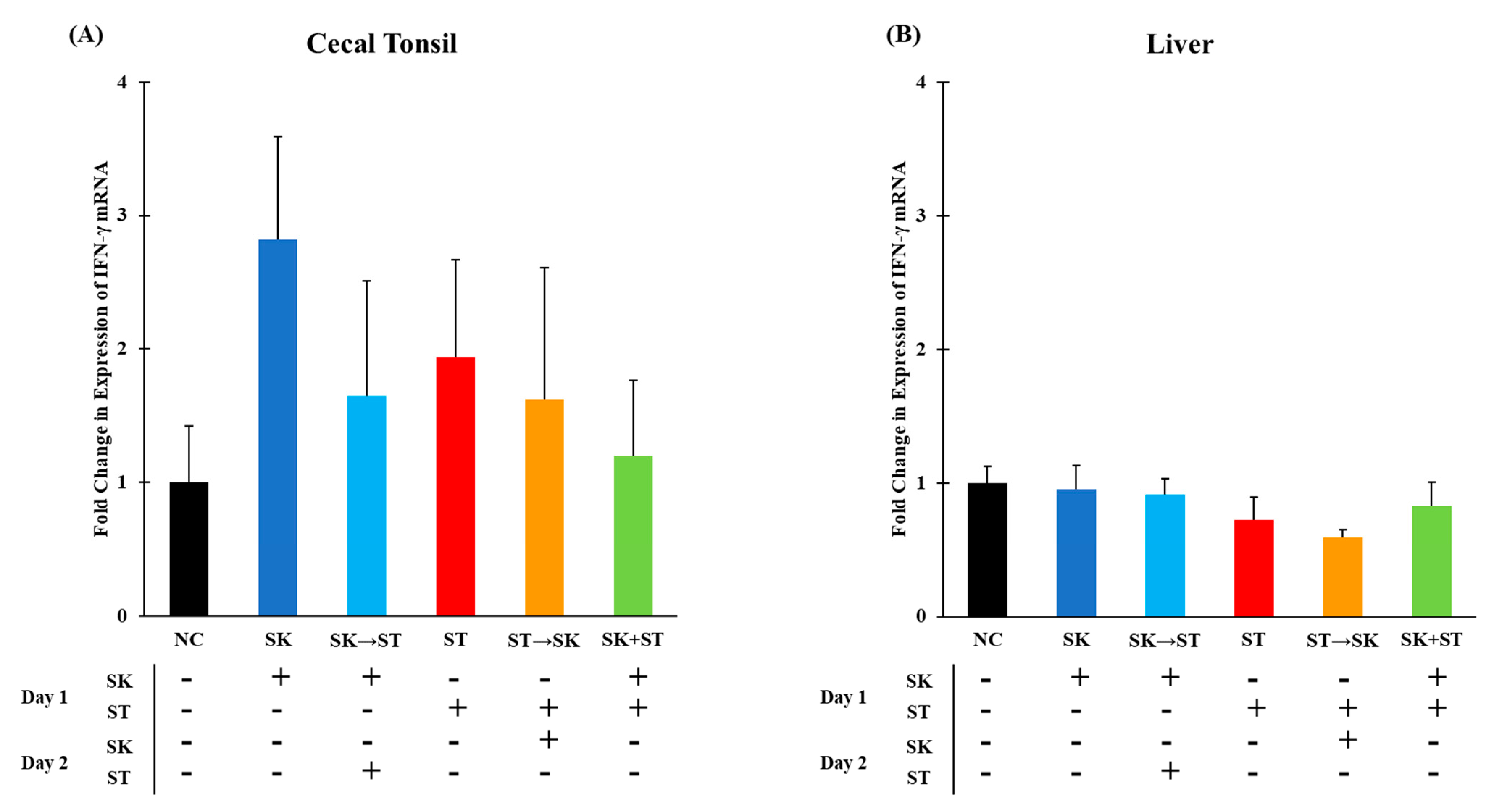
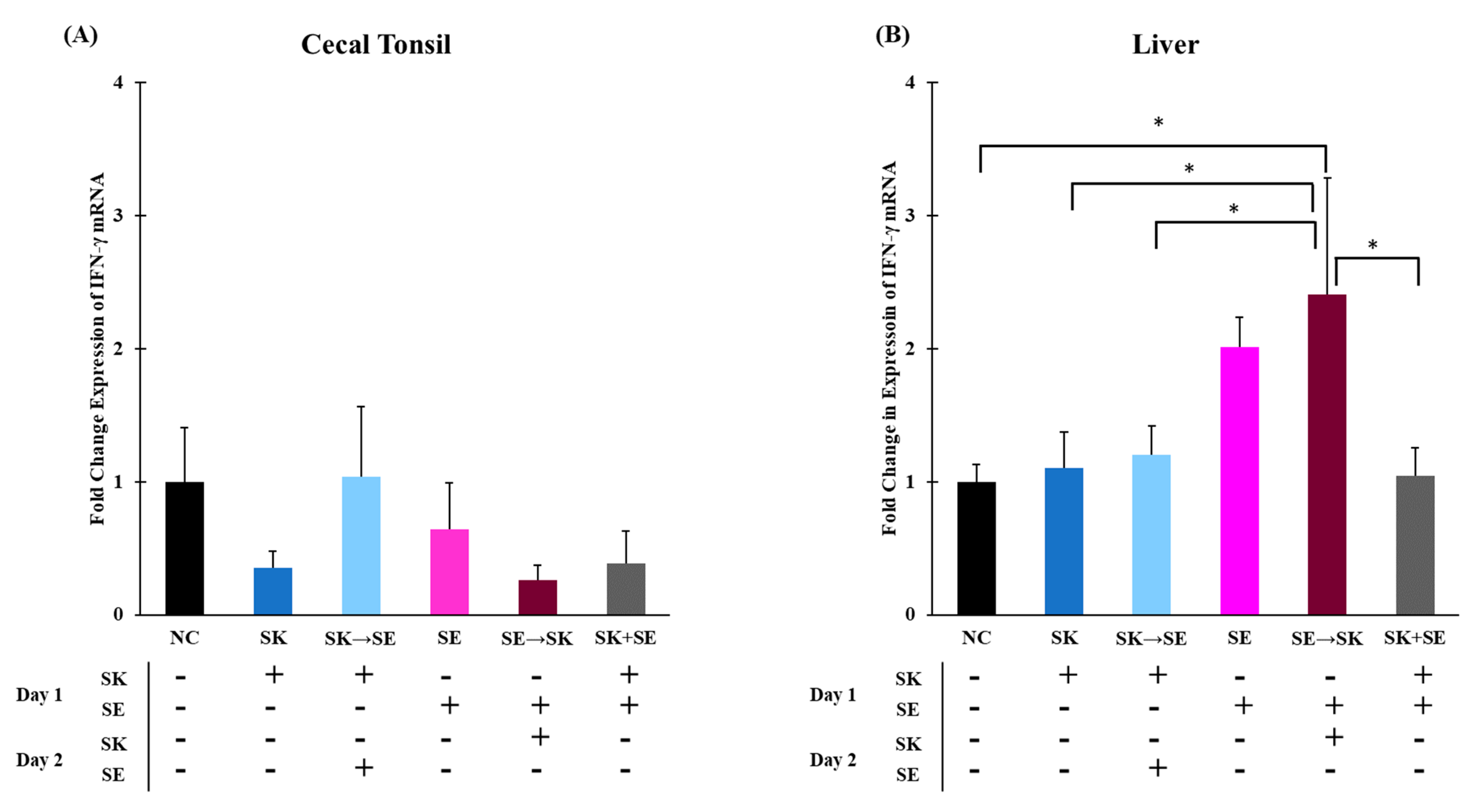
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boore, A.L.; Hoekstra, R.M.; Iwamoto, M.; Fields, P.I.; Bishop, R.D.; Swerdlow, D.L. Salmonella enterica Infections in the United States and Assessment of Coefficients of Variation: A Novel Approach to Identify Epidemiologic Characteristics of Individual Serotypes, 1996–2011. PLoS ONE 2015, 10, e0145416. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.L.; Johnson, T.J.; Ricke, S.C.; Nayak, R.; Danzeisen, J. Salmonella Pathogenicity and Host Adaptation in Chicken-Associated Serovars. Microbiol. Mol. Biol. Rev. 2013, 77, 582–607. [Google Scholar] [CrossRef] [PubMed]
- Food Safety and Inspection Service—United States Department of Agriculture. Serotypes Profile of Salmonella Isolates from Meat and Poultry Products, January 1998 through December 2014. Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/data-collection-and-reports/microbiology/annual-serotyping-reports (accessed on 13 February 2020).
