Metabolome Analysis of Constituents in Membrane Vesicles for Clostridium thermocellum Growth Stimulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions of C. thermocellum and B. subtilis
2.2. Preparation of MV Fraction of C. thermocellum
2.3. Growth Evaluation of C. thermocellum with MV Supplementation
2.4. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analysis of C. thermocellum MV and B. subtilis Broth
3. Results and Discussion
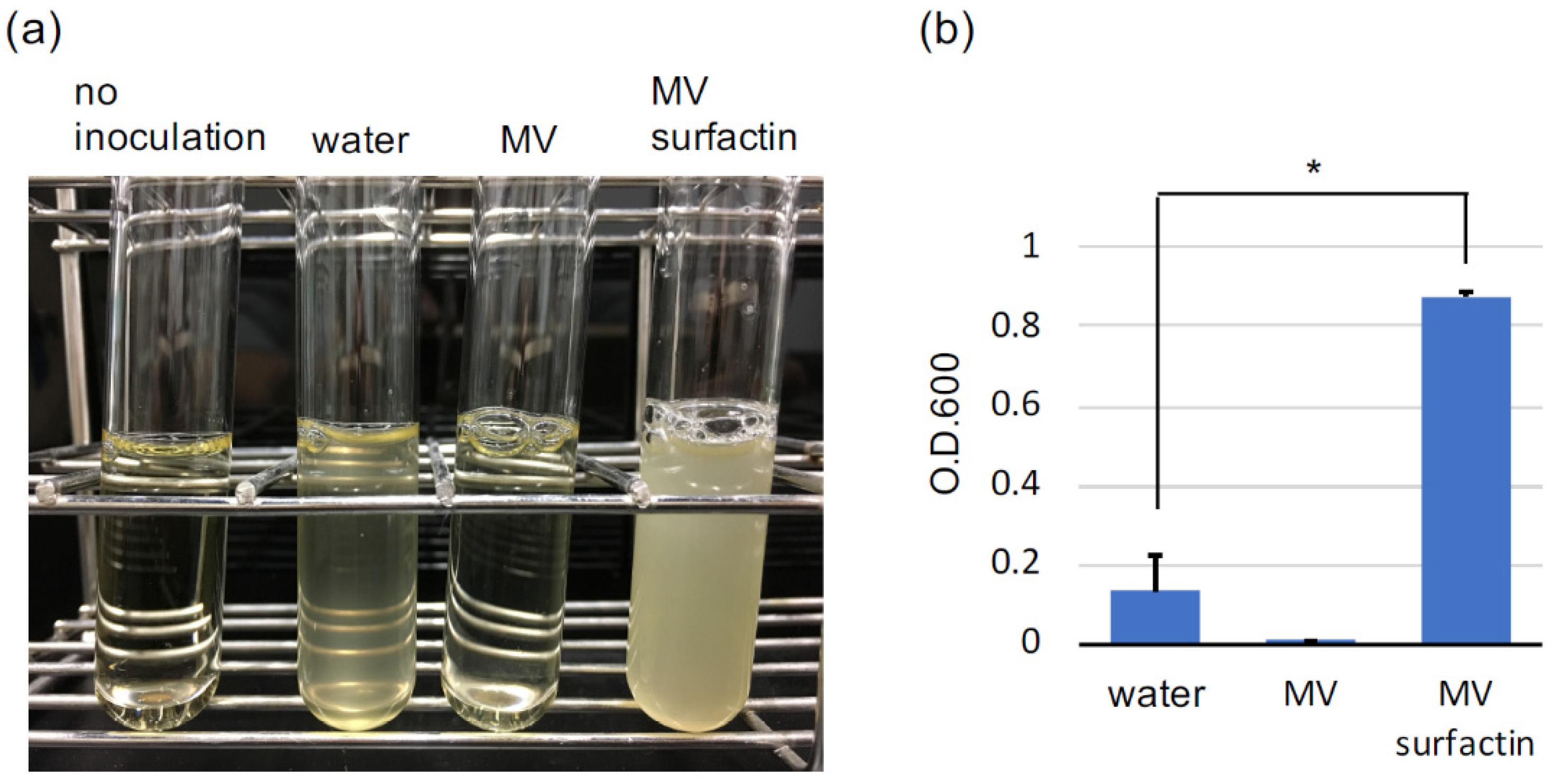
3.1. MV Constituents Promote C. thermocellum Growth
3.2. B. subtilis Broth Promotes C. thermocellum Growth Rate
3.3. Metabolome Analysis of the Constituents in C. thermocellum MV and B. subtilis Broth
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koeck, D.E.; Pechtl, A.; Zverlov, V.V.; Schwarz, W.H. Genomics of cellulolytic bacteria. Curr. Opin. Biotechnol. 2014, 29, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Tindall, B.J. The names Hungateiclostridium Zhang et al. 2018, Hungateiclostridium thermocellum (Viljoen et al. 1926) Zhang et al. 2018, Hungateiclostridium cellulolyticum (Patel et al. 1980) Zhang et al. 2018, Hungateiclostridium aldrichii (Yang et al. 1990) Zhang et al. 2018, Hungateiclostridium alkalicellulosi (Zhilina et al. 2006) Zhang et al. 2018, Hungateiclostridium clariflavum (Shiratori et al. 2009) Zhang et al. 2018, Hungateiclostridium straminisolvens (Kato et al. 2004) Zhang et al. 2018 and Hungateiclostridium saccincola (Koeck et al. 2016) Zhang et al. 2018 contravene Rule 51b of the International Code of Nomenclature of Prokaryotes and require replacement names in the genus Acetivibrio Patel et al. 1980. Int. J. Syst. Evol. Microbiol. 2019, 69, 3927–3932. [Google Scholar] [CrossRef]
- Izquierdo, J.A.; Pattathil, S.; Guseva, A.; Hahn, M.G.; Lynd, L.R. Comparative analysis of the ability of Clostridium clariflavum strains and Clostridium thermocellum to utilize hemicellulose and unpretreated plant material. Biotechnol. Biofuels 2014, 7, 136. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, S.; Nishida, A.; Yasui, S.; Karita, S. Characterization of lignocellulose particles during lignocellulose solubilization by Clostridium thermocellum. Biosci. Biotechnol. Biochem. 2017, 81, 2028–2033. [Google Scholar] [CrossRef] [Green Version]
- Paye, J.M.; Guseva, A.; Hammer, S.K.; Gjersing, E.; Davis, M.F.; Davison, B.H.; Olstad, J.; Donohoe, B.S.; Nguyen, T.Y.; Wyman, C.E.; et al. Biological lignocellulose solubilization: Comparative evaluation of biocatalysts and enhancement via cotreatment. Biotechnol. Biofuels 2016, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Holwerda, E.K.; Thorne, P.G.; Olson, D.G.; Amador-Noguez, D.; Engle, N.L.; Tschaplinski, T.J.; van Dijken, J.P.; Lynd, L.R. The exometabolome of Clostridium thermocellum reveals overflow metabolism at high cellulose loading. Biotechnol. Biofuels 2014, 7, 155. [Google Scholar] [CrossRef]
- Tian, L.; Papanek, B.; Olson, D.G.; Rydzak, T.; Holwerda, E.K.; Zheng, T.; Zhou, J.; Maloney, M.; Jiang, N.; Giannone, R.J.; et al. Simultaneous achievement of high ethanol yield and titer in Clostridium thermocellum. Biotechnol. Biofuels 2016, 9, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Goto, S.; Yonemura, S.; Sekine, K.; Okuma, E.; Takagi, Y.; Hon-Nami, K.; Saiki, T. Effect of yeast extract and vitamin B (12) on ethanol production from cellulose by Clostridium thermocellum I-1-B. Appl. Environ. Microbiol. 1992, 58, 734–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argyros, D.A.; Tripathi, S.A.; Barrett, T.F.; Rogers, S.R.; Feinberg, L.F.; Olson, D.G.; Foden, J.M.; Miller, B.B.; Lynd, L.R.; Hogsett, D.A.; et al. High ethanol titers from cellulose by using metabolically engineered thermophilic, anaerobic microbes. Appl. Environ. Microbiol. 2011, 77, 8288–8294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynd, L.R.; van Zyl, W.H.; McBride, J.E.; Laser, M. Consolidated bioprocessing of cellulosic biomass: An update. Curr. Opin. Biotechnol. 2005, 16, 577–583. [Google Scholar] [CrossRef]
