Mineralosphere Microbiome Leading to Changed Geochemical Properties of Sedimentary Rocks from Aiqigou Mud Volcano, Northwest China
Abstract
:1. Introduction
2. Materials and Methods
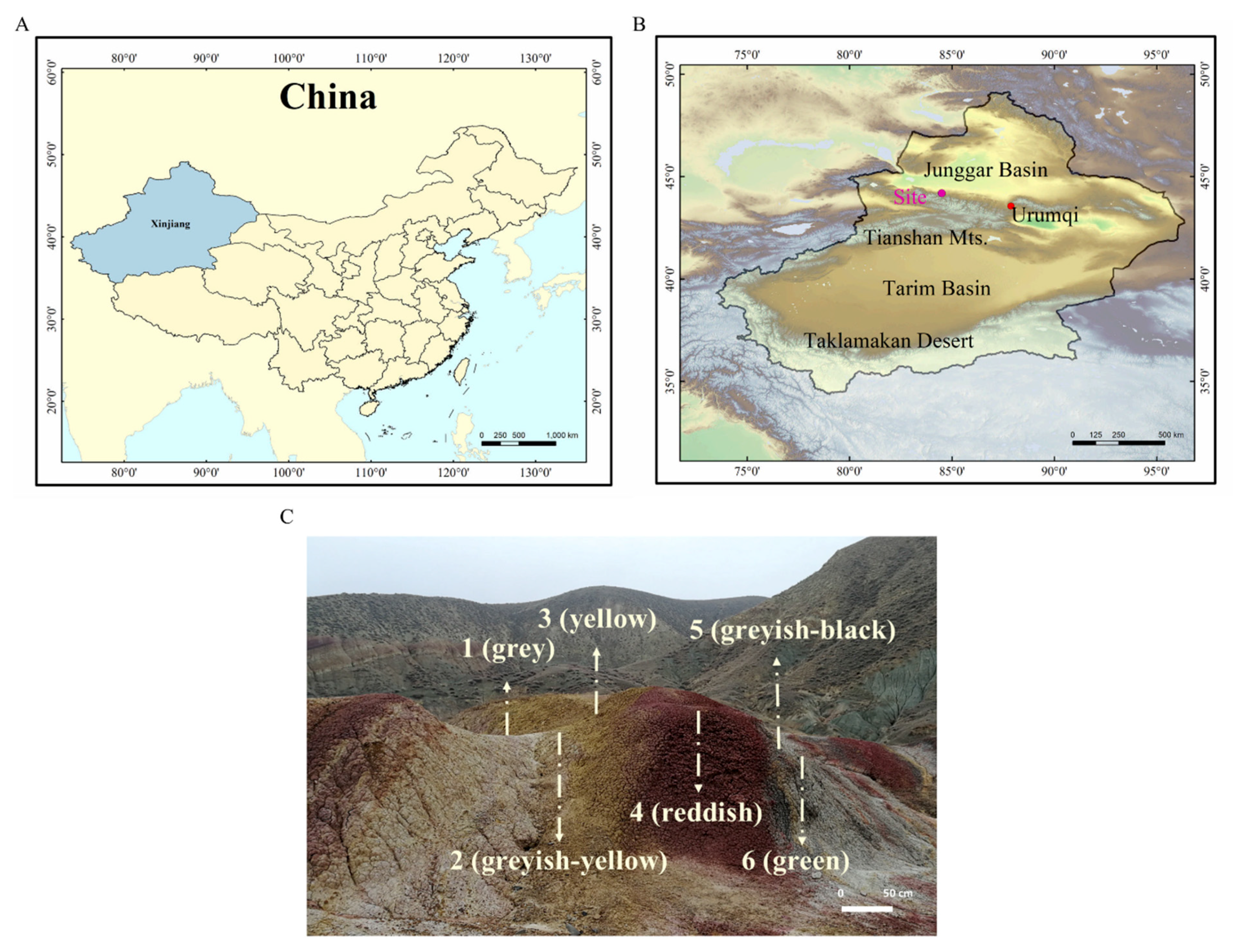
2.1. Site Description and Sample Collection
2.2. Rock Physicochemical Analyses
2.3. Enrichments
2.4. DNA Extraction, Amplification, and High Throughput Sequencing of the 16S rRNA Gene
2.5. Quantitative PCR
- (1)
- The primers were the same as above, and the sequences were synthesized by Sangon Biotech. Takara TaqTM Hot Start Version (Takara, Dalian, China) was selected as the system. The reaction system was 25 μL: 1 μL forward primer (10 μM), 1 μL reverse primer (10 μM), 1 μL DNA (10–40 ng μL−1), 0.2 μL Takara Taq HS, 2.5 μL 10× PCR Buffer, 1.5 μL dNTP mixture and the remaining volume was replenished with sterile deionized water. The procedure recommended in the instructions was used for the reaction, and the PCR products obtained after amplification were verified by 1.5% agarose gel electrophoresis and then recycled by gel cutting by AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Tewksbury, MA, USA).
- (2)
- The purified PCR products were sequenced and cloned into the pGEM-T Easy Vector (Promega, Madison, WI, USA), and then transformed into the Escherichia coli DH5α (TIANGEN, Beijing, China) [19]. The obtained strains were verified by PCR and sequencing, and the DNA length was calculated by BioEdit.
