Seroprevalence of ToRCH Pathogens in Southeast Asia
Abstract
:1. Introduction
| Pathogen | Routes of Transmission Besides Transplacental Spread | Consequences for the Mother | Consequences for the Unborn or Newborn Child | Vaccination Available | Treatment Available | References |
|---|---|---|---|---|---|---|
| T. gondii | Ingestion of oocysts or tissue cysts via contaminated food, or from soil, cat feces etc. | Mostly asymptomatic; Possible complications: lymphadenopathy; myocarditis; pneumonia; hepatitis; encephalitis | Stillbirth; brain damages (intracranial calcifications; hydrocephalus; microcephaly; mental retardation); hepatic enlargement; ocular damages; subclinical infections with development of ocular lesions at later time point | No | Yes | [11,12] |
| VZV | Human-to-human transmission, respiratory or contact with lesions | Mostly harmless rash and mild flu-like symptoms; Possible complications: severe pneumonia and death | Intrauterine death; Congenital varicella syndrome (skin lesions; neurologic defects; eye diseases; skeletal anomalies); Neonatal varicella | Yes | Yes | [13,14] |
| B19V | Human-to-human transmission, respiratory, blood | Mostly asymptomatic; mild illness with fever; rash; malaise, headache; Possible complication: polyarthritis | Preterm delivery; miscarriage; severe anemia; nonimmune hydrops fetalis; meningoencephalitis | No | No | [5] |
| RV | Human-to-human transmission, respiratory | Mostly asymptomatic or mild illness with rash; Possible complication: polyarthritis | Spontaneous abortion; miscarriage; stillbirth; fetal growth restriction; CRS (small for infant age; hearing loss; cataract and heart defects) | Yes | No | [5,15] |
| CMV | Human-to-human transmission by body fluids, e.g., saliva, urine, blood | Mostly asymptomatic; Possible complications: fever; pharyngitis; lymphadenopathy; hepatosplenomegaly; arthralgia; rash | Mostly asymptomatic; Spontaneous abortion; fetal death or preterm birth; mental retardation; hearing loss; fetal growth restriction | No | Yes | [3,4,16] |
| HSV | Human-to-human transmission by contact with lesions | Mostly asymptomatic; Possible complications: Blistering; ulceration in the genital or oral region; fever; lymphadenopathy; systemic infection | Preterm delivery; spontaneous abortion; fetal death; cutaneous symptoms; brain damages (microcephaly; intracranial calcifications; encephalitis); Neonatal infection (skin, eye, central nervous system manifestations); systemic infection | No | Yes | [3,17,18] |
2. Materials and Methods
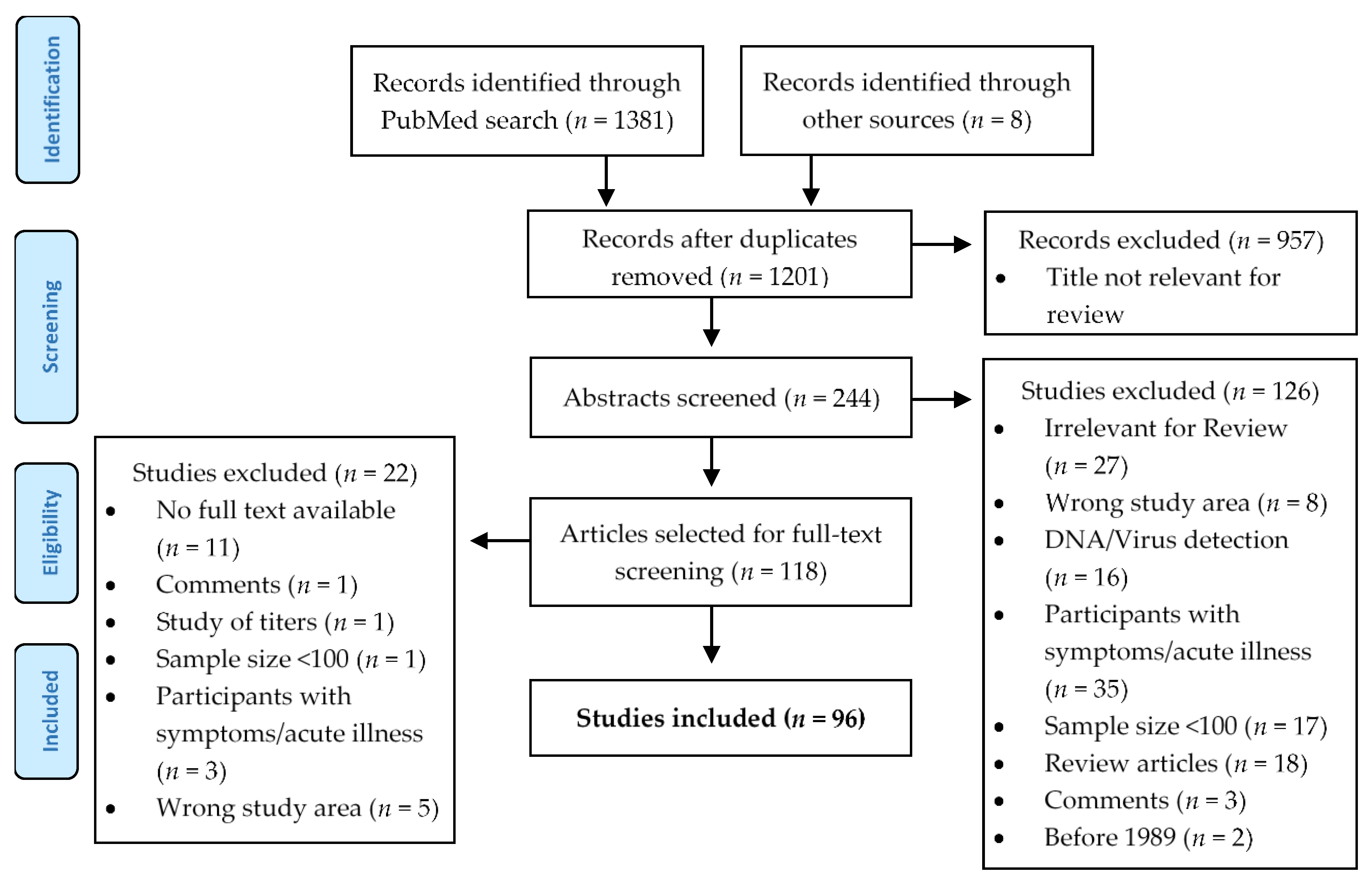
2.1. Literature Search
2.2. Data Processing
2.3. Quality Criteria
3. Results
3.1. Toxoplasma Gondii
| Study Location and Year | Study Population (n, Age Range) | IgG Seroprevalence in % (95% CI) * | Detection Method | Comments and/or Risk Factors for Seropositivity | Reference, Year Published | |
|---|---|---|---|---|---|---|
| Cambodia | Phnom Penh, NA | Adults, (335, NA) | 13.1 (9.49–16.71) * | Direct agglutination test (BioMérieux), Immunoenzymatic test (Platelia IgM and IgG, Sanofi Pasteur) | [26], 1999 | |
| Nationwide, 2012 | Women, (2150, 15–39y) | 5.8 (4.7–7.0) | Multiplex Bead Assay | [27], 2016 | ||
| Laos | Keoudom, NA | General population, (588, 3–70 y) | 15.3 (12.39–18.21) * | CFT | Higher age | [28], 1992 |
| Malaysia Range: 10.6–59.7 | Kuala Lumpur, NA | Pregnant women, (219, 20–41 y) | 39.7 (33.25–46.21) | ELISA (IgM- and IgG-NovaLisa, Dietzenbach, Germany) | Higher age; Low level of education and awareness; Parity (≥1); Lack of awareness of toxoplasmosis; Consumption of undercooked meat | [29], 2014 |
| Selangor, NA | Aborigines, (415, NA) | 10.6 (7.64–13.56) * | IFAT | [30], 1994 | ||
| Kuala Lumpur, NA | Blood donors, (203, 18–65 y) | 28.1 (21.92–34.28) | ELISA | [31], 2002 | ||
| Kuala Lumpur, 1994 to 2001 | HIV-infected, (406, 17–74 y) | 51.2 (46.34–56.06) | ELISA (AxSYM, Abbott Laboratories, USA) | [32], 2003 | ||
| Kuala Lumpur, 2001 to 2002 | HIV-infected, (505, 17–71 y) | 44.8 (42.64–51.76) | ELISA (AxSYM, Abbott Laboratories, USA) | [33], 2004 | ||
| Kuala Lumpur, 2002 | HIV-infected, (301, 18–78 y) | 41.2 (35.5–46.9) | ELISA (Trinity Biotech, Bray, Ireland) | Ethnicity (Malay) | [34], 2003 | |
| Kuala Lumpur, 2002 | Pregnant women, (200, 18–43 y) | 39.0 (32.24–45.76) | ELISA (Trinity Biotech, Bray, Ireland) | Ethnicity (Malay) | [35], 2003 | |
| Kuala Lumpur, 2000 to 2004 | HIV-infected, (162, 1–85 y) | 35.8 (28.42–43.18) | ELISA (Trinity Biotech, Bray, Ireland and Veda-lab, Alencon Cedex, France) | [36], 2005 | ||
| Kuala Lumpur, 2000 to 2004 | Ocular patients, (161, 1–85 y) | 31.1 (23.95–38.25) | ELISA (Trinity Biotech, Bray, Ireland and Veda-lab, Alencon Cedex, France) | [36], 2005 | ||
| NA, NA | Worker, (198, NA) | 44.9 (37.97–51.83) | IFAT | [37], 2008 | ||
| Kuala Lumpur, 2007 to 2008 | Renal patients, (247, 21–89 y) | 46.6 (40–52) | ELISA (IgM and IgG, Trinity Biotech, New York, USA) | Ethnicity (Malay); Marital status (married); Low level of education | [38], 2011 | |
| Kuala Lumpur, 2009 | Oncology patients, (129, 15–88 y) | 38.8 (30.34–47.16) | ELISA | Living in rural areas; Consumption of undercooked meat and/or history of blood transfusion | [39], 2010 | |
| Peninsular, 2007 to 2010 | Indigenous, (495, 1–82 y) | 31.0 (26.9–35.1) | ELISA (IgM and IgG, Trinity Biotech, New York, NY, USA) | Age (>12 y); domestic use of untreated river and mountain water; Close contact with pets | [40], 2011 | |
| Kuala Lumpur, 2010 | Ocular patients, (493, 2–90 y) | 25.0 (21.0–29.0) | ELISA (Trinity Biotech, New York, USA) | Higher age; Ethnicity (Malay) | [41], 2012 | |
| Kuala Lumpur, 2011 | Patients with Schizophrenia, (144, NA) | 37.5 (29.59–45.41) | ELISA (Platelia Toxo IgG ELISA BioRad, USA) | Age (>40 y); Ethnicity (Malay) | [42], 2012 | |
| Kuala Lumpur, 2011 | Healthy patients, (144, NA) | 34.0 (26.26–41.74) | ELISA (Platelia Toxo IgG ELISA BioRad, USA) | [42], 2012 | ||
| Selangor, NA | Patients with Schizophrenia, (101, 18–65 y) | 51.5 (41.75–51.5) | ELISA (IBL Company, Hamburg, Germany) | [43], 2015 | ||
| Pangkor Island, NA | General population, (298, 1–80 y) | 59.7 (54.13–65.27) * | ELISA (IgM and IgG, Trinity Biotech, USA) | Gender (female); Ethnicity (Malay) | [44], 2014 | |
| Kuala Lumpur, 2012 to 2013 | Pregnant women, (281, NA) | 33.5 (27.98–39.02) | ELISA (Platelia Toxo IgM and IgG, BioRad, USA) | [45], 2014 | ||
| NA, 2012 to 2013 | Prison inmates, (303, NA) | 39.3 (33.8–44.8) | ELISA (Platelia Toxo IgM and IgG, BioRad, USA) | Age (>40 y); HIV status (positive); Drug abuse history | [46], 2016 | |
| Selangor, Klang Valley, 2013 to 2014 | Veterinary personnel, pet owner, (312, 17–64 y) | 18.3 (14.01–22.59) | ELISA (IgG-NovaLisa, Dietzenbach, Germany) | Age (≥30 y); Working duration (>10 y) | [47], 2015 | |
| Myanmar | Yangon, NA | Pregnant women, (215, 18–45 y) | 30.2 (24.09–36.37) | ELISA (IgM- and IgG-NovaLisa, Dietzenbach, Germany) | [29], 2014 | |
| Thailand-Myanmar-Border, 2014 to 2015 | Pregnant women, (199, 16–46 y) | 31.7 (25.6–38.4) | ELISA (IgM and IgG, Novatec, Dietzenbach Germany) | Parity (≥3) | [48], 2017 | |
| Singapore | Singapore, 1997 to 1998 | Pregnant women, (120, NA) | 17.2 (10.45–23.95) | IFAT | [49], 2000 | |