- Mazzoli, R.; Lamberti, C.; Pessione, E. Engineering new metabolic capabilities in bacteria: Lessons from recombinant cellulolytic strategies. Trends Biotechnol. 2012, 30, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.G.; McBride, J.E.; Shaw, A.J.; Lynd, L.R. Recent progress in consolidated bioprocessing. Curr. Opin. Biotechnol. 2012, 23, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.A.; Kenig, R.; Lamed, R. Adherence of Clostridium thermocellum to cellulose. J. Bacteriol. 1983, 156, 818–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamed, R.; Setter, E.; Bayer, E.A. Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum. J. Bacteriol. 1983, 156, 828–836. [Google Scholar] [CrossRef] [Green Version]
- Bayer, E.A.; Belaich, J.P.; Shoham, Y.; Lamed, R. The cellulosomes: Multienzyme machines for degradation of plant cell wall polysaccharides. Annu. Rev. Microbiol. 2004, 58, 521–554. [Google Scholar] [CrossRef] [PubMed]
- White, B.A.; Lamed, R.; Bayer, E.A.; Flint, H.J. Biomass utilization by gut microbiomes. Annu. Rev. Microbiol. 2014, 68, 279–296. [Google Scholar] [CrossRef]
- Nataf, Y.; Yaron, S.; Stahl, F.; Lamed, R.; Bayer, E.A.; Scheper, T.H.; Sonenshein, A.L.; Shoham, Y. Cellodextrin and laminaribiose ABC transporters in Clostridium thermocellum. J. Bacteriol. 2009, 191, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Prawitwong, P.; Waeonukul, R.; Tachaapaikoon, C.; Pason, P.; Ratanakhanokchai, K.; Deng, L.; Sermsathanaswadi, J.; Septiningrum, K.; Mori, Y.; Kosugi, A. Direct glucose production from lignocellulose using Clostridium thermocellum cultures supplemented with a thermostable β-glucosidase. Biotechnol. Biofuels 2013, 6, 184. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, S.; Ogawa, S.; Nishida, A.; Kobayashi, Y.; Kurosawa, T.; Karita, S. Cellulosomes localise on the surface of membrane vesicles from the cellulolytic bacterium Clostridium thermocellum. FEMS Microbiol. Lett. 2019, 366. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, J.; Park, J.; Gho, Y.S. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin. Cell Dev. Biol. 2015, 40, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Kong, Q.; Roland, K.L.; Curtiss, R. Membrane vesicles of Clostridium perfringens type A strains induce innate and adaptive immunity. Int. J. Med. Microbiol. 2014, 304, 431–443. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.Y.; Choi, D.Y.; Kim, D.K.; Kim, J.W.; Park, J.O.; Kim, S.; Kim, S.H.; Desiderio, D.M.; Kim, Y.K.; Kim, K.P.; et al. Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 2009, 9, 5425–5436. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, C.W.; Lee, T.; Kim, S.I.; Lee, J.C.; Shin, J.H. Transcription factor σB plays an important role in the production of extracellular membrane-derived vesicles in Listeria monocytogenes. PLoS ONE 2013, 8, e73196. [Google Scholar] [CrossRef]
- Liao, S.; Klein, M.I.; Heim, K.P.; Fan, Y.; Bitoun, J.P.; Ahn, S.J.; Burne, R.A.; Koo, H.; Brady, L.J.; Wen, Z.T. Streptococcus mutans extracellular DNA is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J. Bacteriol. 2014, 196, 2355–2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olaya-Abril, A.; Prados-Rosales, R.; McConnell, M.J.; Martín-Peña, R.; González-Reyes, J.A.; Jiménez-Munguía, I.; Gómez-Gascón, L.; Fernández, J.; Luque-García, J.L.; García-Lidón, C.; et al. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J. Proteom. 2014, 106, 46–60. [Google Scholar] [CrossRef]