- (3)
- The plasmids with target genes were extracted with TIANprep Mini Plasmid Kit (TIANGEN Biotech, Beijing, China), then the Plasmid DNA was used NanoDrop 2000 (Thermo scientific, Wilmington, DE, USA) to determine the purity and concentration. A260/280 between 1.8–2.0 indicates that the plasmid is of good quality and can be used in the production of standard curves. According to the concentration, the copy number of the plasmid can be calculated according to Equation (1):
2.6. Data and Statistical Analyses
3. Results
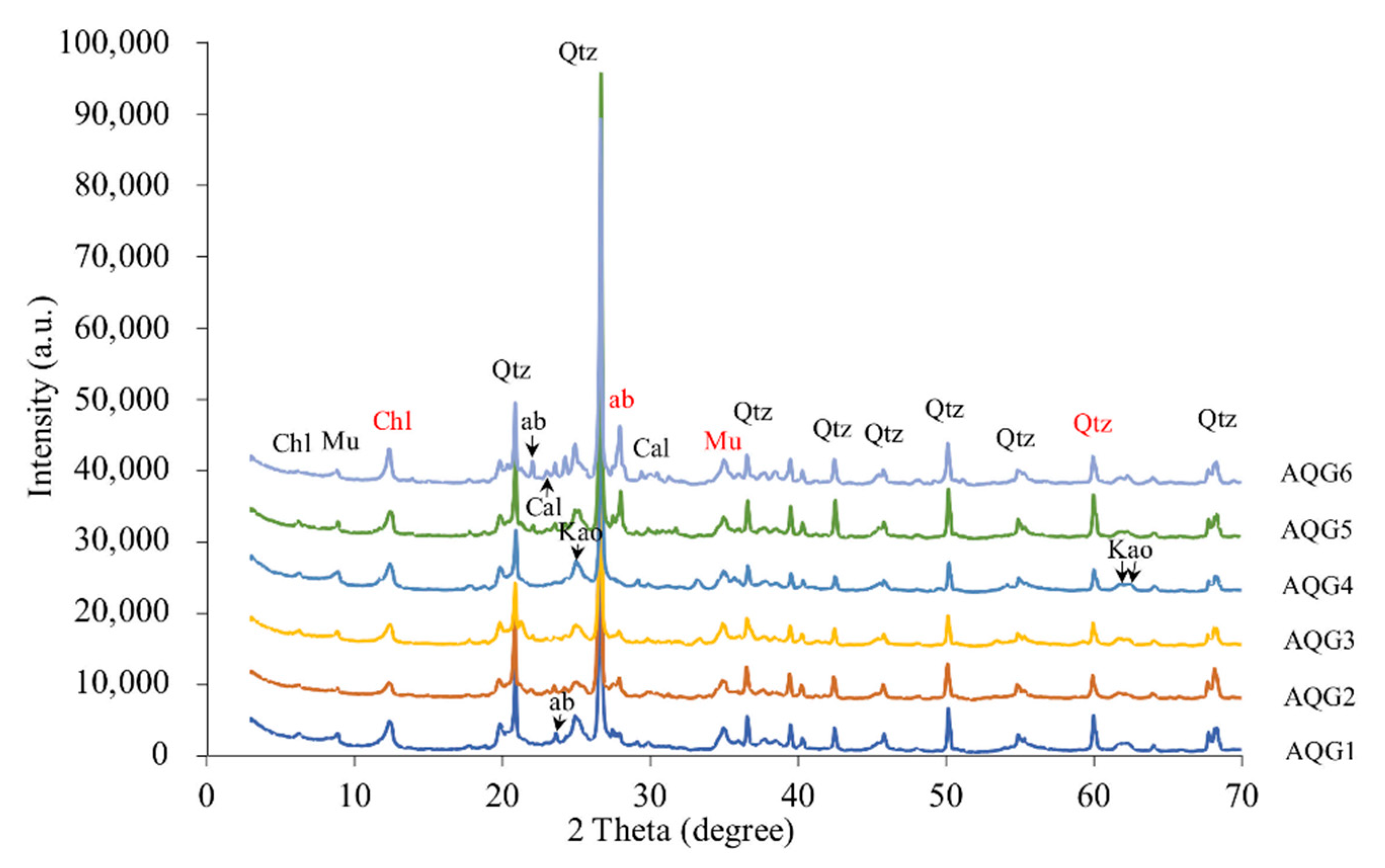
3.1. Mineralogical Compositions
3.2. Chemical Composition of Major Elements
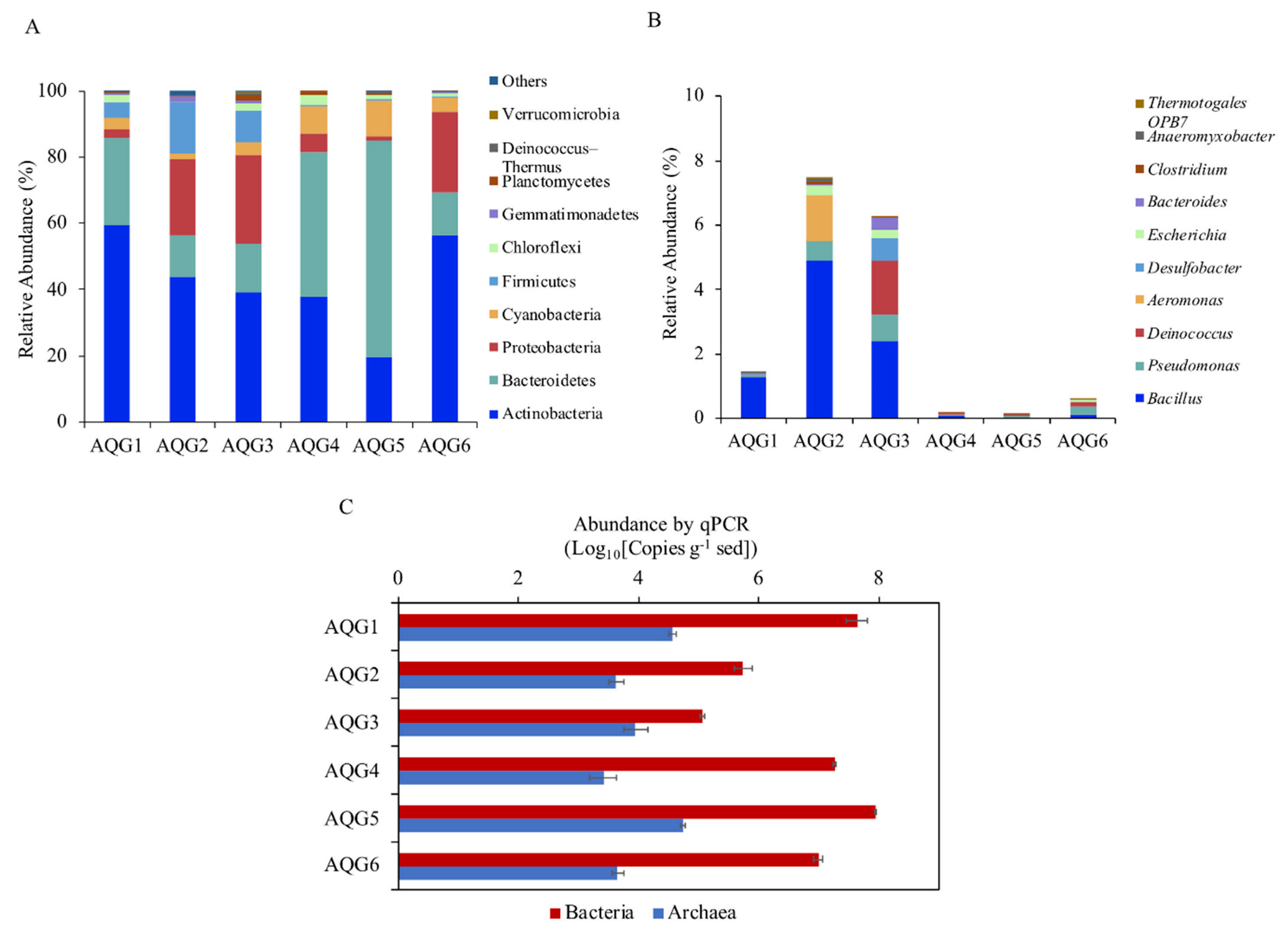
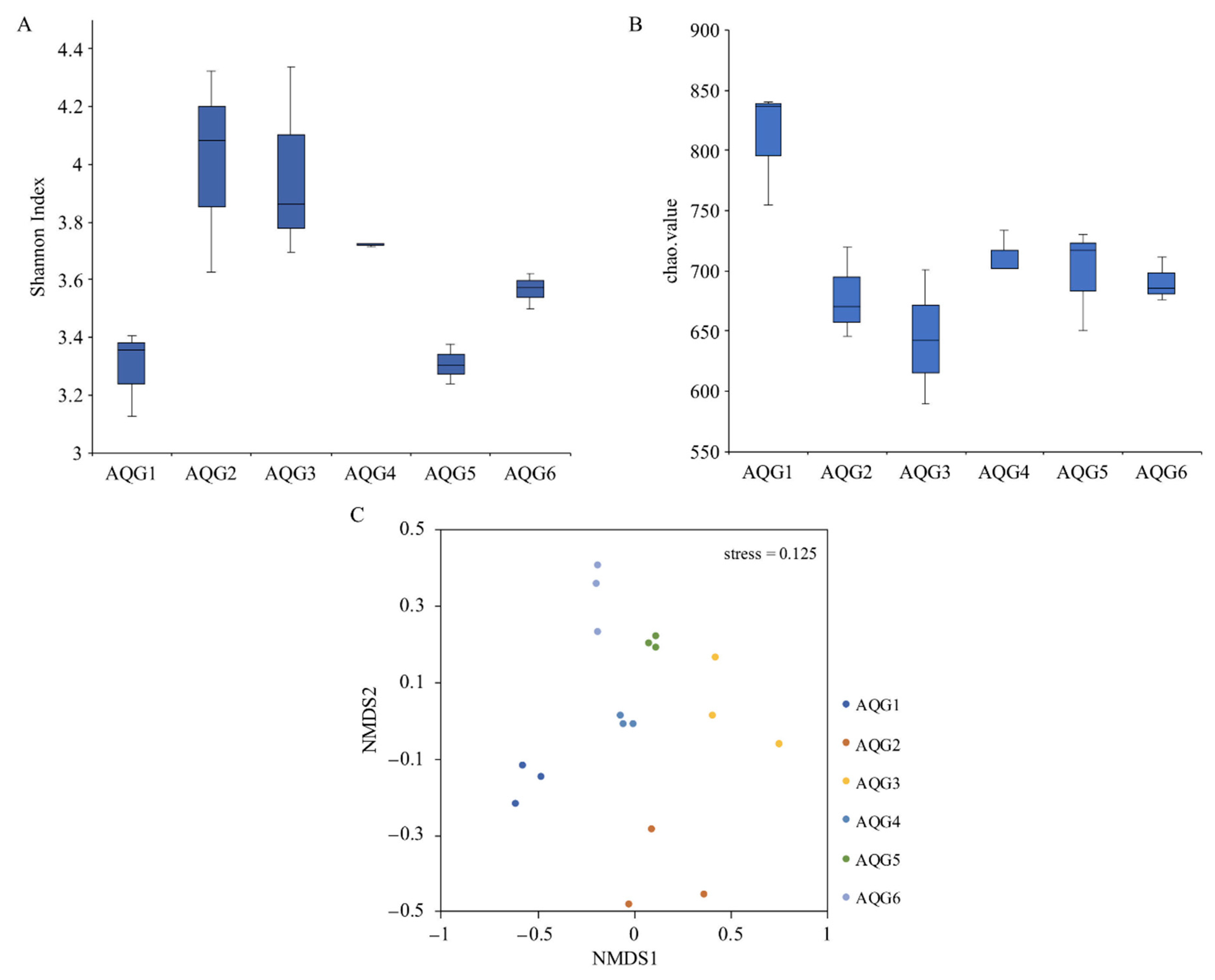
3.3. Microbial Communities in Different Rock Layers
3.4. Environmental Factors Associated with Microbial Community Structure
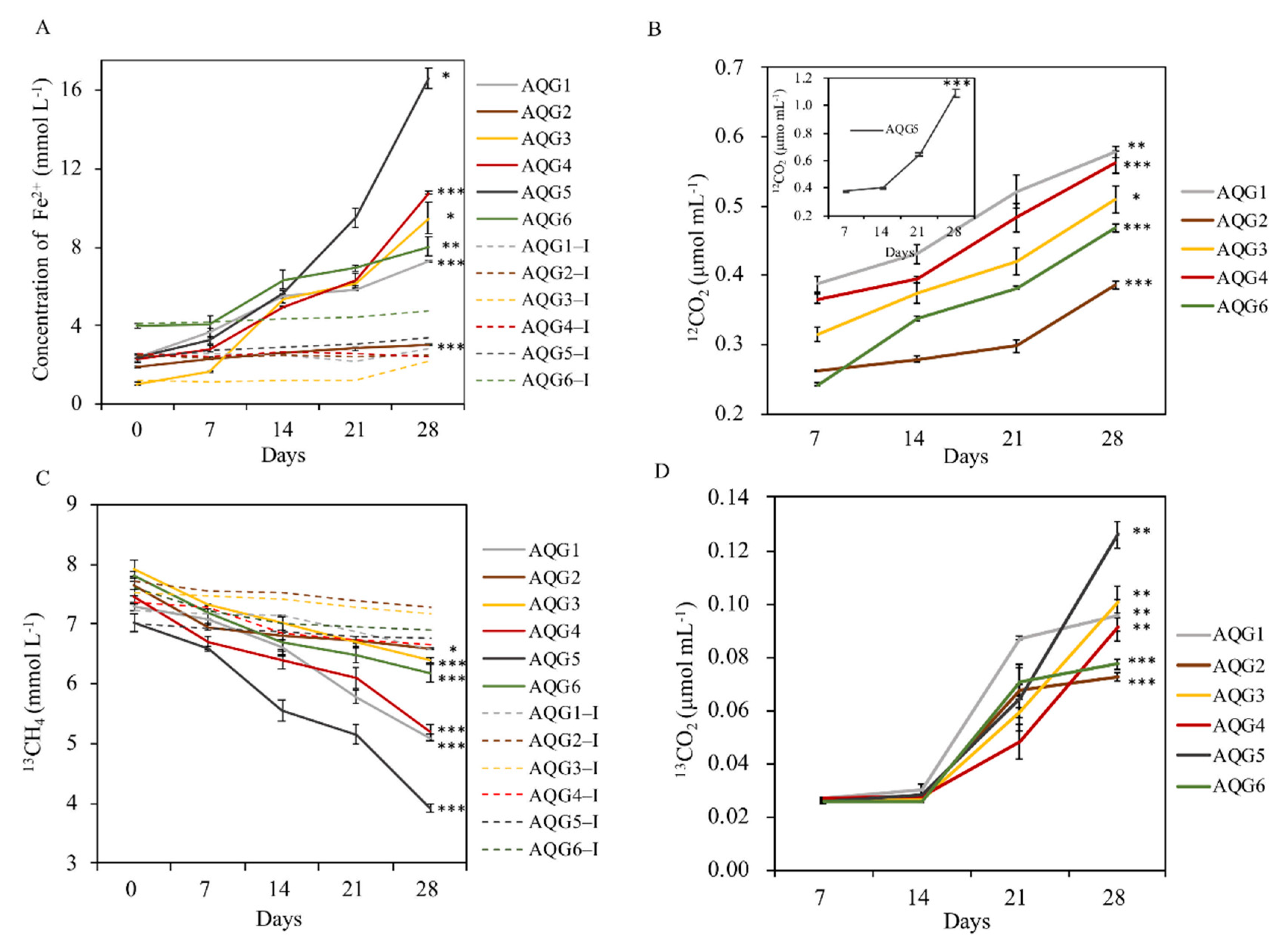
3.5. Biogeochemical Process of Hydrocarbon and Iron in Enrichments
4. Discussion
4.1. Geochemical Properties of AQG Terrestrial Rocks
4.2. Microbial Communities of AQG Terrestrial Rocks
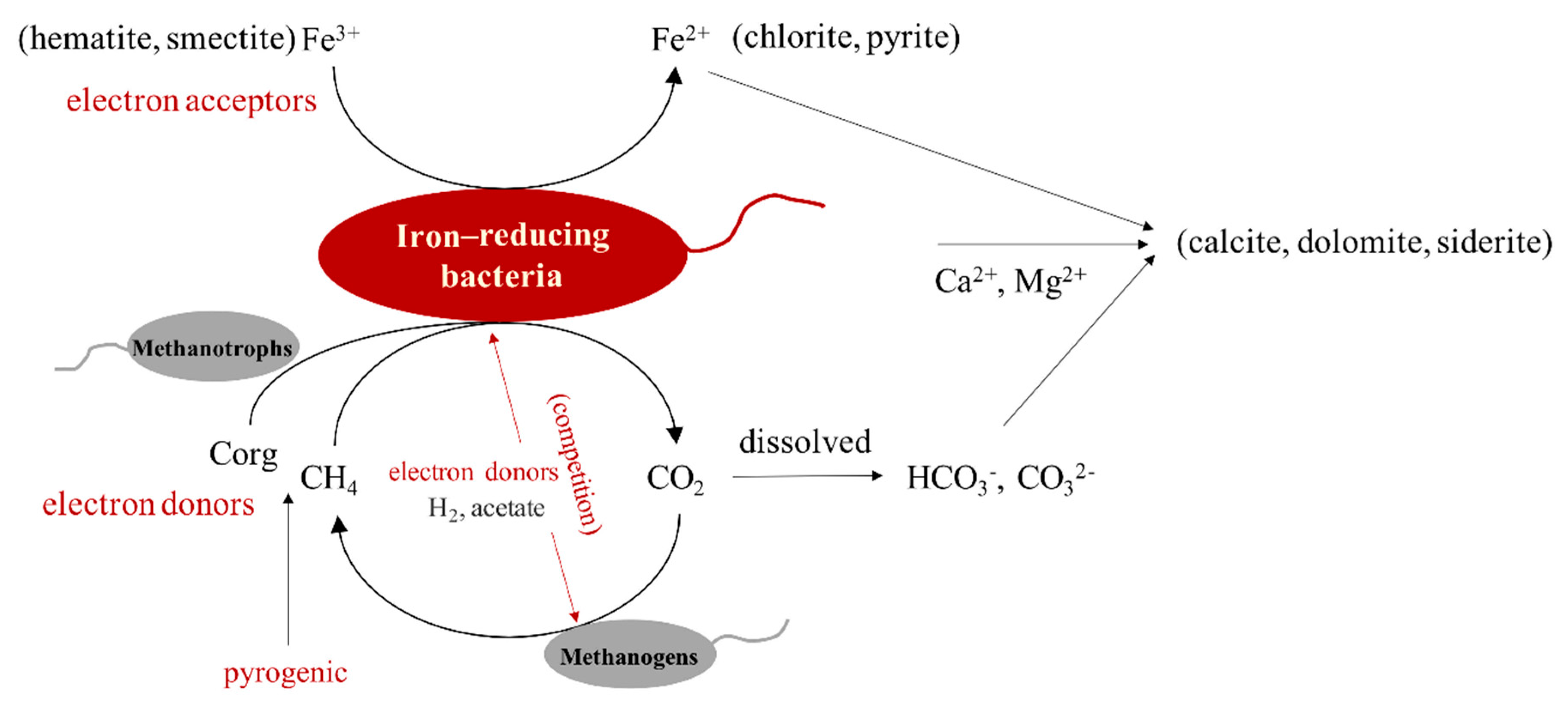
4.3. The Interaction between Hydrocarbons and Iron-Bearing Minerals Mediated by Microorganisms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, W.; Zheng, G.; Ma, X.; Fortin, D.; Hilton, D.R.; Liang, S.; Chen, Z.; Hu, G. Iron speciation of mud breccia from the Dushanzi Mud Volcano in the Xinjiang Uygur Autonomous Region, NW China. Acta Geol. Sin. Engl. Ed. 2018, 92, 2201–2213. [Google Scholar]
- Meer, F.V.D.; Dijk, P.V.; Werff, H.V.D.; Yang, H. Remote sensing and petroleum seepage: A review and case study. Terra Nova 2002, 14, 1–17. [Google Scholar] [CrossRef]
- Etiope, G.; Feyzullayev, A.; Baciu, C.L. Terrestrial methane seeps and mud volcanoes: A global perspective of gas origin. Mar. Pet. Geol. 2009, 26, 333–344. [Google Scholar] [CrossRef]
- Hmiel, B.; Petrenko, V.V.; Dyonisius, M.N.; Buizert, C.; Smith, A.M.; Place, P.F.; Harth, C.; Beaudette, R.; Hua, Q.; Yang, B.; et al. Preindustrial 14CH4 indicates greater anthropogenic fossil CH4 emissions. Nature 2020, 578, 409. [Google Scholar] [CrossRef] [PubMed]
- Saunois, M.; Bousquet, P.; Poulter, B.; Peregon, A.; Ciais, P.; Canadell, J.G.; Dlugokencky, E.J.; Etiope, G.; Bastviken, D.; Houweling, S.; et al. The global methane budget 2000–2012. Earth Syst. Sci. Data 2016, 8, 697–751. [Google Scholar] [CrossRef] [Green Version]
- Saunders, D.F.; Burson, K.R.; Thompson, C.K. Model for hydrocarbon microseepage and related near-surface alterations. AAPG Bull. Am. Assoc. Pet. Geol. 1999, 83, 170–185. [Google Scholar]
- Zheng, G.D.; Fu, B.H.; Takahashi, Y.; Kuno, A.; Matsuo, M.; Zhang, J. Chemical speciation of redox sensitive elements during hydrocarbon leaching in the Junggar Basin, Northwest China. J. Asian Earth Sci. 2010, 39, 713–723. [Google Scholar] [CrossRef]