| Singapore, 2006 to 2011 | HIV-infected, (771, NA) | 23.7 (20.7–26.7) | NA | [50], 2013 | ||
| Thailand Range: 2.6–53.7 | Bangkok, NA | Pregnant women, (468, NA) | 12.6 (9.59–15.61) | LAT (Toxotest MT Eiken, Japan) | [51], 1991 | |
| Bangkok, 1992 to 1995 | Pregnant women, (300, 14–40 y) | 13.7 (9.81–17.59) | ELISA (TOXOELISA II, Biowhittaker, USA) | [52], 1997 | ||
| Samut Sakhon, 1996 | Pregnant women, (1200, NA) | 13.2 (11.28–15.12) | Sabin-Feldman Dye Test | Consumption of undercooked meat | [53], 1998 | |
| Samut Sakhon, NA | Pregnant women, (300, 14–41 y) | 21.7 (17.04–26.36) | Sabin-Feldman Dye Test | [54], 1999 | ||
| Bangkok, 1997 to 1998 | General population, (163, 2–89 y) | 3.1 (0.44–5.76) * | LAT (Toxo Check, Eiken Chemical Co., Ltd., Japan) | [55], 2000 | ||
| Loei Province, 1997 | Blood donors, (345, 17–56 y) | 4.1 (2.01–6.19) | ELISA | Gender (male) | [56], 2000 | |
| Bangkok, 1997 to 1999 | HIV-infected pregnant women, (838, NA) | 53.7 (50.32–57.08) | ELISA (Platelia Toxo IgG, Sanofi Diagnostics Pasteur, France) | HIV status (positive) | [57], 2001 | |
| Bangkok, 1997 to 1999 | Pregnant women, (831, NA) | 5.3 (3.78–6.82) | ELISA (Platelia Toxo IgG, Sanofi Diagnostics Pasteur, France) | [57], 2001 | ||
| Bangkok, 1999 to 2000 | Pregnant women, (200, NA) | 13.6 (8.85–18.35) | ELISA | [58], 2001 | ||
| Bangkok, NA | General population, temple residents (327, 2–75 y) | 6.4 (3.75–9.05) | Sabin-Feldman Dye Test | Cat ownership | [59], 2003 | |
| Khon Kaen, 2009 to 2012 | Women, (493, 21–81 y) | 2.6 (1.2–4.0) * | LAT (TOXOTEST-MT Eiken, Eiken-Kagaku, Tochigi, Japan) | [60], 2013 | ||
| Songhkla Province, Hat Yai, 2009 to 2010 | Pregnant women, (640, 15–45 y) | 21.6 (18.5–24.9) | ELISA (IgG-Trinity Biotech, New York) | Age (≥36 y); Living outside Songkhla province; Contact with cats; Drinking unclean water | [61], 2011 | |
| Songhkla Province, 2009 to 2010 | HIV-infected, (300, 21–78 y) | 36.3 (30.86–41.74) | ELISA (IgG-NovaLisa, Dietzenbach, Germany) | Gender (male) | [62,63], 2013, 2015 | |
| Songhkla Province, Hat Yai, 2012 to 2013 | Pregnant women, (760, 14–47 y) | 22.0 (19.0–25.0) | ELISA (IgG- and IgM- Trinity Biotech, New York) | Age (≥26 y); Working as a laborer; Drinking unclean water | [64], 2014 | |
| Vietnam Range: 4.2–11.2 | Ho Chi Minh City, 1996 | HIV-positive injecting drug users, (235, 24–57 y) | 9.0 (5.34–12.66) | ELISA (IgM and IgG; Behring) | [65], 1999 | |
| Nghe An, Lao Cai and Tien Giang provinces, 2006 | General population, (650, NA) | 4.2 (1.78–4.62) | Sabin-Feldman Dye Test | [66], 2008 | ||
| Ho Chi Minh City, NA | Drug addicted, (300, 18–53 y) | 7.7 (4.68–10.72) | ELISA (Platelia Toxo IgG, BioRad) | [67], 2003 | ||
| Ho Chi Minh City, NA | HIV-negative adults, (150, NA) | 6.5 (2.55–10.45) | ELISA (Platelia Toxo IgG, BioRad) | [67], 2003 | ||
| NhaTrang, NA | Pregnant women, (300, 18–43 y) | 11.2 (7.63–14.77) | ELISA (Platelia Toxo IgG, BioRad) | [67], 2003 |
3.1.1. Seroprevalence by Country
3.1.2. Risk Factors for Seropositivity
3.2. Varicella Zoster Virus
| Study Location and Year | Study Population (n, Age Range) | IgG Seroprevalence in % (95% CI) | Detection Method | Comments and/or Risk Factors for Seropositivity | Reference, Year Published | |
|---|---|---|---|---|---|---|
| Cambodia | NA | |||||
| Laos | Vientiane Capital, Huaphan Province, Boulhikhamxay Province, 2013 | HCW, (1128, 15–69 y) | 95.0 (93.73–96.27) | ELISA (Euroimmun) | Early life exposure (15–24y age group: already 94.1% positive) | [68], 2015 |
| Malaysia | Kuala Lumpur, NA | HIV-infected, (232, 32–43 y) | 86.6 (82.22–90.98) | ELISA (Siemens Enzygnost, Siemens Healthcare GmbH, Germany) | [69], 2017 | |
| Myanmar | NA | |||||
| Singapore Range: 55.3–91.7 | Singapore, 2000 to 2005 | Military men, (2189, 16–36 y) | 76.0 (74.21–77.79) | ELISA | [70], 2007 | |
| Singapore, 2008 to 2010 | General population, (1200, 1–17 y) | 55.3 (52.5–58.1) | EIA (Euroimmun AG, Germany) | Higher age; Ethnicity (Chinese) | [71], 2014 | |
| Singapore,2009 to 2014 | HCW, (6701, NA) | 91.7 (91.04–92.36) | ELISA (Euroimmun Medizinische Labordiagnostika AG, Germany) | Higher age; Ethnicity (Chinese); HCW in nursing vocation | [72], 2015 | |
| Thailand Range: 52.8–92.0 | Bangkok, 1994 | General population, (559, 4M-77 y) | 61.4 (57.36–65.44) | ELISA (Enzygnost, Behringwerke, Germany) | Higher age | [73], 1997 |
| Bangkok, Chiang Mai, Khoen Kaen, Had Yai, 1997 to 1998 | General population, (2093, 9M-29 y) | 52.8 (50.6–54.9) | ELISA (Enzygnost, Dade Behring, Marburg Germany) | Region (North); Central/South: Seroprevalence notably lower in rural areas; Higher age | [74], 2001 | |
| Bangkok, 1998 to 2000 | Healthy children, blood donors, (350, NA) | 64.6 (59.59–69.61) | ELISA (Human, Germany) | Higher age; Increasing number of family members | [75], 2005 | |
| Bangkok, 2006 to 2007 | Medical students, (237, 20–38 y) | 82.3 (77.44–87.16) | EIA | [76], 2009 | ||
| Bangkok, 2008 to 2009 | Medical students, (374, 18–25.8 y) | 92.0 (89.25–94.75) | ELISA (Wiesbaden, Germany) | [77], 2012 | ||
| Vietnam | Ho Chi Minh City, 1996 | Intravenous drug users, (235, 24–57 y) | 99.0 (97.73–100.27) | ELISA (Behring, Germany) | [65], 1999 |
3.2.1. Seroprevalence by Country
3.2.2. Risk Factors for Seropositivity
3.3. Primate Erythroparvovirus 1
| Study Location and Year | Study Population (n, Age Range) | IgG Seroprevalence in % (95% CI) | Detection Method | Comments and/or Risk Factors for Seropositivity | Reference, Year Published | |
|---|---|---|---|---|---|---|
| Cambodia | NA | |||||
| Laos | NA | |||||
| Malaysia | Kuala Lumpur, 1999 to 2000 | Blood donors, undergraduate students, patients, (800, 6M–81 y) | 37.6 (34.24–40.96) | ELISA (Biotrin, Dublin, Ireland) | Higher age | [78], 2002 |
| Myanmar | NA | |||||
| Singapore | Singapore, 1993 | General population, (600, 6M–50 y) | 16.2 (13.25–19.15) | ELISA | Higher age | [79], 1994 |
| Singapore, 1997 to 1998 | Pregnant women, (120, NA) | 30.0 (21.8–38.2) | ELISA | Higher age | [49], 2000 | |
| Thailand Range: 10.9–20.2 | Bangkok, Songkhla Province, 1998 to 1999 | Children and blood donors, (129, 0–51 y) | 20.2 (13.24–27.08) | ELISA (Genzyme Virotech GmbH, Germany) | Higher age | [80], 2000 |
| Bangkok, 1998 to 1999 | Immunocompromised children, (106, 1–15 y) | 16.0 (9.02–22.98) | ELISA (Genzyme Virotech GmbH, Russelsheim, Germany) | [81], 2000 | ||
| Bangkok, 1999 to 2000 | Undergraduate students, (128, 18–24 y) | 10.9 (5.53–16.35) | ELISA (Genzyme Virotech GmbH, Russelsheim, Germany) | [82], 2003 | ||
| Vietnam | NA |
3.3.1. Seroprevalence by Country
3.3.2. Risk Factors for Seropositivity
3.4. Rubella Virus
| Study Location and Year | Study Population (n, Age Range) | IgG Seroprevalence in % (95% CI) | Detection Method | Comments and/or Risk Factors for Seropositivity | Reference, Year Published | |
|---|---|---|---|---|---|---|
| Cambodia | Nationwide, 2012 | Women, (2154, 15–39 y) | 73.3 (70.5–76.1) | ELISA (Enzygnost, Siemens, Germany) | Study prior to RCV introduction; Age (15–19 y); Living area (rural) | [83], 2015 |