- Rivera, J.; Cordero, R.J.; Nakouzi, A.S.; Frases, S.; Nicola, A.; Casadevall, A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc. Natl. Acad. Sci. USA 2010, 107, 19002–19007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrempf, H.; Koebsch, I.; Walter, S.; Engelhardt, H.; Meschke, H. Extracellular Streptomyces vesicles: Amphorae for survival and defence. Microb. Biotechnol. 2011, 4, 286–299. [Google Scholar] [CrossRef] [Green Version]
- Klieve, A.V.; Yokoyama, M.T.; Forster, R.J.; Ouwerkerk, D.; Bain, P.A.; Mawhinney, E.L. Naturally occurring DNA transfer system associated with membrane vesicles in cellulolytic Ruminococcus spp. of ruminal origin. Appl. Environ. Microbiol. 2005, 71, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Elhenawy, W.; Debelyy, M.O.; Feldman, M.F. Preferential packing of acidic glycosidases and proteases into Bacteroides outer membrane vesicles. mBio 2014, 5, e00909–e00914. [Google Scholar] [CrossRef] [Green Version]
- Rakoff-Nahoum, S.; Coyne, M.J.; Comstock, L.E. An ecological network of polysaccharide utilization among human intestinal symbionts. Curr. Biol. 2014, 24, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arntzen, M.; Várnai, A.; Mackie, R.I.; Eijsink, V.G.H.; Pope, P.B. Outer membrane vesicles from Fibrobacter succinogenes S85 contain an array of carbohydrate-active enzymes with versatile polysaccharide-degrading capacity. Environ. Microbiol. 2017, 19, 2701–2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashburn, L.M.; Whiteley, M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 2005, 437, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Schertzer, J.W.; Boulette, M.L.; Whiteley, M. More than a signal: Non-signaling properties of quorum sensing molecules. Trends Microbiol. 2009, 17, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Biller, S.J.; Schubotz, F.; Roggensack, S.E.; Thompson, A.W.; Summons, R.E.; Chisholm, S.W. Bacterial vesicles in marine ecosystems. Science 2014, 343, 183–186. [Google Scholar] [CrossRef]
- Prados-Rosales, R.; Weinrick, B.C.; Piqué, D.G.; Jacobs, W.R.; Casadevall, A.; Rodriguez, G.M. Role for Mycobacterium tuberculosis membrane vesicles in iron acquisition. J. Bacteriol. 2014, 196, 1250–1256. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y. Characterization of a symbiotic coculture of Clostridium thermohydrosulfuricum YM3 and Clostridium thermocellum YM4. Appl. Environ. Microbiol. 1990, 56, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, T.; Kadoya, R.; Endo, K.; Tohata, M.; Sawada, K.; Liu, S.; Ozawa, T.; Kodama, T.; Kakeshita, H.; Kageyama, Y.; et al. Enhanced recombinant protein productivity by genome reduction in Bacillus subtilis. DNA Res. 2008, 15, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Koo, B.M.; Kritikos, G.; Farelli, J.D.; Todor, H.; Tong, K.; Kimsey, H.; Wapinski, I.; Galardini, M.; Cabal, A.; Peters, J.M.; et al. Construction and analysis of two genome-scale deletion libraries for Bacillus subtilis. Cell Syst. 2017, 4, 291–305.e297. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, N.; Ara, T.; Kanaya, S.; Nakamura, Y.; Iijima, Y.; Enomoto, M.; Motegi, T.; Aoki, K.; Suzuki, H.; Shibata, D. An application of a relational database system for high-throughput prediction of elemental compositions from accurate mass values. Bioinformatics 2013, 29, 290–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, L.; Kessler, A.; Cabezas-Sanchez, P.; Luque-Garcia, J.L.; Casadevall, A. Extracellular vesicles produced by the Gram-positive bacterium Bacillus subtilis are disrupted by the lipopeptide surfactin. Mol. Microbiol. 2014, 93, 183–198. [Google Scholar] [CrossRef] [Green Version]