- Zheng, G.D.; Fu, B.H.; Duan, Y.; Wang, Q.; Matsuo, M.; Takano, B. Iron speciation related to color of Jurassic sedimentary rocks in Turpan Basin, northwest China. J. Radioanal. Nucl. Chem. 2004, 261, 421–427. [Google Scholar] [CrossRef]
- Zheng, G.D.; Fu, B.H.; Kuno, A.; Matsuo, M. Iron speciation in bleached rocks by hydrocarbon leaching in Dushanzi Mud Volcano, NW China. J. Phys. Conf. Ser. 2010, 217. [Google Scholar] [CrossRef]
- Zheng, G.D.; Lang, Y.H.; Takano, B.; Matsuo, M.; Kuno, A.; Tsushima, H. Iron speciation of sliding mud in Toyama Prefecture, Japan. J. Asian Earth Sci. 2002, 20, 955–963. [Google Scholar] [CrossRef]
- Zheng, G.; Ma, X.; Guo, Z.; Hilton, D.R.; Xu, W.; Liang, S.; Fan, Q.; Chen, W. Gas geochemistry and methane emission from Dushanzi mud volcanoes in the southern Junggar Basin, NW China. J. Asian Earth Sci. 2017, 149, 184–190. [Google Scholar] [CrossRef]
- Borch, T.; Kretzschmar, R.; Kappler, A.; Van Cappellen, P.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K. Biogeochemical redox processes and their impact on contaminant dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Geomicrobiology of the built environment. Nat. Microbiol. 2017, 2, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryachko, Y. Novel approaches to microbial enhancement of oil recovery. J. Biotechnol. 2018, 266, 118–123. [Google Scholar] [CrossRef]
- Ren, G.; Ma, A.; Zhang, Y.; Deng, Y.; Zheng, G.; Zhuang, X.; Zhuang, G.; Fortin, D. Electron acceptors for anaerobic oxidation of methane drive microbial community structure and diversity in mud volcanoes. Environ. Microbiol. 2018, 20, 2370–2385. [Google Scholar] [CrossRef] [Green Version]
- Uroz, S.; Kelly, L.C.; Turpault, M.-P.; Lepleux, C.; Frey-Klett, P. The mineralosphere concept: Mineralogical control of the distribution and function of mineral-associatec bacterial communities. Trends Microbiol. 2015, 23, 751–762. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Y.J.; Hu, W.X.; Yao, S.P.; Wang, X.L.; Zhang, Y.Q.; Tang, Y. The Permian hybrid petroleum system in the northwest margin of the Junggar Basin, northwest China. Mar. Pet. Geol. 2005, 22, 331–349. [Google Scholar] [CrossRef]
- Wan, Z.; Shi, Q.; Zhang, Q.; Cai, S.; Xia, B. Characteristics and developmental mechanisms of mud volcanoes on the southern margin of the Junggar Basin, NW China. Geol. J. 2015, 50, 434–445. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Bai, Z.; Wu, S.; Li, X.; Wang, N.; Du, X.; Fan, H.; Zhuang, G.; Bohu, T.; et al. Unraveling Mechanisms and Impact of Microbial Recruitment on Oilseed Rape (Brassica napus L.) and the Rhizosphere Mediated by Plant Growth-Promoting Rhizobacteria. Microorganisms 2021, 9, 161. [Google Scholar] [CrossRef]