- Van Immerseel, F.; Methner, U.; Rychlik, I.; Nagy, B.; Velge, P.; Martin, G.; Foster, N.; Ducatelle, R.; Barrow, P.A. Vaccination and early protection against non-host-specific Salmonella serotypes in poultry: Exploitation of innate immunity and microbial activity. Epidemiol. Infect. 2005, 133, 959–978. [Google Scholar] [CrossRef]
- Acheson, D.; Hohmann, E.L. Nontyphoidal Salmonellosis. Clin. Infect. Dis. 2001, 32, 263–269. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Brenner, F.W.; Villar, R.G.; Angulo, F.J.; Tauxe, R.; Swaminathan, B. Salmonella Nomenclature. J. Clin. Microbiol. 2000, 38, 2465–2467. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Enteric Disease Surveillance: Salmonella Annual Report. Available online: https://www.cdc.gov/nationalsurveillance/pdfs/2016-Salmonella-report-508.pdf (accessed on 13 February 2020).
- Gong, J.; Yu, H.; Liu, T.; Gill, J.; Chambers, J.; Wheatcroft, R.; Sabour, P. Effects of zinc bacitracin, bird age and access to range on bacterial microbiota in the ileum and caeca of broiler chickens. J. Appl. Microbiol. 2008, 104, 1372–1382. [Google Scholar] [CrossRef]
- Beal, R.K.; Powers, C.; Wigley, P.; Barrow, P.A.; Smith, A.L. Temporal dynamics of the cellular, humoral and cytokine responses in chickens during primary and secondary infection withSalmonella entericaserovar Typhimurium. Avian Pathol. 2004, 33, 25–33. [Google Scholar] [CrossRef]
- Withanage, G.S.K.; Wigley, P.; Kaiser, P.; Mastroeni, P.; Brooks, H.; Powers, C.; Beal, R.; Barrow, P.; Maskell, D.; McConnell, I. Cytokine and Chemokine Responses Associated with Clearance of a Primary Salmonella enterica Serovar Typhimurium Infection in the Chicken and in Protective Immunity to Rechallenge. Infect. Immun. 2005, 73, 5173–5182. [Google Scholar] [CrossRef] [PubMed]
- Setta, A.; Barrow, P.; Kaiser, P.; Jones, M. Early immune dynamics following infection with Salmonella enterica serovars Enteritidis, Infantis, Pullorum and Gallinarum: Cytokine and chemokine gene expression profile and cellular changes of chicken cecal tonsils. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 397–410. [Google Scholar] [CrossRef]
- Henderson, S.C.; Bounous, D.I.; Lee, M.D. Early Events in the Pathogenesis of Avian Salmonellosis. Infect. Immun. 1999, 67, 3580–3586. [Google Scholar] [CrossRef]
- Shao, Y.; Guo, Y.; Wang, Z. β-1,3/1,6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar Typhimurium. Poult. Sci. 2013, 92, 1764–1773. [Google Scholar] [CrossRef]
- Haraga, A.; Ohlson, M.B.; Miller, S.I. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 2008, 6, 53–66. [Google Scholar] [CrossRef]
- Tohidi, R.; Javanmard, A.; Idris, I. Immunogenetics applied to control salmonellosis in chicken: A review. J. Appl. Anim. Res. 2017, 46, 331–339. [Google Scholar] [CrossRef]
- Lawley, T.D.; Walker, A.W. Intestinal colonization resistance. Immunology 2012, 138, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Van Der Waaij, D.; Berghuis-de Vries, J.M.; Lekkerkerk-Van Der Wees, J.E.C. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. Epidemiol. Infect. 1971, 69, 405–411. [Google Scholar] [CrossRef]
- Kamada, N.; Chen, G.Y.; Inohara, N.; Núñez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013, 14, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.; Nurmi, E. Prevention of the growth ofSalmonella infantisin chicks by the flora of the alimentary tract of chickens. Br. Poult. Sci. 1973, 14, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Barrow, P.; Huggins, M.; Lovell, M.; Simpson, J. Observations on the pathogenesis of experimental Salmonella typhimurium infection in chickens. Res. Vet. Sci. 1987, 42, 194–199. [Google Scholar] [CrossRef]
- Methner, U.; Haase, A.; Berndt, A.; Martin, G.; Nagy, B.; Barrow, P.A. Exploitation of Intestinal Colonization-Inhibition Between Salmonella Organisms for Live Vaccines in Poultry—Potential and Limitations. Zoonoses Public Health 2011, 58, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tellez, G.; Latorre, J.D.; Ray, P.M.; Hernandez, X.; Hargis, B.M.; Ricke, S.C.; Kwon, Y.M. Salmonella Excludes Salmonella in Poultry: Confirming an Old Paradigm Using Conventional and Barcode-Tagging Approaches. Front. Vet. Sci. 2018, 5, 101. [Google Scholar] [CrossRef]