- Mukamolova, G.V.; Kaprelyants, A.S.; Young, D.I.; Young, M.; Kell, D.B. A bacterial cytokine. Proc. Natl. Acad. Sci. USA 1998, 95, 8916–8921. [Google Scholar] [CrossRef] [Green Version]
- Mukamolova, G.V.; Murzin, A.G.; Salina, E.G.; Demina, G.R.; Kell, D.B.; Kaprelyants, A.S.; Young, M. Muralytic activity of Micrococcus luteus Rpf and its relationship to physiological activity in promoting bacterial growth and resuscitation. Mol. Microbiol. 2006, 59, 84–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, I.M.; Laaberki, M.H.; Popham, D.L.; Dworkin, J. A eukaryotic-like Ser/Thr kinase signals bacteria to exit dormancy in response to peptidoglycan fragments. Cell 2008, 135, 486–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K.; Fujita, Y.; Ehrlich, S.D. Three asparagine synthetase genes of Bacillus subtilis. J. Bacteriol. 1999, 181, 6081–6091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K.I.; Aoyama, D.; Ishio, I.; Shibayama, T.; Fujita, Y. Organization and transcription of the myo-inositol operon, iol, of Bacillus subtilis. J. Bacteriol. 1997, 179, 4591–4598. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.I.; Shibayama, T.; Aoyama, D.; Fujita, Y. Interaction of a repressor and its binding sites for regulation of the Bacillus subtilis iol divergon. J. Mol. Biol. 1999, 285, 917–929. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamaguchi, M.; Morinaga, T.; Kinehara, M.; Ikeuchi, M.; Ashida, H.; Fujita, Y. myo-Inositol catabolism in Bacillus subtilis. J. Biol. Chem. 2008, 283, 10415–10424. [Google Scholar] [CrossRef] [Green Version]
- Butcher, B.G.; Lin, Y.P.; Helmann, J.D. The yydFGHIJ operon of Bacillus subtilis encodes a peptide that induces the LiaRS two-component system. J. Bacteriol. 2007, 189, 8616–8625. [Google Scholar] [CrossRef] [Green Version]
- Popp, P.F.; Benjdia, A.; Strahl, H.; Berteau, O.; Mascher, T. The epipeptide YydF intrinsically triggers the cell envelope stress response of Bacillus subtilis and causes severe membrane perturbations. Front. Microbiol. 2020, 11, 151. [Google Scholar] [CrossRef]
- Noirot-Gros, M.F.; Soultanas, P.; Wigley, D.B.; Ehrlich, S.D.; Noirot, P.; Petit, M.A. The beta-propeller protein YxaL increases the processivity of the PcrA helicase. Mol. Genet. Genom. 2002, 267, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, Y.; Oh, Y.Y.; Ha, N.C.; Song, J. Plant growth-promoting activity of beta-propeller protein YxaL secreted from Bacillus velezensis strain GH1-13. PLoS ONE 2019, 14, e0207968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rucker, R.; Chowanadisai, W.; Nakano, M. Potential physiological importance of pyrroloquinoline quinone. Altern. Med. Rev. 2009, 14, 268–277. [Google Scholar] [PubMed]
- Ameyama, M.; Shinagawa, E.; Matsushita, K.; Adachi, O. Growth stimulating substance for microorganisms produced by Escherichia coli causing the reduction of the lag phase in microbial growth and identity of the substance with pyrroloquinoline quinone. Agric. Biol. Chem. 1984, 48, 3099–3107. [Google Scholar] [CrossRef]
- Ameyama, M.; Shinagawa, E.; Matsushita, K.; Adachi, O. Growth stimulating activity for microorganisms in naturally occurring substances and partial characterization of the substance for the activity as pyrroloquinoline quinone. Agric. Biol. Chem. 1985, 49, 699–709. [Google Scholar]
- Shimao, M.; Yamamoto, H.; Ninomiya, K.; Hato, N.; Adachi, O.; Ameyama, M.; Sakazawa, C. Pyrroloquinoline quinone as an essential growth factor for a poly (vinyl alcohol)-degrading symbiont, Pseudomonas sp. VM15C. Agric. Biol. Chem. 1984, 48, 2873–2876. [Google Scholar] [CrossRef]