- Xie, F.; Ma, A.; Zhou, H.; Liang, Y.; Yin, J.; Ma, K.; Zhuang, X.; Zhuang, G. Niche differentiation of denitrifying anaerobic methane oxidizing bacteria and archaea leads to effective methane filtration in a Tibetan alpine wetland. Environ. Int. 2020, 140, 105764. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Organic matter mineralization with reduction of ferric Fe in anaerobic sediments. Appl. Environ. Microbiol. 1986, 51, 683–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stookey, L.L. Ferrozine—A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef] [Green Version]
- Yusoff, M.Z.M.; Hu, A.Y.; Feng, C.J.; Maeda, T.; Shirai, Y.; Hassan, M.A.; Yu, C.P. Influence of pretreated activated sludge for electricity generation in microbial fuel cell application. Bioresour. Technol. 2013, 145, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Horikoshi, K. Rapid detection and quantification of members of the archaeal community by quantitative PCR using fluorogenic probes. Appl. Environ. Microbiol. 2000, 66, 5066–5072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delong, E.F. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 1992, 89, 5685–5689. [Google Scholar] [CrossRef] [Green Version]
- Lane, D.J.; Pace, B.; Olsen, G.J.; Stahl, D.A.; Sogin, M.L.; Pace, N.R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc. Natl. Acad. Sci. USA 1985, 82, 6955–6959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amann, R.I.; Binder, B.J.; Olson, R.J.; Chisholm, S.W.; Devereux, R.; Stahl, D.A. Combination of 16S rRNA-Targeted Oligonucleotide Probes with Flow Cytometry for Analyzing Mixed Microbial Populations. Appl. Environ. Microbiol. 1990, 56, 1919–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giardine, B.; Riemer, C.; Hardison, R.C.; Burhans, R.; Elnitski, L.; Shah, P.; Zhang, Y.; Blankenberg, D.; Albert, I.; Taylor, J.; et al. Galaxy: A platform for interactive large-scale genome analysis. Genome Res. 2005, 15, 1451–1455. [Google Scholar] [CrossRef] [Green Version]
- Goecks, J.; Nekrutenko, A.; Taylor, J.; Galaxy, T. Galaxy: A comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 2010, 11. [Google Scholar] [CrossRef] [Green Version]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Kong, Y. Btrim: A fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics 2011, 98, 152–153. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ma, A.; Liu, G.; Ma, J.; Wei, J.; Zhou, H.; Brandt, K.K.; Zhuang, G. Potential feedback mediated by soil microbiome response to warming in a glacier forefield. Glob. Chang. Biol. 2020, 26, 697–708. [Google Scholar] [CrossRef]
- Dong, H.; Jaisi, D.P.; Kim, J.; Zhang, G. Microbe-clay mineral interactions. Am. Mineral. 2009, 94, 1505–1519. [Google Scholar] [CrossRef]
- Kim, J.; Dong, H.L.; Seabaugh, J.; Newell, S.W.; Eberl, D.D. Role of microbes in the smectite-to-illite reaction. Science 2004, 303, 830–832. [Google Scholar] [CrossRef] [Green Version]
- Choe, Y.H.; Kim, M.; Woo, J.; Lee, M.J.; Lee, J.I.; Lee, E.J.; Lee, Y.K. Comparing rock-inhabiting microbial communities in different rock types from a high arctic polar desert. FEMS Microbiol. Ecol. 2018, 94, fiy070. [Google Scholar] [CrossRef] [Green Version]