| Laos Range: 43.6–86.2 | Vientiane Capital, 2007 to 2008 | School children, (411, 6–12 y) | 43.6 (38.8–48.4) | EIA (Denka Seiken, Japan) | Study prior to RCV introduction; Gender (girls); Age (6 y); Place of birth (hospital) | [84], 2011 |
| Vientiane Capital, Huaphan, Boulhikhamxay, 2013 | HCW, (1128, 15–69 y) | 86.2 (84.2–88.2) | ELISA (Euroimmun) | Childless | [68], 2015 | |
| Nationwide, 2014 | General population, (2135, 1–2y, 5–81 y) | 75.4 (75.3–75.5) | ELISA (Enzygnost, Siemens Healthcare Diagnostics) | Not with been included in the SIA 2011 | [85], 2018 | |
| Malaysia | Kuala Lumpur, 2001 to 2002 | Pregnant women, (414, 15–45 y) | 92.3 (89.7–94.9) | EIA (EIAgen, Italy) | [86], 2005 | |
| Selangor, 2005 | Pregnant women, (500- 16–42 y) | 88.6 (86.8–92.3) | MEIA (AxSYM) | Laborer; No history of vaccination | [87,88], 2008, 2013 | |
| Myanmar | NA | |||||
| Singapore Range: 71.7–88.5 | Singapore, 1993 | General population, (909, NA) | 71.7 (68.77–74.63) | MEIA (Abbott) | [89,90], 2010 | |
| Singapore, 1998 | General population, (928, NA) | 80.2 (77.64–82.76) | MEIA (Abbott) | [89,90], 2010 | ||
| Singapore, 2004 | General population, (4153, 18–74 y) | 84.0 (82.9–85.1) | MEIA (Abbott) | [89,91], 2010, 2015 | ||
| Singapore, 2010 | General population, (3293, 18–79 y) | 85.0 (83.7–86.2) | Chemiluminescent microparticle immunoassay (Abbott Park, Ireland) | Ethnicity (permanent residents); higher age (among women) | [91], 2015 | |
| Singapore, 2008 to 2010 | Children, (1200, 1–17 y) | 88.5 (86.6–90.2) | Chemiluminescent immunoassay (Abbott Architect, Abbott Laboratories, USA) | Ethnicity (Malay) | [91,92], 2015, 2013 | |
| Thailand Range: 74.7–89.4 | Bangkok, 1992 to 1995 | Pregnant women, (300, 14–40 y) | 85.7 (81.74–89.66) | ELISA (Rubelisa II, Biowhittaker, USA) | [52], 1997 | |
| Khon Kaen, 2004 | Pregnant women, (150, 15–40 y) | 74.7 (67.6–81.6) | ELISA | [93], 2005 | ||
| Chiang Rai, Udon Thani, Chon Buri, Nakhon Si Thammarat, 2004 | General population, (899, 0–59 y) | 89.0 (86.6–91.0) | ELISA (RE57081; IBL) | [94], 2009 | ||
| Pathum Thani 2006 to 2007 | Medical students, (237, 20–38 y) | 88.2 (84.1–92.3) | EIA | [76], 2009 | ||
| Chiang Mai, 2011 | HIV-infected, (500, 36–48 y) | 84.6 (81.4–87.8) | ELISA (Enzygnost, Siemens, Marburg, Germany) | [95], 2016 | ||
| Chiang Mai, 2011 | Adults, (132, 30.5–59 y) | 89.4 (84.2–94.7) | ELISA (Enzygnost, Siemens, Marburg, Germany) | [95], 2016 | ||
| Bangkok, 2014 | Women, (289, 28–40 y) | 87.2 (83.4–91.0) | ELISA (Euroimmun, Lübeck, Germany) | [96], 2018 | ||
| Vietnam | Nha Trang, 2009 to 2010 | Pregnant women, (1988, 17–45 y) | 71.1 (69.1–73.1) | EIA (Mini VIDAS) | Study prior to RCV introduction; Study used cord blood; Young age; Primipara; Increased no. of ANC visits; Preterm delivery | [97], 2014 |
3.4.1. Seroprevalence by Country
3.4.2. Risk Factors for Seropositivity
3.5. Cytomegalovirus
| Study Location and Year | Study Population (n, Age Range) | IgG Seroprevalence in % (95% CI) * | Detection Method | Comments and/or Risk Factors for Seropositivity | Reference, Year Published | |
|---|---|---|---|---|---|---|
| Cambodia | NA | |||||
| Laos | NA | |||||
| Malaysia Range: 84.0–97.6 | Kuala Lumpur, NA | Blood donors, (172, 18–47 y) | 97.6 (95.31–99.89) | MEIA (Abbott Axsym System) | [100], 2006 | |
| Kuala Lumpur, NA | HIV-infected, (232, 32–43 y) | 96.1 (93.61–98.59) | Immunoassay (Elecsys, (Roche, Switzerland) | [69], 2017 | ||
| Nationwide, 2007 to 2008 | Pregnant women, (125, NA) | 84.0 (77.57–90.43) | ELISA (DRG Instruments GmbH, Marburg, Germany) | [101], 2011 | ||
| Myanmar | NA | |||||
| Singapore | Singapore, 1997 to 1998 | Pregnant women, (120, NA) | 87.0 (80.98–93.02) | ELISA | Higher age (no statistically significant trend); Ethnicity (Not-Singaporeans) | [49], 2000 |
| Singapore, 2006 to 2011 | HIV-infected, (753, NA) | 96.8 (95.54–98.06) | NA | [50], 2013 | ||
| Thailand Range: 52.4–100 | Khon Kaen, 1990 | Blood donors, (359, 17–59 y) | 93.3 (90.78–95.89) * | ELISA (Abbott Laboratories) | Higher age (no statistically significant trend) | [102], 1993 |
| Bangkok, 1999 to 2000 | Pregnant women, (200, NA) | 79.7 (74.13–85.27) | ELISA | [58], 2001 | ||
| Bangkok, 1992 to 1995 | Pregnant women, (300, 14–40 y) | 100.00 | ELISA (Biowhittaker, USA) | [52], 1997 | ||
| Bangkok, 1997 | Blood donors, (380, 17–50 y) | 71.8 (67.28–76.32) | ELISA | [103], 1999 | ||
| Bangkok, 1997 | Pregnant women, (209, 15–45 y) | 90.9 (87–94.8) | ELISA | Higher age | [103], 1999 | |
| Bangkok, 1998 | Blood donors, (441, 18–55 y) | 52.4 (47.72–57.04) | ELISA | [104], 2001 | ||
| NA, NA | Mothers, (2101, NA) | 86.53 (85.07–87.99) | Immunoassay (Abbott Diagnostics, Abbott Park, IL, USA) | Study used cord blood | [105], 2013 | |
| Bangkok, 1997 | Blood donors, (303, 16–56 y) | 97.0 (95.08–98.92) * | ELISA (Enzygnost, Behring, Germany) | Sex (female) | [106], 1998 | |
| Bangkok, 1995, 1997 | Students, (172, 17–25 y) | 86.0 (80.81–91.19) | ELISA (Enzygnost, Behring, Germany) | Sex (female) | [106], 1998 | |
| Bangkok, 1997 | Pregnant women, (100, 15–40 y) | 100.0 | ELISA (Enzygnost, Behring, Germany) | [106], 1998 | ||
| Bangkok, 1990 | Blood donors, (2196, NA) | 97.3 (96.62–97.98) * | EIA (Abbott) | [107], 1992 | ||
| Vietnam | Ho Chi Minh City, 1996 | Intravenous drug users (235, 24–57 y) | 100.0 | ELISA (Behring) | [65], 1999 |
3.5.1. Seroprevalence by Country
3.5.2. Risk Factors for Seropositivity
3.6. Herpes Simplex Virus
| Study Location and Year | Study Population (n, Age Range) | IgG Seroprevalence in % (95% CI) | Detection Method | Comments and/or Risk Fac tors for Serop Ositivity | Reference, Year Publ ished | |
|---|---|---|---|---|---|---|
| (A) HSV-1 | ||||||
| Cambodia | NA | |||||
| Laos | NA | |||||
| Malaysia | Kuala Lumpur, NA | HIV-infected, (232, 32–43) | 70.7 (64.84–75.56) | ELISA (HerpeSelect, Focus Diagnostics, Cypress, CA, USA) | [69], 2017 | |
| Myanmar | NA | |||||
| Singapore | Singapore, 2003 to 2004 | Sex workers, (300, 22–70 y) | 76.7 (71.92–81.48) | ELISA (HerpeSelect 1, Focus Diagnostics, Cypress, CA 90630, USA) | [108], 2006 | |
| Singapore, 2003 to 2004 | Attendees in STI clinic, (400, 15–80 y) | 55.8 (50.93–60.67) | ELISA (HerpeSelect 1, Focus Diagnostics, Cypress, CA 90630, USA) | Higher age | [109], 2006 | |
| Thailand Range: 56.5–91.0 | Phitsanulok, 1991 | Male army conscripts, (1115, NA) | 77.0 (74.4–79.4) | Immunoblot | [110,111], 1998, 1999 | |
| Chiang Rai Province, 1991 to 1994 | FSW, (500, NA) | 91.0 (88.49–93.51) | Immunoblot | [112], 1999 | ||
| Bangkok, 2006 to 2010 | MSM, (1744, 18–56 y) | 56.5 (54.17–58.83) | ELISA (HerpeSelect 1, Focus Diagnostics, Cypress, CA 90630, USA) | [113], 2013 | ||
| Vietnam | Ho Chi Minh City, 2000 to 2001 | Women, (100,18–55 y) | 98.0 (95.26–100.74) | ELISA (HerpeSelect 1, Focus Diagnostics, Cypress, CA 90630, USA); Western Blot | [114], 2004 | |
| (B) HSV-2 | ||||||
| Cambodia | NA | |||||
| Laos | NA | |||||
| Malaysia | Kuala Lumpur, NA | HIV-infected, (232, 32–43) | 53.9 (47.49–60.31) | ELISA (HerpeSelect, Focus Diagnostics, Cypress, CA 90630, USA) | [69], 2017 | |
| Myanmar | NA | |||||
| Singapore | Singapore, 2003 to 2004 | Sex workers, (300, 22–70 y) | 79.0 (74.39–83.61) | ELISA (HerpeSelect 2, Focus Diagnostics, Cypress, CA 90630, USA) | Higher age; Duration of years of practice as sex worker (>9 y) | [108], 2006 |