- Sassone-Corsi, M.; Raffatellu, M. No vacancy: How beneficial microbes cooperate with immunity to provide colonization resistance to pathogens. J. Immunol. 2015, 194, 4081–4087. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Tellez, G.I.; McGruder, E.D.; Hargis, B.M.; Williams, J.D.; Corrier, D.E.; Deloach, J.R. Heterophils are decisive components in the early responses of chickens to Salmonella enteritidis infections. Microb. Pathog. 1994, 16, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.S.; Kaiser, P.; Fife, M. The chicken IL-1 family: Evolution in the context of the studied vertebrate lineage. Immunogenetics 2014, 66, 427–438. [Google Scholar] [CrossRef]
- Akira, S. The role of IL-18 in innate immunity. Curr. Opin. Immunol. 2000, 12, 59–63. [Google Scholar] [CrossRef]
- Schneider, K.; Klaas, R.; Kaspers, B.; Staeheli, P. Chicken interleukin-6: cDNA structure and biological properties. JBIC J. Biol. Inorg. Chem. 2001, 268, 4200–4206. [Google Scholar] [CrossRef]
- Rahman, M.; Uyangaa, E.; Han, Y.W.; Kim, S.B.; Kim, J.H.; Choi, J.Y.; Eo, S.K.; Rahman, M. Oral co-administration of live attenuated Salmonella enterica serovar Typhimurium expressing chicken interferon-α and interleukin-18 enhances the alleviation of clinical signs caused by respiratory infection with avian influenza virus H9N2. Vet. Microbiol. 2012, 157, 448–455. [Google Scholar] [CrossRef]
- Göbel, T.W.; Schneider, K.; Schaerer, B.; Mejri, I.; Puehler, F.; Weigend, S.; Staeheli, P.; Kaspers, B. IL-18 Stimulates the Proliferation and IFN-γ Release of CD4+ T Cells in the Chicken: Conservation of a Th1-Like System in a Nonmammalian Species. J. Immunol. 2003, 171, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Klasing, K.C.; Peng, R.K. Influence of cell sources, stimulating agents, and incubation conditions on release of interleukin-1 from chicken macrophages. Dev. Comp. Immunol. 1987, 11, 385–394. [Google Scholar] [CrossRef]
- A Janeway, C. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol. Today 1992, 13, 11–16. [Google Scholar] [CrossRef]
- He, H.; Genovese, K.J.; Kogut, M.H. Modulation of chicken macrophage effector function by TH1/TH2 cytokines. Cytokine 2011, 53, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, L.; Kagnoff, M.F. Cytokines in host defense against Salmonella. Microbes Infect. 2001, 3, 1191–1200. [Google Scholar] [CrossRef]
- Kitagawa, H.; Shiraishi, S.; Imagawa, T.; Uehara, M. Ultrastructural characteristics and lectin-binding properties of M cells in the follicle-associated epithelium of chicken caecal tonsils. J. Anat. 2000, 197, 607–616. [Google Scholar] [CrossRef]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.; Eckersall, P. Acute phase proteins: A review of their function, behaviour and measurement in chickens. World’s Poult. Sci. J. 2014, 70, 27–44. [Google Scholar] [CrossRef]
- Withanage, G.S.K.; Kaiser, P.; Wigley, P.; Powers, C.; Mastroeni, P.; Brooks, H.; Barrow, P.; Smith, A.; Maskell, D.; McConnell, I. Rapid Expression of Chemokines and Proinflammatory Cytokines in Newly Hatched Chickens Infected with Salmonella enterica Serovar Typhimurium. Infect. Immun. 2004, 72, 2152–2159. [Google Scholar] [CrossRef]
- Fricke, W.F.; McDermott, P.F.; Mammel, M.K.; Zhao, S.; Johnson, T.J.; Rasko, D.A.; Fedorka-Cray, P.J.; Pedroso, A.; Whichard, J.M.; Leclerc, J.E.; et al. Antimicrobial Resistance-Conferring Plasmids with Similarity to Virulence Plasmids from Avian Pathogenic Escherichia coli Strains in Salmonella enterica Serovar Kentucky Isolates from Poultry. Appl. Environ. Microbiol. 2009, 75, 5963–5971. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Pedroso, A.A.; Porwollik, S.; McClelland, M.; Lee, M.D.; Kwan, T.; Zamperini, K.; Soni, V.; Sellers, H.S.; Russell, S.M.; et al. rpoS-Regulated Core Genes Involved in the Competitive Fitness of Salmonella enterica Serovar Kentucky in the Intestines of Chickens. Appl. Environ. Microbiol. 2014, 81, 502–514. [Google Scholar] [CrossRef]
- Nickerson, C.A.; Curtiss, R. Role of sigma factor RpoS in initial stages of Salmonella Typhimurium infection. Infect. Immun. 1997, 65, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Cobb-Vantress. Broiler Management Guide. 2018. Available online: https://www.cobb-vantress.com/assets/5c7576a214/Broilerguide-R1.pdf (accessed on 4 January 2021).