- Imamura, D.; Kuwana, R.; Takamatsu, H.; Watabe, K. Localization of proteins to different layers and regions of Bacillus subtilis spore coats. J. Bacteriol. 2010, 192, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Kuwana, R.; Takamatsu, H.; Watabe, K. Expression, localization and modification of YxeE spore coat protein in Bacillus subtilis. J. Biochem. 2007, 142, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Antelmann, H.; Engelmann, S.; Schmid, R.; Hecker, M. General and oxidative stress responses in Bacillus subtilis: Cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J. Bacteriol. 1996, 178, 6571–6578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ollinger, J.; Song, K.B.; Antelmann, H.; Hecker, M.; Helmann, J.D. Role of the Fur regulon in iron transport in Bacillus subtilis. J. Bacteriol. 2006, 188, 3664–3673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Onofrio, A.; Crawford, J.M.; Stewart, E.J.; Witt, K.; Gavrish, E.; Epstein, S.; Clardy, J.; Lewis, K. Siderophores from neighboring organisms promote the growth of uncultured bacteria. Chem. Biol. 2010, 17, 254–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietiäinen, M.; Gardemeister, M.; Mecklin, M.; Leskelä, S.; Sarvas, M.; Kontinen, V.P. Cationic antimicrobial peptides elicit a complex stress response in Bacillus subtilis that involves ECF-type sigma factors and two-component signal transduction systems. Microbiology 2005, 151, 1577–1592. [Google Scholar] [CrossRef]
- Hayashi, S.; Itoh, K.; Suyama, K. Genes of Bacillus subtilis 168 that support growth of the cyanobacterium, Synechococcus leopoliensis CCAP1405/1 on agar media. Microb. Ecol. 2015, 70, 849–852. [Google Scholar] [CrossRef]
- Niehaus, T.D.; Folz, J.; McCarty, D.R.; Cooper, A.J.L.; Moraga Amador, D.; Fiehn, O.; Hanson, A.D. Identification of a metabolic disposal route for the oncometabolite. J. Biol. Chem. 2018, 293, 8255–8263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Fang, K.; Hong, S.M.C.; Kim, I.; Jang, I.S.; Hong, S.H. Medium chain unsaturated fatty acid ethyl esters inhibit persister formation of Escherichia coli via antitoxin HipB. Appl. Microbiol. Biotechnol. 2018, 102, 8511–8524. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Dow, J.M. Communication with a growing family: Diffusible signal factor (DSF) signaling in bacteria. Trends Microbiol. 2011, 19, 145–152. [Google Scholar] [CrossRef]
- Boon, C.; Deng, Y.; Wang, L.H.; He, Y.; Xu, J.L.; Fan, Y.; Pan, S.Q.; Zhang, L.H. A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J. 2008, 2, 27–36. [Google Scholar] [CrossRef]
- Davies, D.G.; Marques, C.N. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol. 2009, 191, 1393–1403. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Boon, C.; Eberl, L.; Zhang, L.H. Differential modulation of Burkholderia cenocepacia virulence and energy metabolism by the quorum-sensing signal BDSF and its synthase. J. Bacteriol. 2009, 191, 7270–7278. [Google Scholar] [CrossRef] [Green Version]
- Twomey, K.B.; O’Connell, O.J.; McCarthy, Y.; Dow, J.M.; O’Toole, G.A.; Plant, B.J.; Ryan, R.P. Bacterial cis-2-unsaturated fatty acids found in the cystic fibrosis airway modulate virulence and persistence of Pseudomonas aeruginosa. ISME J. 2012, 6, 939–950. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Wu, J.; Eberl, L.; Zhang, L.H. Structural and functional characterization of diffusible signal factor family quorum-sensing signals produced by members of the Burkholderia cepacia complex. Appl. Environ. Microbiol. 2010, 76, 4675–4683. [Google Scholar] [CrossRef] [Green Version]
- Dean, S.N.; Chung, M.C.; van Hoek, M.L. Burkholderia diffusible signal factor signals to Francisella novicida to disperse biofilm and increase siderophore production. Appl. Environ. Microbiol. 2015, 81, 7057–7066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, D.A.; Cummins, C.S. Nutritional requirements of anaerobic coryneforms. J. Bacteriol. 1978, 135, 858–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenstein, R.J.; Su, L.; Brown, S.T. Growth of M. avium subspecies paratuberculosis in culture is enhanced by nicotinic acid, nicotinamide, and α and β nicotinamide adenine dinucleotide. Dig. Dis. Sci. 2011, 56, 368–375. [Google Scholar] [CrossRef]