| Singapore, 2003 to 2004 | Attendees of STI clinic, (400, 15–80 y) | 28.5 (24.08–32.92) | ELISA (HerpeSelect 2, Focus Diagnostics, Cypress, CA 90630, USA) | [109], 2006 | ||
| Thailand Range: 14.9–80.0 | Phitsanulok, 1991 | Male army conscripts, (1115, 21–27) | 14.9 (12.9–17.1) | Immunoblot | Higher age; Occupation (Businessmen, skilled laborers); Living area (upper North); Start of sexual activity (≤16 y); (early) sexual contact with FSW; Frequency of sexual contact with FSW (≥4 times/y) | [110,111], 1998, 1999 |
| Chiang Rai Province, 1991 to 1994 | FSW, (500, NA) | 75.6 (71.84–79.36) | Immunoblot | HIV status (positive) | [112], 1999 | |
| Bangkok, 1992 to 1995 | Pregnant women, (300, 14–40 y) | 80.0 (75.47–84.53) | ELISA (Herpelisa II, Biowhittaker, USA) | [52], 1997 | ||
| Bangkok, 1996 to 1997 | HIV-infected pregnant women, (307, 17–39 y) | 74.3 (69.41–79.19) | ELISA (HerpeSelect 2, Focus Diagnostics, Cypress, CA 90630, USA) | [115], 2008 | ||
| Bangkok, 2006 to 2010 | MSM, (1544, NA) | 20.7 (18.68–22.72) | ELISA (HerpeSelect 2, Focus Diagnostics, Cypress, CA 90630, USA) | Age (≥30 y); Low level of education; Past use of drugs; Meeting casual sexual partners at a public venue; Syphilis seropositivity | [116], 2012 | |
| Bangkok, 2006 to 2012 | MSM, (1744, 18–56 y) | 21.3 (19.38–23.22) | ELISA (HerpeSelect 2, Focus Diagnostics, Cypress, CA 90630, USA) | [113], 2013 | ||
| Vietnam Range: 2.0–30.8 | HCMC, 1997 | Married women, (1106, 16–69 y) | 30.8 (28.1–33.4) | ELISA (Focus Diagnostics, Cypress, CA) | Higher age; Low level of education; Age at first intercourse (age <19 y); Age at first pregnancy (age <21 y); Nulliparous; Number of lifetime sexual partner (>1) | [117,118], 2009, 2003 |
| Hanoi, 1997 | Married women, (1170, 17–82 y) | 8.8 (7.1–10.5) | ELISA (Focus Diagnostics, Cypress, CA) | [117,118], 2009, 2003 | ||
| Bac Ninh Province, 2003 | Injection drug user, (309, 18–45 y) | 22.4 (17.6–27.9) | ELISA (HerpSelect 2, MRL; Focus Technologies, Los Anglees, CA) | Resident of Bac Ninh town; Injection frequency (daily) | [119], 2006 | |
| Lai Chau, Quang Tri, An Giang, Dong Thap, Kien Giang Province, 2002 to 2003 | FSW, (904, NA) | 27.7 (24.8–30.7) | ELISA (Genzyme Virotech GmbH, Russelsheim, Germany 2003) | Ethnicity (Kinh); Sex work; Number of clients (≥9/week); Ever worked outside Vietnam; >1 pregnancy termination; Syphilis seropositivity HIV status (positive) | [120], 2006 | |
| Lai Chau, Quang Tri, An Giang, Dong Thap, Kien Giang Province, 2004 | FSW, (982, NA) | 24.9 (22.2–27.6) | NA | [121], 2007 | ||
| Hanoi, 2004 | Married women, (1238, NA) | 2.0 (1.22–2.78) | ELISA (HerpSelect 2, MRL; Focus Technologies, Los Anglees, CA), Western Blot | [122], 2008 | ||
| Hai Phong city, Do Son beach, 2007 | Clients of FSW, (292, 18–60 y) | 16.35 (12.11–20.59) | ELISA (HerpeSelect 2, Focus Diagnostics, Cypress, CA 90630, USA) | Active and potential bridgers (males with sex with FSW and lower-risk women, not using condoms) | [123], 2009 | |
| (C) Unclassified HSV | ||||||
| Vietnam | Ho Chi Minh City, 1996 | HIV-infected drug users, (235, 24–57 y) | 99.0 (97.72–100–28) | ELISA (Behring) | [65], 1999 |
3.6.1. Seroprevalence by Country
3.6.2. Risk Factors for Seropositivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Coyne, C.B.; Lazear, H.M. Zika virus-reigniting the TORCH. Nat. Rev. Microbiol. 2016, 14, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, D.A. The Origins and Emergence of Zika Virus, the Newest TORCH Infection: What’s Old Is New Again. Arch. Pathol. Lab Med. 2017, 141, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams Waldorf, K.M.; McAdams, R.M. Influence of infection during pregnancy on fetal development. Reproduction 2013, 146, R151-62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madrid, L.; Varo, R.; Sitoe, A.; Bassat, Q. Congenital and perinatally-acquired infections in resource-constrained settings. Expert Rev. Anti Infect. Ther. 2016, 14, 845–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neu, N.; Duchon, J.; Zachariah, P. TORCH infections. Clin. Perinatol. 2015, 42, 77–103. [Google Scholar] [CrossRef] [PubMed]
- Menson, E.; Lyall, H. Clinical presentation of congenital viral infection. Curr. Paediatr. 2005, 15, 163–170. [Google Scholar] [CrossRef]
- Vynnycky, E.; Adams, E.J.; Cutts, F.T.; Reef, S.E.; Navar, A.M.; Simons, E.; Yoshida, L.M.; Brown, D.W.; Jackson, C.; Strebel, P.M.; et al. Using Seroprevalence and Immunisation Coverage Data to Estimate the Global Burden of Congenital Rubella Syndrome, 1996-2010: A Systematic Review. PLoS ONE 2016, 11, e0149160. [Google Scholar] [CrossRef] [Green Version]
- Khanal, S.; Bahl, S.; Sharifuzzaman, M.; Dhongde, D.; Pattamadilok, S.; Reef, S.; Morales, M.; Dabbagh, A.; Kretsinger, K.; Patel, M. Progress Toward Rubella and Congenital Rubella Syndrome Control-South-East Asia Region, 2000–2016. MMWR Morb. Mortal Wkly. Rep. 2018, 67, 602–606. [Google Scholar] [CrossRef]
- Knapp, J.K.; Mariano, K.M.; Pastore, R.; Grabovac, V.; Takashima, Y.; Alexander, J.P., Jr.; Reef, S.E.; Hagan, J.E. Progress Toward Rubella Elimination-Western Pacific Region, 2000–2019. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 744–750. [Google Scholar] [CrossRef]
- World Health Organization. JRF Supplementary Questionnaire on Surveillance 2017; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/JRF_Supplementary_Questionnaire_Surveillance_18Mar.pdf?ua=1 (accessed on 10 March 2019).
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Wallon, M.; Peyron, F. Congenital Toxoplasmosis: A Plea for a Neglected Disease. Pathogens 2018, 7, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauerbrei, A.; Wutzler, P. Neonatal varicella. J. Perinatol. 2001, 21, 545–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauerbrei, A.; Wutzler, P. Herpes simplex and varicella-zoster virus infections during pregnancy: Current concepts of prevention, diagnosis and therapy. Part 2: Varicella-zoster virus infections. Med. Microbiol. Immunol. 2007, 196, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Dontigny, L.; Arsenault, M.Y.; Martel, M.J.; Clinical Practice Obstetrics Committee. Rubella in pregnancy. J. Obstet. Gynaecol. Can. 2008, 30, 152–158. [Google Scholar] [CrossRef]
- Akpan, U.S.; Pillarisetty, L.S. Congenital Cytomegalovirus Infection (Congenital CMV Infection). Available online: https://www.ncbi.nlm.nih.gov/books/NBK541003/ (accessed on 9 September 2019).
- Sauerbrei, A.; Wutzler, P. Herpes simplex and varicella-zoster virus infections during pregnancy: Current concepts of prevention, diagnosis and therapy. Part 1: Herpes simplex virus infections. Med. Microbiol. Immunol. 2007, 196, 89–94. [Google Scholar] [CrossRef]
- Bhatta, A.K.; Keyal, U.; Liu, Y.; Gellen, E. Vertical transmission of herpes simplex virus: An update. J. Dtsch. Derm. Ges 2018, 16, 685–692. [Google Scholar] [CrossRef]
- UNICEF; United Nations Inter-Agency Group for Child Mortality Estimation (UN IGME). Child Mortality Estimates Country-Specific Neonatal Mortality Rate; UNICEF, 2019; Available online: https://data.unicef.org/resources/dataset/neonatal-mortality-data/ (accessed on 23 October 2019).