- Cobb-Vantress. Cobb700 Broiler Performance and Nutrition Supplement. 2018. Available online: https://www.cobb-vantress.com/assets/Cobb-Files/c7c812114a/Cobb700_Broiler_Supplement.pdf (accessed on 4 January 2021).
- Kogut, M.H.; McGruder, E.D.; Hargis, B.M.; E Corrier, D.; Deloach, J.R.; Corner, D.E. In vivo activation of heterophil function in chickens following injection with Salmonella enteritidis -immune lymphokines. J. Leukoc. Biol. 1995, 57, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.A.; Deloach, J.R.; Corrier, D.E.; Nisbet, D.J.; Stanker, L.H. Evaluation of Salmonella Serotype Distributions from Commercial Broiler Hatcheries and Grower Houses. Avian Dis. 1999, 43, 39–47. [Google Scholar] [CrossRef]
- Byrd, J.A.; Corrier, D.E.; Deloach, J.R.; Nisbet, D.J.; Stanker, L.H. Horizontal Transmission of Salmonella typhimurium in Broiler Chicks. J. Appl. Poult. Res. 1998, 7, 75–80. [Google Scholar] [CrossRef]
- Zhao, D.; Kogut, M.H.; Genovese, K.J.; Hsu, C.-Y.; Lee, J.T.; Farnell, Y.Z. Altered expression of lactate dehydrogenase and monocarboxylate transporter involved in lactate metabolism in broiler wooden breast. Poult. Sci. 2020, 99, 11–20. [Google Scholar] [CrossRef]
- Hong, Y.H.; Lillehoj, H.S.; Lillehoj, E.P.; Lee, S.H. Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Vet. Immunol. Immunopathol. 2006, 114, 259–272. [Google Scholar] [CrossRef]
- Khampeerathuch, T.; Mudsak, A.; Srikok, S.; Vannamahaxay, S.; Chotinun, S.; Chuammitri, P. Differential gene expression in heterophils isolated from commercial hybrid and Thai indigenous broiler chickens under quercetin supplementation. J. Appl. Anim. Res. 2017, 46, 804–812. [Google Scholar] [CrossRef]
- Kumar, S.; Buza, J.J.; Burgess, S.C. Genotype-Dependent Tumor Regression in Marek’s Disease Mediated at the Level of Tumor Immunity. Cancer Microenviron. 2009, 2, 23–31. [Google Scholar] [CrossRef][Green Version]
- Markazi, A.; Luoma, A.; Shanmugasundaram, R.; Mohnl, M.; Murugesan, G.R.; Selvaraj, R. Effects of drinking water synbiotic supplementation in laying hens challenged with Salmonella. Poult. Sci. 2018, 97, 3510–3518. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Philbin, V.J.; Smith, A.L. Expression patterns of chicken Toll-like receptor mRNA in tissues, immune cell subsets and cell lines. Vet. Immunol. Immunopathol. 2005, 104, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Careem, M.; Hunter, B.; Sarson, A.; Mayameei, A.; Zhou, H.; Sharif, S. Marek’s Disease Virus–Induced Transient Paralysis Is Associated with Cytokine Gene Expression in the Nervous System. Viral Immunol. 2006, 19, 167–176. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Pamer, E.G. Interbacterial mechanisms of colonization resistance and the strategies pathogens use to overcome them. Mucosal Immunol. 2019, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barrow, P.A.; Tucker, J.F.; Simpson, J.M. Inhibition of colonization of the chicken alimentary tract with Salmonella typhimurium gram-negative facultatively anaerobic bacteria. Epidemiol. Infect. 1987, 98, 311–322. [Google Scholar] [CrossRef]
- Foster, N.; Lovell, M.A.; Marston, K.L.; Hulme, S.D.; Frost, A.J.; Bland, P.; Barrow, P.A. Rapid Protection of Gnotobiotic Pigs against Experimental Salmonellosis following Induction of Polymorphonuclear Leukocytes by Avirulent Salmonella enterica. Infect. Immun. 2003, 71, 2182–2191. [Google Scholar] [CrossRef]