- Iandolo, J.J.; Clark, C.W.; Bluhm, L.; Ordal, Z.J. Repression of Staphylococcus aureus in associative culture. Appl. Microbiol. 1965, 13, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Sacks, L.E.; Thompson, P.A. Germination requirements of Bacillus macerans spores. J. Bacteriol. 1971, 105, 739–746. [Google Scholar] [CrossRef] [Green Version]
- Rajalaxmi, M.; Beema Shafreen, R.; Iyer, P.M.; Sahaya Vino, R.; Balamurugan, K.; Pandian, S.K. An in silico, in vitro and in vivo investigation of indole-3-carboxaldehyde identified from the seawater bacterium Marinomonas sp. as an anti-biofilm agent against Vibrio cholerae O1. Biofouling 2016, 32, 439–450. [Google Scholar] [CrossRef]
- Kaznowski, A. Identification of Aeromonas strains of different origin to the genomic species level. J. Appl. Microbiol. 1998, 84, 423–430. [Google Scholar] [CrossRef]
- Zhang, X.X.; Chang, H.; Tran, S.L.; Gauntlett, J.C.; Cook, G.M.; Rainey, P.B. Variation in transport explains polymorphism of histidine and urocanate utilization in a natural Pseudomonas population. Environ. Microbiol. 2012, 14, 1941–1951. [Google Scholar] [CrossRef]
- El Dine, R.S.; Elfaky, M.A.; Asfour, H.; El Halawany, A.M. Anti-adhesive activity of Aframomum melegueta major phenolics on lower respiratory tract pathogens. Nat. Prod. Res. 2019, 35, 539–547. [Google Scholar] [CrossRef]

| No. | Formula | Exact Mass | Name | Database | Database ID |
|---|---|---|---|---|---|
| 3203 | C16H31O1N1 | 253.241 | EX-HR2 | ||
| 3013 | C12H22O2 | 198.162 | Ethyl 2-decenoate | UC2 | HMDB0037329 |
| C12H22O2 | 198.162 | Ethyl 4-decenoate | UC2 | HMDB0039220 | |
| C12H22O2 | 198.162 | Methyl 9-undecenoate | UC2 | HMDB0037305 | |
| C12H22O2 | 198.162 | Methyl 10-undecenoate | UC2 | HMDB0029585 | |
| C12H22O2 | 198.162 | Allyl nonanoate | UC2 | HMDB0029763 | |
| C12H22O2 | 198.162 | cis-3-Hexenyl hexanoate | UC2 | HMDB0033378 | |
| C12H22O2 | 198.162 | 2-Hexenyl hexanoate | UC2 | HMDB0038924 | |
| C12H22O2 | 198.162 | Hexyl 2E-hexenoate | UC2 | HMDB0038269 | |
| C12H22O2 | 198.162 | Hexyl 2-methyl-3-pentenoate | UC2 | HMDB0040158 | |
| C12H22O2 | 198.162 | Hexyl 2-methyl-4-pentenoate | UC2 | HMDB0040163 | |
| C12H22O2 | 198.162 | 1-Ethenylhexyl butanoate | UC2 | HMDB0037498 | |
| C12H22O2 | 198.162 | 2-Octenyl butyrate | UC2 | HMDB0038081 | |
| C12H22O2 | 198.162 | cis-4-Decenyl acetate | UC2 | HMDB0032214 | |
| C12H22O2 | 198.162 | Menthyl acetate | UC2 | C00036314 | |
| C12H22O2 | 198.162 | Rhodinyl acetate | UC2 | HMDB0037186 | |
| C12H22O2 | 198.162 | Citronellyl acetate | UC2 | C00035564 | |
| C12H22O2 | 198.162 | 2-Dodecenoic acid | UC2 | HMDB0010729 | |
| C12H22O2 | 198.162 | 4-dodecenoic acid | UC2 | C00051284 | |
| C12H22O2 | 198.162 | 5-dodecenoic acid | UC2 | HMDB0000529 | |
| C12H22O2 | 198.162 | 11-Dodecenoic acid | UC2 | HMDB0032248 | |
| C12H22O2 | 198.162 | 5-dodecalactone | UC2 | HMDB0037742 | |
| C12H22O2 | 198.162 | gamma-Dodecalactone | UC2 | C00030347 | |
| C12H22O2 | 198.162 | epsilon-Dodecalactone | UC2 | HMDB0038895 | |
| C12H22O2 | 198.162 | alpha-Heptyl-gamma-valerolactone | UC2 | HMDB0037813 | |
| C12H22O2 | 198.162 | 4-butyl-4-hydroxyoctanoic acid lactone | UC2 | HMDB0036182 | |
| C12H22O2 | 198.162 | 2,6-Dimethyl-5-heptenal propyleneglycol acetal | UC2 | HMDB0032235 | |
| C12H22O2 | 198.162 | citral dimethyl acetal | UC2 | HMDB0040361 | |
| C12H22O2 | 198.162 | citronelloxyacetaldehyde | UC2 | HMDB0041449 | |
| C12H22O2 | 198.162 | Chokol A | UC2 | C00011518 | |