- United Nations Inter-Agency Group for Child Mortality Estimation (UN IGME). Levels & Trends in Child Mortality: Report 2019; United Nations Inter-Agency Group for Child Mortality Estimation. 2019; Available online: https://www.unicef.org/media/60561/file/UN-IGME-child-mortality-report-2019.pdf (accessed on 27 June 2019).
- Cutts, F.T.; Hanson, M. Seroepidemiology: An underused tool for designing and monitoring vaccination programmes in low- and middle-income countries. Trop. Med. Int. Health 2016, 21, 1086–1098. [Google Scholar] [CrossRef]
- Thompson, K.M.; Odahowski, C.L. Systematic Review of Measles and Rubella Serology Studies. Risk Anal. Off. Publ. Soc. Risk Anal. 2016, 36, 1459–1486. [Google Scholar] [CrossRef]
- Creative Research Systems. The Survey System. Available online: https://www.surveysystem.com/sscalc.htm (accessed on 11 January 2019).
- Dimech, W.; Mulders, M.N. A 16-year review of seroprevalence studies on measles and rubella. Vaccine 2016, 34, 4110–4118. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 21, e1000097. [Google Scholar] [CrossRef]
- Richard-Lenoble, D.; Cheng, H.K.; Sire, J.M.; Duong, T.H.; Cheng, T.V.; Phanny, I.; Rainsy, T.; Cauchoix, C. Toxoplasmosis in Cambodia: Initial serological evaluation at Phnom Penh. Sante 1999, 9, 377–382. [Google Scholar]
- Priest, J.W.; Jenks, M.H.; Moss, D.M.; Mao, B.; Buth, S.; Wannemuehler, K.; Soeung, S.C.; Lucchi, N.W.; Udhayakumar, V.; Gregory, C.J.; et al. Integration of Multiplex Bead Assays for Parasitic Diseases into a National, Population-Based Serosurvey of Women 15-39 Years of Age in Cambodia. PLoS Negl. Trop. Dis. 2016, 10, e0004699. [Google Scholar] [CrossRef] [PubMed]
- Catar, G.; Giboda, M.; Gutvirth, J.; Hongvanthong, B. Seroepidemiological study of toxoplasmosis in Laos. Southeast Asian J. Trop. Med. Public Health 1992, 23, 491–492. [Google Scholar] [PubMed]
- Andiappan, H.; Nissapatorn, V.; Sawangjaroen, N.; Nyunt, M.H.; Lau, Y.L.; Khaing, S.L.; Aye, K.M.; Mon, N.C.; Tan, T.C.; Kumar, T.; et al. Comparative study on Toxoplasma infection between Malaysian and Myanmar pregnant women. Parasites Vectors 2014, 7, 564. [Google Scholar] [CrossRef]
- Hakim, S.L.; Radzan, T.; Nazma, M. Distribution of anti-Toxoplasma gondii antibodies among Orang Asli (aborigines) in Peninsular Malaysia. Southeast Asian J. Trop. Med. Public Health 1994, 25, 485–489. [Google Scholar]
- Nissapatorn, V.; Kamarulzaman, A.; Init, I.; Tan, L.H.; Rohela, M.; Norliza, A.; Chan, L.L.; Latt, H.M.; Anuar, A.K.; Quek, K.F. Seroepidemiology of toxoplasmosis among HIV-infected patients and healthy blood donors. Med. J. Malays. 2002, 57, 304–310. [Google Scholar]
- Nissapatorn, V.; Lee, C.K.; Khairul, A.A. Seroprevalence of toxoplasmosis among AIDS patients in Hospital Kuala Lumpur, 2001. Singap. Med. J. 2003, 44, 194–196. [Google Scholar]
- Nissapatorn, V.; Lee, C.; Quek, K.F.; Leong, C.L.; Mahmud, R.; Abdullah, K.A. Toxoplasmosis in HIV/AIDS patients: A current situation. Jpn. J. Infect. Dis. 2004, 57, 160–165. [Google Scholar] [PubMed]
- Nissapatorn, V.; Lee, C.K.; Cho, S.M.; Rohela, M.; Anuar, A.K.; Quek, K.F.; Latt, H.M. Toxoplasmosis in HIV/AIDS patients in Malaysia. Southeast Asian J. Trop. Med. Public Health 2003, 34 (Suppl. 2), 80–85. [Google Scholar]
- Nissapatorn, V.; Noor Azmi, M.A.; Cho, S.M.; Fong, M.Y.; Init, I.; Rohela, M.; Khairul Anuar, A.; Quek, K.F.; Latt, H.M. Toxoplasmosis: Prevalence and risk factors. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2003, 23, 618–624. [Google Scholar] [CrossRef]
- Nissapatorn, V.; Lim, Y.A.; Jamaiah, I.; Agnes, L.S.; Amyliana, K.; Wen, C.C.; Nurul, H.; Nizam, S.; Quake, C.T.; Valartmathi, C.; et al. Parasitic infections in Malaysia: Changing and challenges. Southeast Asian J. Trop. Med. Public Health 2005, 36 (Suppl. 4), 50–59. [Google Scholar] [PubMed]
- Chan, B.T.; Amal, R.N.; Hayati, M.I.; Kino, H.; Anisah, N.; Norhayati, M.; Sulaiman, O.; Abdullah, M.M.; Fatmah, M.S.; Roslida, A.R.; et al. Seroprevalence of toxoplasmosis among migrant workers from different Asian countries working in Malaysia. Southeast Asian J. Trop. Med. Public Health 2008, 39, 9–13. [Google Scholar]
- Nissapatorn, V.; Leong, T.H.; Lee, R.; Init, I.; Ibrahim, J.; Yen, T.S. Seroepidemiology of toxoplasmosis in renal patients. Southeast Asian J. Trop. Med. Public Health 2011, 42, 237–247. [Google Scholar] [PubMed]
- Nimir, A.; Othman, A.; Ee, S.; Musa, Z.; Majid, I.A.; Kamarudin, Z.; Xian, C.; Isa, N.H. Latent toxoplasmosis in patients with different malignancy: A hospital based study. J. Clin. Med. Res. 2010, 2, 117–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngui, R.; Lim, Y.A.; Amir, N.F.; Nissapatorn, V.; Mahmud, R. Seroprevalence and sources of toxoplasmosis among Orang Asli (indigenous) communities in Peninsular Malaysia. Am. J. Trop. Med. Hyg. 2011, 85, 660–666. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Khang, T.F.; Andiappan, H.; Nissapatorn, V.; Subrayan, V. An age-adjusted seroprevalence study of Toxoplasma antibody in a Malaysian ophthalmology unit. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 322–326. [Google Scholar] [CrossRef]
- Emelia, O.; Amal, R.N.; Ruzanna, Z.Z.; Shahida, H.; Azzubair, Z.; Tan, K.S.; Noor Aadila, S.; Siti, N.A.; Aisah, M.Y. Seroprevalence of anti-Toxoplasma gondii IgG antibody in patients with schizophrenia. Trop. Biomed. 2012, 29, 151–159. [Google Scholar]
- Omar, A.; Bakar, O.C.; Adam, N.F.; Osman, H.; Osman, A.; Suleiman, A.H.; Manaf, M.R.; Selamat, M.I. Seropositivity and serointensity of Toxoplasma gondii antibodies and DNA among patients with schizophrenia. Korean J. Parasitol. 2015, 53, 29–34. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Ngui, R.; Muhammad Aidil, R.; Lim, Y.A.; Rohela, M. Current status of parasitic infections among Pangkor Island community in Peninsular Malaysia. Trop. Biomed. 2014, 31, 836–843. [Google Scholar] [PubMed]
- Emelia, O.; Rahana, A.R.; Mohamad Firdaus, A.; Cheng, H.S.; Nursyairah, M.S.; Fatinah, A.S.; Azmawati, M.N.; Siti, N.A.; Aisah, M.Y. IgG avidity assay: A tool for excluding acute toxoplasmosis in prolonged IgM titer sera from pregnant women. Trop. Biomed. 2014, 31, 633–640. [Google Scholar] [PubMed]
- Angal, L.; Lim, Y.A.; Yap, N.J.; Ngui, R.; Amir, A.; Kamarulzaman, A.; Rohela, M. Toxoplasmosis in HIV and non HIV prisoners in Malaysia. Trop. Biomed. 2016, 33, 159–169. [Google Scholar] [PubMed]
- Brandon-Mong, G.J.; Che Mat Seri, N.A.; Sharma, R.S.; Andiappan, H.; Tan, T.C.; Lim, Y.A.; Nissapatorn, V. Seroepidemiology of Toxoplasmosis among People Having Close Contact with Animals. Front. Immunol. 2015, 6, 143. [Google Scholar] [CrossRef] [Green Version]
- van Enter, B.J.D.; Lau, Y.L.; Ling, C.L.; Watthanaworawit, W.; Sukthana, Y.; Lee, W.C.; Nosten, F.; McGready, R. Seroprevalence of Toxoplasma gondii Infection in Refugee and Migrant Pregnant Women along the Thailand-Myanmar Border. Am. J. Trop. Med. Hyg. 2017, 97, 232–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, A.; Tan, K.H.; Tee, C.S.; Yeo, G.S. Seroprevalence of cytomegalovirus, toxoplasma and parvovirus in pregnancy. Singap. Med. J. 2000, 41, 151–155. [Google Scholar]
- Lim, R.B.; Tan, M.T.; Young, B.; Lee, C.C.; Leo, Y.S.; Chua, A.; Ng, O.T. Risk factors and time-trends of cytomegalovirus (CMV), syphilis, toxoplasmosis and viral hepatitis infection and seroprevalence in human immunodeficiency virus (HIV) infected patients. Ann. Acad. Med. Singap. 2013, 42, 667–673. [Google Scholar]
- Chintana, T. Pattern of antibodies in toxoplasmosis of pregnant women and their children in Thailand. Southeast Asian J. Trop. Med. Public Health 1991, 22, 107–110. [Google Scholar]
- Taechowisan, T.; Sutthent, R.; Louisirirotchanakul, S.; Puthavathana, P.; Wasi, C. Immune status in congenital infections by TORCH agents in pregnant Thais. Asian Pac. J. Allergy Immunol. 1997, 15, 93–97. [Google Scholar] [PubMed]
- Chintana, T.; Sukthana, Y.; Bunyakai, B.; Lekkla, A. Toxoplasma gondii antibody in pregnant women with and without HIV infection. Southeast Asian J. Trop. Med. Public Health 1998, 29, 383–386. [Google Scholar]
- Sukthana, Y. Difference of Toxoplasma gondii antibodies between Thai and Austrian pregnant women. Southeast Asian J. Trop. Med. Public Health 1999, 30, 38–41. [Google Scholar]
- Maruyama, S.; Boonmar, S.; Morita, Y.; Sakai, T.; Tanaka, S.; Yamaguchi, F.; Kabeya, H.; Katsube, Y. Seroprevalence of Bartonella henselae and Toxoplasma gondii among healthy individuals in Thailand. J. Vet. Med. Sci. 2000, 62, 635–637. [Google Scholar] [CrossRef] [Green Version]