- Van Immerseel, F.; De Buck, J.; De Smet, I.; Mast, J.; Haesebrouck, F.; Ducatelle, R. Dynamics of immune cell infiltration in the caecal lamina propria of chickens after neonatal infection with a Salmonella Enteritidis strain. Dev. Comp. Immunol. 2002, 26, 355–364. [Google Scholar] [CrossRef]
- Akbari, M.R.; Haghighi, H.R.; Chambers, J.R.; Brisbin, J.; Read, L.R.; Sharif, S. Expression of Antimicrobial Peptides in Cecal Tonsils of Chickens Treated with Probiotics and Infected with Salmonella enterica Serovar Typhimurium. Clin. Vaccine Immunol. 2008, 15, 1689–1693. [Google Scholar] [CrossRef]
- Michailidis, G. Expression of chicken LEAP-2 in the reproductive organs and embryos and in response to Salmonella enterica infection. Vet. Res. Commun. 2010, 34, 459–471. [Google Scholar] [CrossRef]
- Cuperus, T.; Coorens, M.; Van Dijk, A.; Haagsman, H.P. Avian host defense peptides. Dev. Comp. Immunol. 2013, 41, 352–369. [Google Scholar] [CrossRef] [PubMed]
- Gast, R.K. Serotype-Specific and Serotype-Independent Strategies for Preharvest Control of Food-Borne Salmonella in Poultry. Avian Dis. 2007, 51, 817–828. [Google Scholar] [CrossRef]
- He, H.; Genovese, K.J.; Swaggerty, C.L.; Nisbet, D.J.; Kogut, M.H. A Comparative Study on Invasion, Survival, Modulation of Oxidative Burst, and Nitric Oxide Responses of Macrophages (HD11), and Systemic Infection in Chickens by Prevalent Poultry Salmonella Serovars. Foodborne Pathog. Dis. 2012, 9, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Gast, R.K.; Beard, C. Age-Related Changes in the Persistence and Pathogenicity of Salmonella typhimurium in Chicks. Poult. Sci. 1989, 68, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.K.; Lovell, M.A.; Hulme, S.D.; Zhang-Barber, L.; Barrow, P.A. Identification of Salmonella typhimuriumGenes Required for Colonization of the Chicken Alimentary Tract and for Virulence in Newly Hatched Chicks. Infect. Immun. 1998, 66, 2099–2106. [Google Scholar] [CrossRef]
- Uni, Z.; Geyra, A.; Ben-Hur, H.; Sklan, D. Small intestinal development in the young chick: Crypt formation and enterocyte proliferation and migration. Br. Poult. Sci. 2000, 41, 544–551. [Google Scholar] [CrossRef]
- Geyra, A.; Uni, Z.; Sklan, D. Enterocyte Dynamics and Mucosal Development in the Posthatch Chick. Poult. Sci. 2001, 80, 776–782. [Google Scholar] [CrossRef]
- Fasina, Y.O.; Holt, P.S.; Moran, E.T.; Moore, R.W.; Conner, D.E.; McKee, S.R. Intestinal Cytokine Response of Commercial Source Broiler Chicks to Salmonella Typhimurium Infection. Poult. Sci. 2008, 87, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Crhanova, M.; Hradecka, H.; Faldynova, M.; Matulova, M.; Havlickova, H.; Sisak, F.; Rychlik, I. Immune Response of Chicken Gut to Natural Colonization by Gut Microflora and to Salmonella enterica Serovar Enteritidis Infection. Infect. Immun. 2011, 79, 2755–2763. [Google Scholar] [CrossRef]
- Nishimichi, N.; Kawashima, T.; Hojyo, S.; Horiuchi, H.; Furusawa, S.; Matsuda, H. Characterization and expression analysis of a chicken interleukin-6 receptor alpha. Dev. Comp. Immunol. 2006, 30, 419–429. [Google Scholar] [CrossRef]
- Millet, S.; Bennett, J.; Lee, K.A.; Hau, M.; Klasing, K.C. Quantifying and comparing constitutive immunity across avian species. Dev. Comp. Immunol. 2007, 31, 188–201. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Zlotnik, A.; Mosmann, T.R.; Howard, M.; O’Garra, A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991, 147, 3815–3822. [Google Scholar]