- Uroz, S.; Calvaruso, C.; Turpault, M.-P.; Frey-Klett, P. Mineral weathering by bacteria: Ecology, actors and mechanisms. Trends Microbiol. 2009, 17, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology-SGM 2010, 156, 609–643. [Google Scholar] [CrossRef]
- Yang, X.R.; Yan, D.T.; Wei, X.S.; Zhang, L.W.; Zhang, B.; Xu, H.W.; Gong, Y.; He, J. Different formation mechanism of quartz in siliceous and argillaceous shales: A case study of Longmaxi Formation in South China. Mar. Pet. Geol. 2018, 94, 80–94. [Google Scholar]
- Kamp, P.C.v.d. Smectite-illite-muscovite transformations, quartz dissolution, and silica release in shales. Clays Clay Miner. 2008, 56, 66–81. [Google Scholar] [CrossRef]
- Thyberg, B.; Jahren, J.; Winje, T.; Bjorlykke, K.; Faleide, J.I.; Marcussen, O. Quartz cementation in Late Cretaceous mudstones, northern North Sea: Changes in rock properties due to dissolution of smectite and precipitation of micro-quartz crystals. Mar. Pet. Geol. 2010, 27, 1752–1764. [Google Scholar] [CrossRef]
- Small, J.S. Fluid composition, mineralogy and morphological changes associated with the smectite-to-illite reaction; an experimental investigation of the effect of organic acid anions. Clay Miner. 1994, 29, 539–554. [Google Scholar] [CrossRef]
- Worden, R.H.; Morad, S. Quartz Cementation in Oil Field Sandstones: A Review of the Key Controversies; Blackwell Science Publisher: Oxford, UK, 2000; pp. 1–20. [Google Scholar]
- Gadd, G.M. Microbial Roles in Mineral Transformations and Metal Cycling in the Earth’s Critical Zone. In Molecular Environmental Soil Science; Xu, J., Sparks, D.L., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 115–165. [Google Scholar]
- Zheng, G.D.; Lang, Y.; Miyahara, M.; Nozaki, T.; Haruaki, T. Iron oxide precipitate in seepage of groundwater from a landslide slip zone. Environ. Geol. 2007, 51, 1455–1464. [Google Scholar] [CrossRef]
- Coutinho, M.L.; Miller, A.Z.; Macedo, M.F. Biological colonization and biodeterioration of architectural ceramic materials: An overview. J. Cult. Herit. 2015, 16, 759–777. [Google Scholar] [CrossRef] [Green Version]
- Jurado, V.; Miller, A.Z.; Cuezva, S.; Fernandez-Cortes, A.; Benavente, D.; Rogerio-Candelera, M.A.; Reyes, J.; Canaveras, J.C.; Sanchez-Moral, S.; Saiz-Jimenez, C. Recolonization of mortars by endolithic organisms on the walls of San Roque church in Campeche (Mexico): A case of tertiary bioreceptivity. Constr. Build. Mater. 2014, 53, 348–359. [Google Scholar] [CrossRef]