- Pinlaor, S.; Ieamviteevanich, K.; Pinlaor, P.; Maleewong, W.; Pipitgool, V. Seroprevalence of specific total immunoglobulin (Ig), IgG and IgM antibodies to Toxoplasma gondii in blood donors from Loei Province, Northeast Thailand. Southeast Asian J. Trop. Med. Public Health 2000, 31, 123–127. [Google Scholar] [PubMed]
- Wanachiwanawin, D.; Sutthent, R.; Chokephaibulkit, K.; Mahakittikun, V.; Ongrotchanakun, J.; Monkong, N. Toxoplasma gondii antibodies in HIV and non-HIV infected Thai pregnant women. Asian Pac. J. Allergy Immunol. 2001, 19, 291–293. [Google Scholar] [PubMed]
- Tantivanich, S.; Amarapal, P.; Suphadtanaphongs, W.; Siripanth, C.; Sawatmongkonkun, W. Prevalence of congenital cytomegalovirus and Toxoplasma antibodies in Thailand. Southeast Asian J. Trop. Med. Public Health 2001, 32, 466–469. [Google Scholar] [PubMed]
- Sukthana, Y.; Kaewkungwal, J.; Jantanavivat, C.; Lekkla, A.; Chiabchalard, R.; Aumarm, W. Toxoplasma gondii antibody in Thai cats and their owners. Southeast Asian J. Trop. Med. Public Health 2003, 34, 733–738. [Google Scholar] [PubMed]
- Sakae, C.; Natphopsuk, S.; Settheetham-Ishida, W.; Ishida, T. Low prevalence of Toxoplasma gondii infection among women in northeastern Thailand. J. Parasitol. 2013, 99, 172–173. [Google Scholar] [CrossRef]
- Nissapatorn, V.; Suwanrath, C.; Sawangjaroen, N.; Ling, L.Y.; Chandeying, V. Toxoplasmosis-serological evidence and associated risk factors among pregnant women in southern Thailand. Am. J. Trop. Med. Hyg. 2011, 85, 243–247. [Google Scholar] [CrossRef]
- Chemoh, W.; Sawangjaroen, N.; Nissapatorn, V.; Suwanrath, C.; Chandeying, V.; Hortiwakul, T.; Andiappan, H.; Sermwittayawong, N.; Charoenmak, B.; Siripaitoon, P.; et al. Toxoplasma gondii infection: What is the real situation? Exp. Parasitol. 2013, 135, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Chemoh, W.; Sawangjaroen, N.; Siripaitoon, P.; Andiappan, H.; Hortiwakul, T.; Sermwittayawong, N.; Charoenmak, B.; Nissapatorn, V. Toxoplasma gondii-Prevalence and Risk Factors in HIV-infected Patients from Songklanagarind Hospital, Southern Thailand. Front. Microbiol. 2015, 6, 1304. [Google Scholar] [CrossRef]
- Andiappan, H.; Nissapatorn, V.; Sawangjaroen, N.; Chemoh, W.; Lau, Y.L.; Kumar, T.; Onichandran, S.; Suwanrath, C.; Chandeying, V. Toxoplasma infection in pregnant women: A current status in Songklanagarind hospital, southern Thailand. Parasites Vectors 2014, 7, 239. [Google Scholar] [CrossRef] [Green Version]
- Follezou, J.Y.; Lan, N.Y.; Lien, T.X.; Lafon, M.E.; Tram, L.T.; Hung, P.V.; Aknine, X.; Lowenstein, W.; Ngai, N.V.; Theodorou, I.; et al. Clinical and biological characteristics of human immunodeficiency virus-infected and uninfected intravascular drug users in Ho Chi Minh City, Vietnam. Am. J. Trop. Med. Hyg. 1999, 61, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Udonsom, R.; Lekkla, A.; Chung, P.T.; Cam, P.D.; Sukthana, Y. Seroprevalence of Toxoplasma gondii antibody in Vietnamese villagers. Southeast Asian J. Trop. Med. Public Health 2008, 39, 14–18. [Google Scholar]
- Buchy, P.; Follezou, J.Y.; Lien, T.X.; An, T.T.; Tram, L.T.; Tri, D.V.; Cuong, N.M.; Glaziou, P.; Chien, B.T. Serological study of toxoplasmosis in Vietnam in a population of drug users (Ho Chi Minh city) and pregnant women (Nha Trang). Bull. De La Soc. De Pathol. Exot. 2003, 96, 46–47. [Google Scholar]
- Black, A.P.; Vilivong, K.; Nouanthong, P.; Souvannaso, C.; Hubschen, J.M.; Muller, C.P. Serosurveillance of vaccine preventable diseases and hepatitis C in healthcare workers from Lao PDR. PLoS ONE 2015, 10, e0123647. [Google Scholar] [CrossRef] [Green Version]
- Yap, S.H.; Abdullah, N.K.; McStea, M.; Takayama, K.; Chong, M.L.; Crisci, E.; Larsson, M.; Azwa, I.; Kamarulzaman, A.; Leong, K.H.; et al. HIV/Human herpesvirus co-infections: Impact on tryptophan-kynurenine pathway and immune reconstitution. PLoS ONE 2017, 12, e0186000. [Google Scholar] [CrossRef] [Green Version]
- Dashraath, P.; Ong, E.S.; Lee, V.J. Seroepidemiology of varicella and the reliability of a self-reported history of varicella infection in Singapore military recruits. Ann. Acad. Med. Singap. 2007, 36, 636–641. [Google Scholar] [PubMed]
- Fatha, N.; Ang, L.W.; Goh, K.T. Changing seroprevalence of varicella zoster virus infection in a tropical city state, Singapore. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2014, 22, 73–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorny, A.W.; Mittal, C.; Saw, S.; Venkatachalam, I.; Fisher, D.A.; Tambyah, P.A. Varicella seroprevalence in healthcare workers in a tertiary hospital: An audit of cross-sectional data. BMC Res. Notes 2015, 8, 664. [Google Scholar] [CrossRef] [Green Version]
- Migasena, S.; Simasathien, S.; Desakorn, V.; Phonrat, B.; Suntharasamai, P.; Pitisuttitham, P.; Aree, C.; Naksrisook, S.; Supeeranun, L.; Samakoses, R.; et al. Seroprevalence of Varicella-Zoster Virus Antibody in Thailand. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 1997, 2, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Lolekha, S.; Tanthiphabha, W.; Sornchai, P.; Kosuwan, P.; Sutra, S.; Warachit, B.; Chup-Upprakarn, S.; Hutagalung, Y.; Weil, J.; Bock, H.L. Effect of climatic factors and population density on varicella zoster virus epidemiology within a tropical country. Am. J. Trop. Med. Hyg. 2001, 64, 131–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowitdamrong, E.; Pancharoen, C.; Thammaborvorn, R.; Bhattarakosol, P. The prevalence of varicella-zoster virus infection in normal healthy individuals aged above 6 months. J. Med Assoc. Thail. Chotmaihet Thangphaet 2005, 88 (Suppl. 4), S7–S11. [Google Scholar]
- Srichomkwun, P.; Apisarnthanarak, A.; Thongphubeth, K.; Yuekyen, C.; Mundy, L.M. Evidence of vaccine protection among thai medical students and implications for occupational health. Infect. Control Hosp. Epidemiol. 2009, 30, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Suwanpakdee, D.; Laohapand, C.; Moolasart, V.; Lomtong, P.; Krairojananan, N.; Srisawat, P.; Watanaveeradej, V. Serosurveillance of varicella and hepatitis B infection after reported cases in medical students and the relationship between past varicella disease history and immunity status. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2012, 95 (Suppl. 5), S80–S85. [Google Scholar]
- Ooi, S.L.; Hooi, P.S.; Chua, B.H.; Lam, S.K.; Chua, K.B. Seroprevalence of human parvovirus B19 infection in an urban population in Malaysia. Med. J. Malays. 2002, 57, 97–103. [Google Scholar]
- Matsunaga, Y.; Goh, K.T.; Utagawa, E.; Muroi, N. Low prevalence of antibody to human parvovirus B19 in Singapore. Epidemiol. Infect. 1994, 113, 537–540. [Google Scholar] [CrossRef] [Green Version]
- Poovorawan, Y.; Theamboonlers, A.; Suandork, P.; Hirsch, P. Prevalence of antibodies to parvovirus B 19 in Thailand. Southeast Asian J. Trop. Med. Public Health 2000, 31, 422–424. [Google Scholar]
- Suandork, P.; Theamboonlers, A.; Likitnukul, S.; Hirsch, P.; Poovorawan, Y. Parvovirus B19 antibodies in immunocompromized children in Thailand. Asian Pac. J. Allergy Immunol. 2000, 18, 161–164. [Google Scholar] [PubMed]
- Bhattarakosol, P.; Pancharoen, C.; Kowitdamrong, E.; Thammaborvorn, R.; Mungmee, V. Prevalence of parvovirus B19 infection in Thai young adults. Southeast Asian J. Trop. Med. Public Health 2003, 34, 585–588. [Google Scholar]
- Mao, B.; Chheng, K.; Wannemuehler, K.; Vynnycky, E.; Buth, S.; Soeung, S.C.; Reef, S.; Weldon, W.; Quick, L.; Gregory, C.J. Immunity to polio, measles and rubella in women of child-bearing age and estimated congenital rubella syndrome incidence, Cambodia, 2012. Epidemiol. Infect. 2015, 143, 1858–1867. [Google Scholar] [CrossRef] [Green Version]
- Phengxay, M.; Hayakawa, Y.; Phan, T.G.; Uneno-Yamamoto, K.; Tanaka-Taya, K.; Vongphrachanh, P.; Komase, K.; Ushijima, H. Seroprevalence of rubella and measles antibodies in Lao PDR. Clin. Lab. 2011, 57, 237–244. [Google Scholar] [PubMed]
- Hachiya, M.; Miyano, S.; Mori, Y.; Vynnycky, E.; Keungsaneth, P.; Vongphrachanh, P.; Xeuatvongsa, A.; Sisouk, T.; Som-Oulay, V.; Khamphaphongphane, B.; et al. Evaluation of nationwide supplementary immunization in Lao People’s Democratic Republic: Population-based seroprevalence survey of anti-measles and anti-rubella IgG in children and adults, mathematical modelling and a stability testing of the vaccine. PLoS ONE 2018, 13, e0194931. [Google Scholar] [CrossRef] [PubMed]
- Sekawi, Z.; Muizatul, W.M.; Marlyn, M.; Jamil, M.A.; Ilina, I. Rubella vaccination programme in Malaysia: Analysis of a seroprevalence study in an antenatal clinic. Med. J. Malays. 2005, 60, 345–348. [Google Scholar]
- Cheong, A.T.; Khoo, E.M. Prevalence of rubella susceptibility among pregnant mothers in a community-based antenatal clinic in Malaysia: A cross-sectional study. Asia Pac. J. Public Health 2008, 20, 340–346. [Google Scholar] [CrossRef]