- Rothwell, L.; Young, J.R.; Zoorob, R.; Whittaker, C.A.; Hesketh, P.; Archer, A.; Smith, A.L.; Kaiser, P. Cloning and Characterization of Chicken IL-10 and Its Role in the Immune Response toEimeria maxima. J. Immunol. 2004, 173, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2003, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Rothwell, L.; Kaiser, P. IFN-γ Priming of Chicken Heterophils Upregulates the Expression of Proinflammatory and Th1 Cytokine mRNA Following Receptor-Mediated Phagocytosis of Salmonella entericaserovarenteritidis. J. Interf. Cytokine Res. 2005, 25, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Kagaya, K.; Watanabe, K.; Fukazawa, Y. Capacity of recombinant gamma interferon to activate macrophages for Salmonella-killing activity. Infect. Immun. 1989, 57, 609–615. [Google Scholar] [CrossRef]
- Kaiser, P.; Stäheli, P. Avian cytokines and chemokines. In Avian Immunology, 2nd ed.; Schat, K., Kaspers, B., Kaiser, P., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 1, Chapter 1; pp. 189–204. [Google Scholar] [CrossRef]
- Dar, M.A.; Urwat, U.; Ahmad, S.M.; Ahmad, R.; Kashoo, Z.A.; Dar, T.A.; Bhat, S.A.; Mumtaz, P.T.; Shabir, N.; Shah, R.A.; et al. Gene expression and antibody response in chicken against Salmonella Typhimurium challenge. Poult. Sci. 2019, 98, 2008–2013. [Google Scholar] [CrossRef]
- Smith, A.L.; Powers, C.; Beal, R.K. The Avian Enteric Immune System in Health and Disease. In Avian Immunology, 2nd ed.; Schat, K., Kaspers, B., Kaiser, P., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 1, Chapter 13; pp. 227–250. [Google Scholar] [CrossRef]
- Juul-Madsen, H.R.; Viertlboeck, B.C.; Härtle, S.; Smith, A.L.; Göbel, T.W. Innate Immune Responses. In Avian Immunology, 2nd ed.; Schat, K., Kaspers, B., Kaiser, P., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 1, Chapter 7; pp. 121–147. [Google Scholar] [CrossRef]
- Cheeseman, J.H.; Kaiser, M.G.; Ciraci, C.; Kaiser, P.; Lamont, S.J. Breed effect on early cytokine mRNA expression in spleen and cecum of chickens with and without Salmonella enteritidis infection. Dev. Comp. Immunol. 2007, 31, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.; Rothwell, L.; Galyov, E.E.; Barrow, P.A.; Burnside, J.; Wigley, P. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum The GenBank accession numbers for the sequences reported in this paper are AI982185 for chicken IL-6 cDNA and AJ250838 for the partial chicken IL-6 genomic sequence, respectively. Microbiology 2000, 146, 3217–3226. [Google Scholar] [CrossRef] [PubMed]
- Buffie, C.G.; Pamer, E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef]
- Phalipon, A.; Sansonetti, P.J. Microbial-Host Interactions at Mucosal Sites. Host Response to Pathogenic Bacteria at Mucosal Sites. In Defense of Mucosal Surfaces: Pathogenesis, Immunity and Vaccines, 1st ed.; Kraehenbuhl, J.P., Neutra, M.R., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 1, pp. 163–189. [Google Scholar] [CrossRef]




| Bacteria | Strain | Source of Strain | Reference |
|---|---|---|---|
| S. Enteritidis | Primary poultry isolate, #97-11771 | National Veterinary Services Laboratory Ames, Iowa, USA | [44] |
| S. Kentucky | Broiler field isolate | Southern USA Farm | [45] |
| S. Typhimurium | Primary poultry isolate | National Veterinary Services Laboratory Ames, Iowa, USA | [46] |
| Treatment | NC | SK | SK → ST | ST | ST → SK | SK + ST |
|---|---|---|---|---|---|---|
| D0 | Place chicks | |||||
| D1 (Challenge) | PBS | 104 CFU SK | 104 CFU SK | 104 CFU ST | 104 CFU ST | 104 CFU SK + ST |
| D2 (Challenge) | PBS | PBS | 105 CFU ST | PBS | 105 CFU SK | PBS |