- Tu, T.-H.; Wu, L.-W.; Lin, Y.-S.; Imachi, H.; Lin, L.-H.; Wang, P.-L. Microbial Community Composition and Functional Capacity in a Terrestrial Ferruginous, Sulfate-Depleted Mud Volcano. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Wong, F.K.Y.; Lau, M.C.Y.; Lacap, D.C.; Aitchison, J.C.; Cowan, D.A.; Pointing, S.B. Endolithic microbial colonization of limestone in a high-altitude arid environment. Microb. Ecol. 2010, 59, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Bajerski, F.; Wagner, D. Bacterial succession in Antarctic soils of two glacier forefields on Larsemann Hills, East Antarctica. FEMS Microbiol. Ecol. 2013, 85, 128–142. [Google Scholar] [CrossRef]
- Osman, J.R.; Wang, Y.; Jaubert, C.; Nguyen, T.-N.; Fernandes, G.R.; DuBow, M.S. The bacterial communities of surface soils from desert sites in the eastern Utah (USA) portion of the Colorado Plateau. Microbiol. Res. 2021, 244, 126664. [Google Scholar] [CrossRef]
- Kulichevskaya, I.S.; Zaichikova, M.V.; Detkova, E.N.; Dedysh, S.N.; Zavarzin, G.A. Larkinella arboricola sp nov., a new spiral-shaped bacterium of the phylum Bacteroidetes isolated from the microbial community of decomposing wood. Microbiology 2009, 78, 741–746. [Google Scholar] [CrossRef]
- Rughoeft, S.; Herrmann, M.; Lazar, C.S.; Cesarz, S.; Levick, S.R.; Trumbore, S.E.; Kuesel, K. Community composition and abundance of bacterial, archaeal and nitrifying populations in savanna soils on vontrasting bedrock material in Kruger National Park, South Africa. Front. Microbiol. 2016, 7, 1638. [Google Scholar]
- Fuerst, J.A.; Sagulenko, E. Beyond the bacterium: Planctomycetes challenge our concepts of microbial structure and function. Nat. Rev. Microbiol. 2011, 9, 403–413. [Google Scholar] [CrossRef]
- Weber, K.A.; Achenbach, L.A.; Coates, J.D. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 2006, 4, 752–764. [Google Scholar] [CrossRef] [Green Version]
- Esther, J.; Sukla, L.B.; Pradhan, N.; Panda, S. Fe (III) reduction strategies of dissimilatory iron reducing bacteria. Korean J. Chem. Eng. 2015, 32, 1–14. [Google Scholar] [CrossRef]
- Horvath, A.S.; Garrick, L.V.; Moreau, J.W. Manganese-reducing Pseudomonas fluorescens-group bacteria control arsenic mobility in gold mining-contaminated groundwater. Environ. Earth Sci. 2014, 71, 4187–4198. [Google Scholar] [CrossRef]
- Petrie, L.; North, N.N.; Dollhopf, S.L.; Balkwill, D.L.; Kostka, J.E. Enumeration and characterization of iron(III)-reducing microbial communities from acidic subsurface sediments contaminated with uranium(VI). Appl. Environ. Microbiol. 2003, 69, 7467–7479. [Google Scholar] [CrossRef] [Green Version]
- Dobbin, P.S.; Warren, L.H.; Cook, N.J.; McEwan, A.G.; Powell, A.K.; Richardson, D.J. Dissimilatory iron(III) reduction by Rhodobacter capsulatus. Microbiology 1996, 142, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Gorby, Y.A.; Yanina, S.; McLean, J.S.; Rosso, K.M.; Moyles, D.; Dohnalkova, A.; Beveridge, T.J.; Chang, I.S.; Kim, B.H.; Kim, K.S.; et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 2006, 103, 11358–11363. [Google Scholar] [CrossRef] [Green Version]