- Cheong, A.T.; Tong, S.F.; Khoo, E.M. How useful is a history of rubella vaccination for determination of disease susceptibility? A cross-sectional study at a public funded health clinic in Malaysia. BMC Fam. Pract. 2013, 14, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, L.W.; Chua, L.T.; James, L.; Goh, K.T. Epidemiological surveillance and control of rubella in Singapore, 1991–2007. Ann. Acad. Med. Singap. 2010, 39, 95–101. [Google Scholar] [PubMed]
- Liew, F.; Ang, L.W.; Cutter, J.; James, L.; Goh, K.T. Evaluation on the effectiveness of the national childhood immunisation programme in Singapore, 1982–2007. Ann. Acad. Med. Singap. 2010, 39, 532. [Google Scholar]
- Chua, Y.X.; Ang, L.W.; Low, C.; James, L.; Cutter, J.L.; Goh, K.T. An epidemiological assessment towards elimination of rubella and congenital rubella syndrome in Singapore. Vaccine 2015, 33, 3150–3157. [Google Scholar] [CrossRef]
- Ang, L.W.; Lai, F.Y.; Tey, S.H.; Cutter, J.; James, L.; Goh, K.T. Prevalence of antibodies against measles, mumps and rubella in the childhood population in Singapore, 2008–2010. Epidemiol. Infect. 2013, 141, 1721–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonruang, S.; Buppasiri, P. Rubella antibodies in normal pregnant women at Srinagarind Hospital, Khon Kaen, Thailand. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2005, 88, 455–459. [Google Scholar]
- Tharmaphornpilas, P.; Yoocharean, P.; Rasdjarmrearnsook, A.O.; Theamboonlers, A.; Poovorawan, Y. Seroprevalence of antibodies to measles, mumps, and rubella among Thai population: Evaluation of measles/MMR immunization programme. J. Health Popul. Nutr. 2009, 27, 80–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaiwarith, R.; Praparattanapan, J.; Nuket, K.; Kotarathitithum, W.; Supparatpinyo, K. Seroprevalence of antibodies to measles, mumps, and rubella, and serologic responses after vaccination among human immunodeficiency virus (HIV)-1 infected adults in Northern Thailand. BMC Infect. Dis. 2016, 16, 190. [Google Scholar] [CrossRef] [Green Version]
- Sreepian, P.M.; Sreepian, A. Seroprevalence of Rubella Immunity among women of childbearing age in Bangkok, Thailand. Southeast Asian J. Trop. Med. Public Health 2018, 49, 76–81. [Google Scholar]
- Miyakawa, M.; Yoshino, H.; Yoshida, L.M.; Vynnycky, E.; Motomura, H.; Tho le, H.; Thiem, V.D.; Ariyoshi, K.; Anh, D.D.; Moriuchi, H. Seroprevalence of rubella in the cord blood of pregnant women and congenital rubella incidence in Nha Trang, Vietnam. Vaccine 2014, 32, 1192–1198. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Vaccine-Preventable Diseases: Monitoring System. 2020 Global Summary. Available online: https://apps.who.int/immunization_monitoring/globalsummary/schedules (accessed on 11 October 2020).
- World Health Organization. Vaccine Introduction; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/immunization/monitoring_surveillance/data/year_vaccine_introduction.xls (accessed on 27 June 2019).
- Ahmed, S.A.; Al-Joudi, F.S.; Zaidah, A.W.; Roshan, T.M.; Rapiaah, M.; Abdullah, Y.M.; Rosline, H. The prevalence of human cytomegalovirus seropositivity among blood donors at the Unit of Blood Transfusion Medicine, Hospital Universiti Sains Malaysia. Southeast Asian J. Trop. Med. Public Health 2006, 37, 294–296. [Google Scholar] [PubMed]
- Saraswathy, T.S.; Az-Ulhusna, A.; Asshikin, R.N.; Suriani, S.; Zainah, S. Seroprevalence of cytomegalovirus infection in pregnant women and associated role in obstetric complications: A preliminary study. Southeast Asian J. Trop. Med. Public Health 2011, 42, 320–322. [Google Scholar]
- Urwijitaroon, Y.; Teawpatanataworn, S.; Kitjareontarm, A. Prevalence of cytomegalovirus antibody in Thai-northeastern blood donors. Southeast Asian J. Trop. Med. Public Health 1993, 24 (Suppl. 1), 180–182. [Google Scholar] [PubMed]
- Tantivanich, S.; Suphadtanaphongs, V.; Siripanth, C.; Desakorn, V.; Suphanit, I.; Phromin, S.; Panakitsuwan, S.; Amarapand, P. Prevalence of cytomegalovirus antibodies among various age groups of Thai population. Southeast Asian J. Trop. Med. Public Health 1999, 30, 265–268. [Google Scholar] [PubMed]
- Amarapal, P.; Tantivanich, S.; Balachandra, K. Prevalence of cytomegalovirus in Thai blood donors by monoclonal staining of blood leukocytes. Southeast Asian J. Trop. Med. Public Health 2001, 32, 148–153. [Google Scholar]
- Fongsarun, J.E.; Ekkapongpisit, M.; Paisan, M.; Chanthachorn, S.; Papadopoulos, K.I. Prevalence of transmissible viral disease in maternal blood samples of autologous umbilical cord blood in a private cord blood bank. Transplant. Technol. 2013. [Google Scholar] [CrossRef]
- Bhattarakosol, P.; Sithidajporn, M.; Bhattarakosol, P. Seroprevalence of cytomegalovirus infection in Thai adults detecting by ELISA. Chula Med. J. 1998, 42, 935–943. Available online: http://clmjournal.org/_fileupload/journal/298-2-3.pdf (accessed on 19 April 2019).
- O’Charoen; Nuchprayoon, C.; Chumnijarakij, T.; Ganapi, S. Cytomegalovirus Antibody Screening Program of Thai Blood Donors for Bone-Marrow Transplant Patients. Available online: http://www.tsh.or.th/file_upload/files/v2%20n1%20023.pdf (accessed on 19 April 2019).
- Theng, T.S.; Sen, P.R.; Tan, H.H.; Wong, M.L.; Chan, K.W. Seroprevalence of HSV-1 and 2 among sex workers attending a sexually transmitted infection clinic in Singapore. Int. J. STD AIDS 2006, 17, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Theng, C.T.; Sen, P.R.; Chio, T.W.; Tan, H.H.; Wong, M.L.; Chan, R.K. Seroprevalence of herpes simplex virus-1 and -2 in attendees of a sexually transmitted infection clinic in Singapore. Sex. Health 2006, 3, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Nopkesorn, T.; Mock, P.A.; Mastro, T.D.; Sangkharomya, S.; Sweat, M.; Limpakarnjanarat, K.; Laosakkitiboran, J.; Young, N.L.; Morse, S.A.; Schmid, S.; et al. HIV-1 subtype E incidence and sexually transmitted diseases in a cohort of military conscripts in northern Thailand. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. Off. Publ. Int. Retrovirol. Assoc. 1998, 18, 372–379. [Google Scholar] [CrossRef]
- Dobbins, J.G.; Mastro, T.D.; Nopkesorn, T.; Sangkharomya, S.; Limpakarnjanarat, K.; Weniger, B.G.; Schmid, D.S. Herpes in the time of AIDS: A comparison of the epidemiology of HIV-1 and HSV-2 in young men in northern Thailand. Sex. Transm. Dis. 1999, 26, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Limpakarnjanarat, K.; Mastro, T.D.; Saisorn, S.; Uthaivoravit, W.; Kaewkungwal, J.; Korattana, S.; Young, N.L.; Morse, S.A.; Schmid, D.S.; Weniger, B.G.; et al. HIV-1 and other sexually transmitted infections in a cohort of female sex workers in Chiang Rai, Thailand. Sex. Transm. Infect. 1999, 75, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Griensven, F.; Thienkrua, W.; McNicholl, J.; Wimonsate, W.; Chaikummao, S.; Chonwattana, W.; Varangrat, A.; Sirivongrangson, P.; Mock, P.A.; Akarasewi, P.; et al. Evidence of an explosive epidemic of HIV infection in a cohort of men who have sex with men in Thailand. Aids 2013, 27, 825–832. [Google Scholar] [CrossRef]
- Ashley-Morrow, R.; Nollkamper, J.; Robinson, N.J.; Bishop, N.; Smith, J. Performance of focus ELISA tests for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2004, 10, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Bollen, L.J.; Whitehead, S.J.; Mock, P.A.; Leelawiwat, W.; Asavapiriyanont, S.; Chalermchockchareonkit, A.; Vanprapar, N.; Chotpitayasunondh, T.; McNicholl, J.M.; Tappero, J.W.; et al. Maternal herpes simplex virus type 2 coinfection increases the risk of perinatal HIV transmission: Possibility to further decrease transmission? Aids 2008, 22, 1169–1176. [Google Scholar] [CrossRef]
- Holtz, T.H.; Thienkrua, W.; McNicholl, J.M.; Wimonsate, W.; Chaikummao, S.; Chonwattana, W.; Wasinrapee, P.; Varangrat, A.; Mock, P.A.; Sirivongrangson, P.; et al. Prevalence of Treponema pallidum seropositivity and herpes simplex virus type 2 infection in a cohort of men who have sex with men, Bangkok, Thailand, 2006–2010. Int. J. STD AIDS 2012, 23, 424–428. [Google Scholar] [CrossRef]
- Le, H.V.; Schoenbach, V.J.; Herrero, R.; Hoang Pham, A.T.; Nguyen, H.T.; Nguyen, T.T.; Munoz, N.; Franceschi, S.; Vaccarella, S.; Parkin, M.D.; et al. Herpes simplex virus type-2 seropositivity among ever married women in South and north Vietnam: A population-based study. Sex. Transm. Dis. 2009, 36, 616–620. [Google Scholar] [CrossRef] [Green Version]