| C12H22O2 | 198.162 | Cybullol | UC2 | C00013221 | |
| 42 | C12H4O3S1 | 227.988 | EX-HR2 | ||
| 3000 | C18H35O2N | 297.267 | Lepadin D | UC2 | C00026353 |
| C18H35O2N | 297.267 | Cassine | UC2 | C00002027 | |
| 2834 | C8H13ON | 139.100 | 5-Pentyloxazole | UC2 | HMDB0038792 |
| C8H13ON | 139.100 | 4,5-Dimethyl-2-propyloxazole | UC2 | HMDB0037869 | |
| C8H13ON | 139.100 | 4,5-Dimethyl-2-isopropyloxazole | UC2 | HMDB0037871 | |
| C8H13ON | 139.100 | 4-Butyl-2-methyloxazole | UC2 | HMDB0037855 | |
| C8H13ON | 139.100 | 2,4-Dimethyl-5-propyloxazole | UC2 | HMDB0037868 | |
| C8H13ON | 139.100 | 4,5-Diethyl-2-methyloxazole | UC2 | HMDB0037870 | |
| C8H13ON | 139.100 | 2-Pentyloxazole | UC2 | HMDB0037818 | |
| C8H13ON | 139.100 | 7beta-Hydroxy-1-methylene-8alpha-pyrrolizidine | UC2 | C00026172 | |
| C8H13ON | 139.100 | 2-propionyltetrahydropyridine | UC2 | HMDB0034884 | |
| C8H13ON | 139.100 | alpha-Phosphinylbenzyl alcohol | UC2 | HMDB0029613 | |
| C8H13ON | 139.100 | Supinidine | UC2 | C00002120 | |
| C8H13ON | 139.100 | Tropinone | UC2 | C00037960 |
| No. | Formula | Exact Mass | Name | Database | Database ID |
|---|---|---|---|---|---|
| 1938 | C12H23O8N | 309.142 | 4-O-beta-D-Glucopyranosylfagomine | UC2 | C00049954 |
| 1980 | C15H39O2N7S3 | 445.233 | EX-HR2 | ||
| 453 | C16H30O6N6 | 402.223 | EX-HR2 | ||
| 2242 | C17H31O4N3 | 341.231 | Diprotin A | UC2 | C00018579 |
| 1607 | C25H40O7 | 452.277 | Briarellin P | UC2 | C00044586 |
| 799 | C10H20O3N2S | 248.119 | Valyl-Methionine | UC2 | HMDB0029133 |
| C10H20O3N2S | 248.119 | Methionyl-Valine | UC2 | HMDB0028986 | |
| 510 | C11H22O4N4 | 274.164 | Glutaminyllysine | UC2 | HMDB0028802 |
| C11H22O4N4 | 274.164 | Lysyl-Gamma-glutamate | UC2 | HMDB0028965 | |
| C11H22O4N4 | 274.164 | Lysyl-Glutamine | UC2 | HMDB0028949 | |
| 960 | C21H40O1N3P3 | 443.238 | EX-HR2 | ||
| 2575 | C8H13N3P2 | 213.058 | EX-HR2 | ||
| 2345 | C19H29N3O4S1 | 395.188 | V1M1F1 | Pep1000 | |
| 2536 | C29H36O5N4 | 520.269 | Lotusine F | UC2 | C00027221 |
| C29H36O5N4 | 520.269 | Nummularine S | UC2 | C00029150 | |
| 2237 | C33H44O11 | 616.288 | Neoazedarachin A | UC2 | C00039833 |
| C33H44O11 | 616.288 | YM 47524 | UC2 | C00016365 | |
| 2633 | C23H55O12N1P2 | 599.320 | EX-HR2 | ||
| 2673 | C46H67O2N10P1S1 | 854.491 | EX-HR2 | ||
| 1271 | C27H44O9 | 512.299 | Butyrolactol B | UC2 | C00016754 |
| C27H44O9 | 512.299 | Integristerone B | UC2 | C00048431 | |
| C27H44O9 | 512.299 | Platenolide B mycarose | UC2 | C00018288 | |
| 162 | C6H6ON2 | 122.048 | Nicotinamide | UC2 | C00000209 |
| C6H6ON2 | 122.048 | 2-Acetylpyrazine | UC2 | HMDB0031861 | |
| 1710 | C15H24O4N4 | 324.180 | EX-HR2 | ||
| 211 | C6H9O3N | 143.058 | SQ 26517 | UC2 | C00018434 |
| C6H9O3N | 143.058 | Trimethadione | UC2 | HMDB0014491 | |
| C6H9O3N | 143.058 | 6-Oxopiperidine-2-carboxylic acid | UC2 | HMDB0061705 | |
| C6H9O3N | 143.058 | 5-ethyl-5-methyl-2,4-oxazolidinedione | UC2 | HMDB0061082 | |
| C6H9O3N | 143.058 | Vinylacetylglycine | UC2 | HMDB0000894 | |
| C6H9O3N | 143.058 | Methyl pyroglutamate | UC2 | C00051578 | |
| 1258 | C22H66N2P2S6 | 612.303 | EX-HR2 | ||
| 994 | C20H33N5O8 | 471.233 | G2[L|I]1E1P1, G1A1V1E1P1, G1A1[L|I]1D1P1, G1T2P2, A2V1D1P1, A1S1T1P2, V1E1Q1P1, [L|I]1D1Q1P1, [L|I]1E1N1P1 | Pep1000 | |
| 655 | C16H27N5O6 | 385.196 | G3V1P1, G1A3P1, G1V1N1P1, A2Q1P1 | Pep1000 | |
| 1034 | C10H16O3N2 | 212.116 | Butabarbital | UC2 | HMDB0014382 |
| C10H16O3N2 | 212.116 | L-prolyl-L-proline | UC2 | HMDB0011180 | |
| C10H16O3N2 | 212.116 | Butethal | UC2 | HMDB0015442 | |
| 457 | C32H48O5N2S1 | 572.328 | EX-HR2 | ||
| 2755 | C9H7ON | 145.053 | Indole-3-carboxaldehyde | UC2 | C00000112 |
| C9H7ON | 145.053 | 2-Quinolone | UC2 | C00044432 | |
| 2680 | C67H108O6N2S5 | 1196.681 | EX-HR2 | ||
| 115 | C6H6O2N2 | 138.043 | 4-Methoxylonchocarpin | UC2 | HMDB0031338 |