- Gralnick, J.A.; Newman, D.K. Extracellular respiration. Mol. Microbiol. 2007, 65, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, G.-J.; He, T.-R.; Yin, D.-L.; Li, T.; An, Y.-L. Seasonal distribution of dissimilatory iron-reducing bacteria in reservoir sediments of Guiyang City. Chin. J. Ecol. 2014, 33, 2153–2160. [Google Scholar]
- Suter, E.A.; Pachiadaki, M.; Taylor, G.T.; Astor, Y.; Edgcomb, V.P. Free-living chemoautotrophic and particle-attached heterotrophic prokaryotes dominate microbial assemblages along a pelagic redox gradient. Environ. Microbiol. 2018, 20, 693–712. [Google Scholar] [CrossRef]
- Hamady, M.; Lozupone, C.; Knight, R. Fast UniFrac: Facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J. 2010, 4, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Stucki, J.W.; Lee, K.; Goodman, B.A.; Kostka, J.E. Effects of in situ biostimulation on iron mineral speciation in a sub-surface soil. Geochim. Cosmochim. Acta 2007, 71, 835–843. [Google Scholar] [CrossRef]
- O’Reilly, S.E.; Watkins, J.; Furukawa, Y. Secondary mineral formation associated with respiration of nontronite, NAu-1 by iron reducing bacteria. Geochem. Trans. 2005, 6, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Jaisi, D.P.; Eberl, D.D.; Dong, H.; Kim, J. The formation of Illite from nontronite by mesophilic and thermophilic bacterial reaction. Clays Clay Miner. 2011, 59, 21–33. [Google Scholar] [CrossRef]
- Kashefi, K.; Shelobolina, E.S.; Elliott, W.C.; Lovley, D.R. Growth of thermophilic and hyperthermophilic Fe(III)-reducing microorganisms on a ferruginous smectite as the sole electron acceptor. Appl. Environ. Microbiol. 2008, 74, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Yue, X.; Li, F.; Wei, C. Bio-current as an indicator for biogenic Fe(II) generation driven by dissimilatory iron reducing bacteria. Biosens. Bioelectron. 2013, 39, 51–56. [Google Scholar] [CrossRef]
- Kato, S. Biotechnological aspects of microbial extracellular electron transfer. Microbes Environ. 2015, 30, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Roden, E.E.; Wetzel, R.G. Competition between Fe(III)-reducing and methanogenic bacteria for acetate in iron-rich freshwater sediments. Microb. Ecol. 2003, 45, 252–258. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Q.; Feng, Y.; Luo, H.; Pan, X.; Gadd, G.M. Microbiological and environmental significance of metal-dependent anaerobic oxidation of methane. Sci. Total Environ. 2018, 610, 759–768. [Google Scholar] [CrossRef]
- Beal, E.J.; House, C.H.; Orphan, V.J. Manganese- and Iron-Dependent Marine Methane Oxidation. Science 2009, 325, 184–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Li, S.W.; Ding, Z.W.; Ding, J.; Lu, Y.Z.; Zeng, R.J. Iron reduction in the DAMO/Shewanella oneidensis MR-1 coculture system and the fate of Fe(II). Water Res. 2016, 88, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Ettwig, K.F.; Zhu, B.; Speth, D.; Keltjens, J.T.; Jetten, M.S.M.; Kartal, B. Archaea catalyze iron-dependent anaerobic oxidation of methane. Proc. Natl. Acad. Sci. USA 2016, 113, 12792–12796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luke, C.; Speth, D.R.; Kox, M.A.R.; Villanueva, L.; Jetten, M.S.M. Metagenomic analysis of nitrogen and methane cycling in the Arabian Sea oxygen minimum zone. Peerj 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.