- Anh, P.T.H.; Hieu, N.T.; Herrero, R.; Vaccarella, S.; Smith, J.S.; Nguyen Thuy, T.T.; Nguyen, H.N.; Nguyen, B.D.; Ashley, R.; Snijders, P.J.; et al. Human papillomavirus infection among women in South and North Vietnam. Int. J. Cancer 2003, 104, 213–220. [Google Scholar] [CrossRef]
- Go, V.F.; Frangakis, C.; Nam le, V.; Bergenstrom, A.; Sripaipan, T.; Zenilman, J.M.; Celentano, D.D.; Quan, V.M. High HIV sexual risk behaviors and sexually transmitted disease prevalence among injection drug users in Northern Vietnam: Implications for a generalized HIV epidemic. J. Acquir. Immune Defic. Syndr. 2006, 42, 108–115. [Google Scholar] [CrossRef]
- O’Farrell, N.; Thuong, N.V.; Nghia, K.V.; Tram, L.T.; Long, N.T. HSV-2 antibodies in female sex workers in Vietnam. Int. J. STD AIDS 2006, 17, 755–758. [Google Scholar] [CrossRef]
- Thuong, N.V.; Van Nghia, K.; Hau, T.P.; Long, N.T.; Van, C.T.; Duc, B.H.; Tram, L.T.; Tuan, N.A.; Tien, N.T.; Godwin, P.; et al. Impact of a community sexually transmitted infection/HIV intervention project on female sex workers in five border provinces of Vietnam. Sex. Transm. Infect. 2007, 83, 376–382. [Google Scholar] [CrossRef]
- Ngo, T.D.; Laeyendecker, O.; La, H.; Hogrefe, W.; Morrow, R.A.; Quinn, T.C. Use of commercial enzyme immunoassays to detect antibodies to the herpes simplex virus type 2 glycoprotein G in a low-risk population in Hanoi, Vietnam. Clin. Vaccine Immunol. CVI 2008, 15, 382–384. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.T.; Nguyen, H.T.; Trinh, H.Q.; Mills, S.J.; Detels, R. Clients of female sex workers as a bridging population in Vietnam. AIDS Behav. 2009, 13, 881–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, D.; Dubey, J.P. Toxoplasma gondii: Transmission, diagnosis and prevention. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2002, 8, 634–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleyer, U.; Gross, U.; Schluter, D.; Wilking, H.; Seeber, F. Toxoplasmosis in Germany. Dtsch. Arztebl. Int. 2019, 116, 435–444. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Parasites-Toxoplasmosis (Toxoplasma Infection) Prevention & Control. Available online: https://www.cdc.gov/parasites/toxoplasmosis/prevent.html (accessed on 10 June 2019).
- Jones, J.L.; Ogunmodede, F.; Scheftel, J.; Kirkland, E.; Lopez, A.; Schulkin, J.; Lynfield, R. Toxoplasmosis-related knowledge and practices among pregnant women in the United States. Infect Dis Obs. Gynecol 2003, 11, 139–145. [Google Scholar] [CrossRef]
- Pereboom, M.T.; Mannien, J.; Spelten, E.R.; Schellevis, F.G.; Hutton, E.K. Observational study to assess pregnant women’s knowledge and behaviour to prevent toxoplasmosis, listeriosis and cytomegalovirus. BMC Pregnancy Childbirth 2013, 13, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andiappan, H.; Nissapatorn, V.; Sawangjaroen, N.; Khaing, S.L.; Salibay, C.C.; Cheung, M.M.; Dungca, J.Z.; Chemoh, W.; Xiao Teng, C.; Lau, Y.L.; et al. Knowledge and practice on Toxoplasma infection in pregnant women from Malaysia, Philippines, and Thailand. Front. Microbiol. 2014, 5, 291. [Google Scholar] [CrossRef]
- Mun San, L. Vaccination for HCWs. SMA News. 2015. Available online: https://www.sma.org.sg/UploadedImg/files/Publications%20-%20SMA%20News/4701/ES.pdf (accessed on 27 April 2019).
- Ministry of Health Malaysia. Adult Vaccination. 2003. Available online: https://de.slideshare.net/Rubzzzz/malaysia-cpg-for-adult-vaccination (accessed on 28 April 2019).
- Daulagala, S.; Noordeen, F. Epidemiology and factors influencing varicella infections in tropical countries including Sri Lanka. Virusdisease 2018, 29, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W. Review of varicella zoster seroepidemiology in India and Southeast Asia. Trop. Med. Int. Health 1998, 3, 886–890. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly. Epidemiol. Rec. 2014, 89, 265–288. Available online: https://www.who.int/wer/2014/wer8925.pdf (accessed on 21 March 2019).
- World Health Organization. Rubella vaccines: WHO position paper. Wkly. Epidemiol. Rec. 2011, 86, 301–316. [Google Scholar] [CrossRef]
- Regional Strategy and Plan of Action for Measles and Rubella Elimination in the Western Pacific; World Health Organization: Manila, PA, USA, 2017.
- Singapore Ministry of Health. Congenital rubella prevention in Singapore: A success story. Epidemiol. News Bull. 2006, 32, 41–61. Available online: https://www.semanticscholar.org/paper/Congenital-rubella-prevention-in-Singapore%3A-a-story/01ea66098675339d84a34f44af4262cc9f7a7f94?p2df (accessed on 9 January 2019).
- World Health Organization (Old Version). EPI Fact Sheet Thailand. World Health Organization. New Dheli, Most Recent Version. 2016. Available online: https://apps.who.int/iris/handle/10665/336764?locale-attribute=de& (accessed on 9 January 2019).
- Centers for Disease Control and Prevention. Success in Cambodia: The Disappearance of Measles! (With Rubella Not Far Behind). Available online: https://www.cdc.gov/globalhealth/immunization/stories/success-in-cambodia.htm (accessed on 9 January 2019).
- World Health Organization (Old Version). Summary of Supplementary Immunization Activities from 2000 to 2019. World Health Organization. Most Recent Version. 2018. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/immunization-analysis-and-insights/global-monitoring/data-statistics-and-graphics (accessed on 8 March 2019).
- World Health Organization (Old Version). EPI Fact Sheet Myanmar. World Health Organization. Most Recent Version. 2016. Available online: https://apps.who.int/iris/handle/10665/329987 (accessed on 9 January 2019).
- Vynnycky, E.; Yoshida, L.M.; Huyen, D.T.; Trung, N.D.; Toda, K.; Cuong, N.V.; Thi Hong, D.; Ariyoshi, K.; Miyakawa, M.; Moriuchi, H.; et al. Modeling the impact of rubella vaccination in Vietnam. Hum. Vaccines Immunother. 2016, 12, 150–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef]
- Manicklal, S.; Emery, V.C.; Lazzarotto, T.; Boppana, S.B.; Gupta, R.K. The “silent” global burden of congenital cytomegalovirus. Clin. Microbiol. Rev. 2013, 26, 86–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanzieri, T.M.; Dollard, S.C.; Bialek, S.R.; Grosse, S.D. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2014, 22, 44–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vries, J.J.; van Zwet, E.W.; Dekker, F.W.; Kroes, A.C.; Verkerk, P.H.; Vossen, A.C. The apparent paradox of maternal seropositivity as a risk factor for congenital cytomegalovirus infection: A population-based prediction model. Rev. Med. Virol. 2013, 23, 241–249. [Google Scholar] [CrossRef]
- Lim, S.L.; Tan, W.C.; Tan, L.K. Awareness of and attitudes toward congenital cytomegalovirus infection among pregnant women in Singapore. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2012, 117, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Victor, M.; Adler, S.P.; Arwady, A.; Demmler, G.; Fowler, K.; Goldfarb, J.; Keyserling, H.; Massoudi, M.; Richards, K.; et al. Knowledge and awareness of congenital cytomegalovirus among women. Infect. Dis. Obs. Gynecol. 2006, 2006, 80383. [Google Scholar] [CrossRef]
- Ross, D.S.; Victor, M.; Sumartojo, E.; Cannon, M.J. Women’s knowledge of congenital cytomegalovirus: Results from the 2005 HealthStyles survey. J. Womens Health (Larchmt.) 2008, 17, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Revello, M.G.; Tibaldi, C.; Masuelli, G.; Frisina, V.; Sacchi, A.; Furione, M.; Arossa, A.; Spinillo, A.; Klersy, C.; Ceccarelli, M.; et al. Prevention of Primary Cytomegalovirus Infection in Pregnancy. EBioMedicine 2015, 2, 1205–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Looker, K.J.; Garnett, G.P.; Schmid, G.P. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull. World Health Organ. 2008, 86, 805–812. [Google Scholar] [CrossRef]
- Smith, J.S.; Robinson, N.J. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: A global review. J. Infect. Dis. 2002, 186 (Suppl. 1), S3–S28. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuchs, F.E.; Pauly, M.; Black, A.P.; Hübschen, J.M. Seroprevalence of ToRCH Pathogens in Southeast Asia. Microorganisms 2021, 9, 574. https://doi.org/10.3390/microorganisms9030574
Fuchs FE, Pauly M, Black AP, Hübschen JM. Seroprevalence of ToRCH Pathogens in Southeast Asia. Microorganisms. 2021; 9(3):574. https://doi.org/10.3390/microorganisms9030574
Chicago/Turabian StyleFuchs, Franziska E., Maude Pauly, Antony P. Black, and Judith M. Hübschen. 2021. "Seroprevalence of ToRCH Pathogens in Southeast Asia" Microorganisms 9, no. 3: 574. https://doi.org/10.3390/microorganisms9030574
APA StyleFuchs, F. E., Pauly, M., Black, A. P., & Hübschen, J. M. (2021). Seroprevalence of ToRCH Pathogens in Southeast Asia. Microorganisms, 9(3), 574. https://doi.org/10.3390/microorganisms9030574