| D3 (Kill) | Collect ceca, liver, and spleen tissues. | |||||
| Treatment | NC | SK | SK → SE | SE | SE → SK | SK + SE |
|---|---|---|---|---|---|---|
| D0 | Place chicks | |||||
| D1 (Challenge) | PBS | 104 CFU SK | 104 CFU SK | 104 CFU SE | 104 CFU SE | 104 CFU SK + SE |
| D2 (Challenge) | PBS | PBS | 105 CFU SE | PBS | 105 CFU SK | PBS |
| D3 (Kill) | Collect ceca, liver, and spleen tissues. | |||||
| Treatment 2 | SK Ceca Log10(cfu/g) | SK Ceca Incidence | SK L/S Incidence | ST Ceca Log10(cfu/g) | ST Ceca Incidence | ST L/S Incidence |
|---|---|---|---|---|---|---|
| NC | 0.00 | 0/20 | 0/20 | 0.00 | 0/20 | 0/20 |
| SK PC | 5.36 | 20/20 | 2/20 | 0.00 | 0/20 | 0/20 |
| SK → ST | 4.69 1 | 20/20 | 1/20 | 0.00 1 | 0/20 | 0/20 |
| ST PC | 0.00 | 0/20 | 0/20 | 4.90 | 20/20 | 3/20 |
| ST → SK | 4.40 1 | 20/20 | 1/20 | 5.35 | 20/20 | 0/20 |
| SK + ST | 5.58 | 20/20 | 5/20 1 | 3.74 1 | 20/20 | 2/20 |
| Treatment 2 | SK Ceca Log10(cfu/g) | SK Ceca Incidence | SK L/S Incidence | ST Ceca Log10(cfu/g) | ST Ceca Incidence | ST L/S Incidence |
|---|---|---|---|---|---|---|
| NC | 0.00 | 0/20 | 0/20 | 0.00 | 0/20 | 0/20 |
| SK PC | 5.24 | 20/20 | 1/20 | 0.00 | 0/20 | 0/20 |
| SK → ST | 5.20 | 20/20 | 3/20 | 2.23 1 | 20/20 | 0/20 |
| ST PC | 0.00 | 0/20 | 0/20 | 4.79 | 20/20 | 1/20 |
| ST → SK | 4.60 1 | 20/20 | 9/20 1 | 5.02 | 20/20 | 9/20 1 |
| SK + ST | 4.79 1 | 20/20 | 4/20 | 3.75 1 | 20/20 | 2/20 |
| Treatment 2 | SK Ceca Log10(cfu/g) | SK Ceca Incidence | SK L/S Incidence | SE Ceca Log10(cfu/g) | SE Ceca Incidence | SE L/S Incidence |
|---|---|---|---|---|---|---|
| NC | 0.00 | 0/20 | 0/20 | 0.00 | 0/20 | 0/20 |
| SK PC | 5.94 | 20/20 | 7/20 1 | 0.00 | 0/20 | 0/20 |
| SK → SE | 5.07 1 | 20/20 | 2/20 | 2.89 1 | 20/20 | 0/20 |
| SE PC | 0.00 | 0/20 | 0/20 | 4.52 | 20/20 | 0/20 |
| SE → SK | 3.90 1 | 20/20 | 0/20 | 4.21 | 20/20 | 0/20 |
| SK + SE | 5.40 | 20/20 | 2/20 | 3.92 1 | 20/20 | 2/20 |
| Treatment 2 | SK Ceca Log10(cfu/g) | SK Ceca Incidence | SK L/S Incidence | SE Ceca Log10(cfu/g) | SE Ceca Incidence | SE L/S Incidence |
|---|---|---|---|---|---|---|
| NC | 0.00 | 0/20 | 0/20 | 0.00 | 0/20 | 0/20 |
| SK PC | 6.74 | 20/20 | 3/20 | 0.00 | 0/20 | 0/20 |
| SK → SE | 6.62 | 20/20 | 1/20 | 2.48 1 | 20/20 | 0/20 |
| SE PC | 0.00 | 0/20 | 0/20 | 5.10 | 20/20 | 0/20 |
| SE → SK | 3.97 1 | 20/20 | 2/20 | 5.20 | 20/20 | 0/20 |
| SK + SE | 6.33 | 20/20 | 1/20 | 3.98 1 | 20/20 | 0/20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda, M.; Kogut, M.; Genovese, K.; Farnell, Y.Z.; Zhao, D.; Wang, X.; Milby, A.; Farnell, M. Competitive Exclusion of Intra-Genus Salmonella in Neonatal Broilers. Microorganisms 2021, 9, 446. https://doi.org/10.3390/microorganisms9020446
Pineda M, Kogut M, Genovese K, Farnell YZ, Zhao D, Wang X, Milby A, Farnell M. Competitive Exclusion of Intra-Genus Salmonella in Neonatal Broilers. Microorganisms. 2021; 9(2):446. https://doi.org/10.3390/microorganisms9020446
Chicago/Turabian StylePineda, Megan, Michael Kogut, Kenneth Genovese, Yuhua Z. Farnell, Dan Zhao, Xi Wang, Allison Milby, and Morgan Farnell. 2021. "Competitive Exclusion of Intra-Genus Salmonella in Neonatal Broilers" Microorganisms 9, no. 2: 446. https://doi.org/10.3390/microorganisms9020446
APA StylePineda, M., Kogut, M., Genovese, K., Farnell, Y. Z., Zhao, D., Wang, X., Milby, A., & Farnell, M. (2021). Competitive Exclusion of Intra-Genus Salmonella in Neonatal Broilers. Microorganisms, 9(2), 446. https://doi.org/10.3390/microorganisms9020446