-L.; Jiang, X.; Wu, Q.L.; Zhou, N.-Y. Intragenomic Heterogeneity of 16S rRNA Genes Causes Overestimation of Prokaryotic Diversity. Appl. Environ. Microbiol. 2013, 79, 5962–5969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Ge, T.; Hu, Y. A review on the coupling of bio-geochemical process for key elements and microbial regulation mechanisms in paddy rice ecosystems. Acta Ecol. Sin. 2015, 35, 6626–6634. [Google Scholar]
| Sample ID | SiO2 (%) | Al2O3 (%) | Fe2O3 (%) | K2O (%) | MgO (%) | Na2O (%) | TiO2 (%) | CaO (%) | MnO (%) | P2O5 (%) | LOI * (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AQG1 | 62.22 | 24.75 | 4.77 | 2.98 | 1.83 | 1.03 | 1.09 | 0.426 | 0.045 | 0.069 | 0.538 |
| AQG2 | 62.39 | 22.68 | 6.15 | 2.66 | 2.17 | 1.42 | 1.03 | 0.608 | 0.063 | 0.076 | 0.345 |
| AQG3 | 55.68 | 22.12 | 9.76 | 2.52 | 2.22 | 3.18 | 0.98 | 0.684 | 0.191 | 0.143 | 0.445 |
| AQG4 | 57.89 | 23.62 | 9.06 | 3.07 | 2.07 | 1.16 | 0.97 | 0.816 | 0.056 | 0.160 | 0.517 |
| AQG5 | 56.61 | 20.28 | 5.26 | 2.48 | 2.04 | 6.22 | 1.02 | 0.692 | 0.057 | 0.074 | 0.615 |
| AQG6 | 57.75 | 27.29 | 3.90 | 2.68 | 2.05 | 2.52 | 1.18 | 0.943 | 0.073 | 0.067 | 0.334 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, K.; Ma, A.; Zheng, G.; Ren, G.; Xie, F.; Zhou, H.; Yin, J.; Liang, Y.; Zhuang, X.; Zhuang, G. Mineralosphere Microbiome Leading to Changed Geochemical Properties of Sedimentary Rocks from Aiqigou Mud Volcano, Northwest China. Microorganisms 2021, 9, 560. https://doi.org/10.3390/microorganisms9030560
Ma K, Ma A, Zheng G, Ren G, Xie F, Zhou H, Yin J, Liang Y, Zhuang X, Zhuang G. Mineralosphere Microbiome Leading to Changed Geochemical Properties of Sedimentary Rocks from Aiqigou Mud Volcano, Northwest China. Microorganisms. 2021; 9(3):560. https://doi.org/10.3390/microorganisms9030560
Chicago/Turabian StyleMa, Ke, Anzhou Ma, Guodong Zheng, Ge Ren, Fei Xie, Hanchang Zhou, Jun Yin, Yu Liang, Xuliang Zhuang, and Guoqiang Zhuang. 2021. "Mineralosphere Microbiome Leading to Changed Geochemical Properties of Sedimentary Rocks from Aiqigou Mud Volcano, Northwest China" Microorganisms 9, no. 3: 560. https://doi.org/10.3390/microorganisms9030560
APA StyleMa, K., Ma, A., Zheng, G., Ren, G., Xie, F., Zhou, H., Yin, J., Liang, Y., Zhuang, X., & Zhuang, G. (2021). Mineralosphere Microbiome Leading to Changed Geochemical Properties of Sedimentary Rocks from Aiqigou Mud Volcano, Northwest China. Microorganisms, 9(3), 560. https://doi.org/10.3390/microorganisms9030560