| C6H6O2N2 | 138.043 | 2-Aminonicotinic acid | UC2 | HMDB0061680 | |
| C6H6O2N2 | 138.043 | Urocanic acid | UC2 | HMDB0062562 | |
| C6H6O2N2 | 138.043 | Nicotinamide N-oxide | UC2 | HMDB0002730 | |
| 2949 | C11H21ON | 183.162 | Tecostanin | UC2 | C00001984 |
| C11H21ON | 183.162 | Incarvilline | UC2 | C00050294 | |
| 1600 | C20H57O4N9S3 | 583.370 | EX-HR2 | ||
| 1727 | C11H20O6N4 | 304.138 | Nopaline | UC2 | C00001548 |
| 526 | C21H56O14N10P2 | 734.345 | EX-HR2 | ||
| 3061 | C17H26O3 | 278.188 | 1-Acetoxy-3,15-epoxygymnomitrane | UC2 | C00021889 |
| C17H26O3 | 278.188 | Litsealactone B | UC2 | C00044889 | |
| C17H26O3 | 278.188 | 9beta-Acetoxy-10(14)-aromadendren-4beta-ol | UC2 | C00021235 | |
| C17H26O3 | 278.188 | Furoscrobiculin C | UC2 | C00021531 | |
| C17H26O3 | 278.188 | [S-[R *,S *-(E)]]-6-[6-(Acetyloxy)-1,5-dimethyl-4-hexenyl]-3-methyl-2-cyclohexen-1-one | UC2 | C00011679 | |
| C17H26O3 | 278.188 | Panaxytriol | UC2 | C00030923 | |
| C17H26O3 | 278.188 | Panaxacol | UC2 | HMDB0039251 | |
| C17H26O3 | 278.188 | Parahigginol C | UC2 | C00049252 | |
| C17H26O3 | 278.188 | [1S-(1R *,2E,4R *,5R *,6E,10R *)]-3, 7, 11, 11-Tetramethylbicyclo [8.1.0]undeca-2,6-diene-4,5-diol 5-acetate | UC2 | C00012427 | |
| C17H26O3 | 278.188 | Isoobtusilactone | UC2 | C00050966 | |
| C17H26O3 | 278.188 | 8beta-Acetoxy-9beta-hydroxyverboccidenten | UC2 | C00020229 | |
| C17H26O3 | 278.188 | Lincomolide B | UC2 | C00047968 | |
| C17H26O3 | 278.188 | [1S-(1R *,2E,4R *,5R *,6E,10R *)]-3, 7, 11, 11-Tetramethylbicyclo[8.1.0]undeca-2,6-diene-4,5-diol 4-acetate | UC2 | C00012428 | |
| C17H26O3 | 278.188 | 4alpha-Hydroxygymnomitryl acetate | UC2 | C00021894 | |
| C17H26O3 | 278.188 | 4-[(4E)-3-hydroxydec-4-en-1-yl]-2-methoxyphenol | UC2 | HMDB0137260 | |
| C17H26O3 | 278.188 | Ro 09-1544 | UC2 | C00017230 | |
| C17H26O3 | 278.188 | 6-Paradol | UC2 | C00002764 | |
| C17H26O3 | 278.188 | Paralemnolin D | UC2 | C00030924 | |
| C17H26O3 | 278.188 | Fenoksan; Fenoxan; Fenozan; Fenozan acid; Irganox 1310; Phenosan; Phenoxan; Phenozan | UC2 | C00016759 | |
| C17H26O3 | 278.188 | [4aR-(4aalpha,5alpha,8abeta,9abeta)]-9a-Ethoxy-4a, 5, 6, 7, 8, 8a, 9, 9a-octahydro-3,4a,5-trimethyl-naphtho[2,3-b]furan-2(4H)-one | UC2 | C00017405 | |
| C17H26O3 | 278.188 | Petasipalin B | UC2 | C00020246 | |
| C17H26O3 | 278.188 | 4-epi-7alpha,15-dihydroxypodocarp-8(14)-en-13-one;(-)-4-epi-7alpha,15-dihydroxypodocarp-8(14)-en-13-one | UC2 | C00035020 | |
| C17H26O3 | 278.188 | 3-[(Acetyloxy)methyl]-6-(1,5-dimethyl-4-hexenyl)-2-cyclohexen-1-one | UC2 | C00011682 | |
| C17H26O3 | 278.188 | Cyclokessyl acetate | UC2 | C00020354 | |
| C17H26O3 | 278.188 | 8-Acetoxy-4-acoren-3-one | UC2 | HMDB0030974 | |
| 832 | C12H34O3N6S3 | 406.185 | EX-HR2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichikawa, S.; Tsuge, Y.; Karita, S. Metabolome Analysis of Constituents in Membrane Vesicles for Clostridium thermocellum Growth Stimulation. Microorganisms 2021, 9, 593. https://doi.org/10.3390/microorganisms9030593
Ichikawa S, Tsuge Y, Karita S. Metabolome Analysis of Constituents in Membrane Vesicles for Clostridium thermocellum Growth Stimulation. Microorganisms. 2021; 9(3):593. https://doi.org/10.3390/microorganisms9030593
Chicago/Turabian StyleIchikawa, Shunsuke, Yoichiro Tsuge, and Shuichi Karita. 2021. "Metabolome Analysis of Constituents in Membrane Vesicles for Clostridium thermocellum Growth Stimulation" Microorganisms 9, no. 3: 593. https://doi.org/10.3390/microorganisms9030593
APA StyleIchikawa, S., Tsuge, Y., & Karita, S. (2021). Metabolome Analysis of Constituents in Membrane Vesicles for Clostridium thermocellum Growth Stimulation. Microorganisms, 9(3), 593. https://doi.org/10.3390/microorganisms9030593






