Yield Losses Caused by Barley Yellow Dwarf Virus-PAV Infection in Wheat and Barley: A Three-Year Field Study in South-Eastern Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sites and Experiments
2.2. Virus Propagation and Aphid Colony
2.3. Inoculation of Virus-Infected Plots with BYDV-PAV
2.4. Assessment of BYDV-PAV Incidence
2.5. Harvest Assessments
2.6. Weather Data
2.7. Data Analysis
3. Results
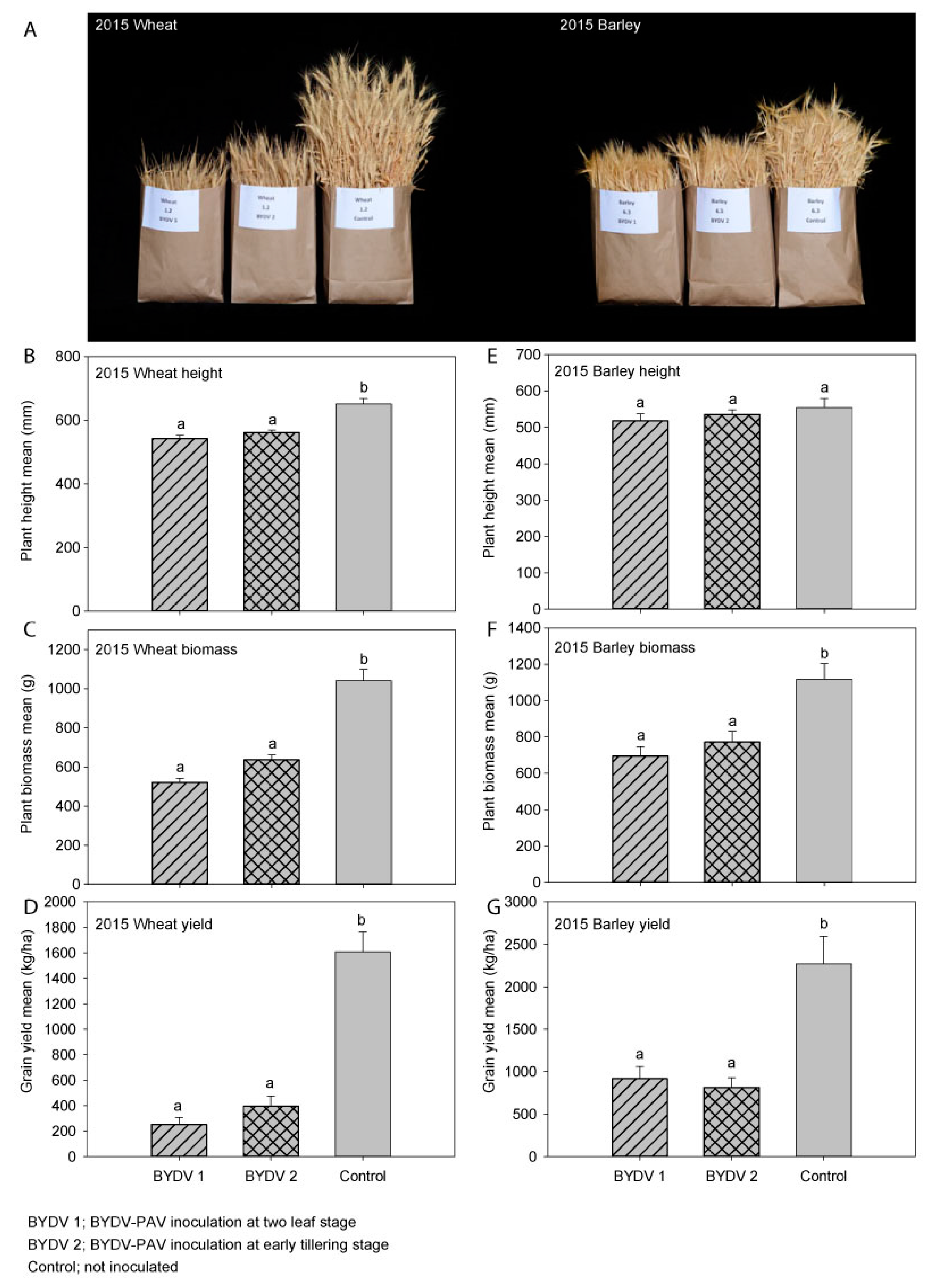
3.1. Experiment 1 (2015); Wheat (cv. Yitpi)
3.2. Experiment 2 (2015); Barley (cv. Hindmarsh)
3.3. Experiment 3 (2017); Wheat (cv. Mace)
3.4. Experiment 4 (2018); Wheat (cv. Mace)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Plant Health and Food Security. Available online: http://www.fao.org/3/a-i7829e.pdf (accessed on 3 March 2021).
- Bos, L. Crop losses caused by viruses. Crop Prot. 1982, 1, 263–282. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses (ICTV). ICTV Master Species List 2019 v1. Checklist Dataset. Available online: https://talk.ictvonline.org/files/master-species-lists/m/msl/9601; https://doi.org/10.15468/i4jnfv (accessed on 3 March 2021).
- Sward, R.J.; Lister, R.M. The identity of barley yellow dwarf virus isolates in cereals and grasses from mainland Australia. Aust. J. Agric. Res. 1988, 39, 375–384. [Google Scholar] [CrossRef]
- McKirdy, S.J.; Jones, R.A.C. Occurrence of barley yellow dwarf virus serotypes MAV and RMV in over-summering grasses. Aust. J. Agric. Res. 1993, 44, 1195–1209. [Google Scholar] [CrossRef]
- Jones, R.A.C.; McKirdy, S.J.; Shivas, R.G. Occurrence of barley yellow dwarf viruses in over-summering grasses and cereal crops in Western Australia. Australas Plant Pathol. 1990, 19, 90–96. [Google Scholar] [CrossRef]
- Nancarrow, N.; Aftab, M.; Freeman, A.; Rodoni, B.; Hollaway, G.; Trębicki, P. Prevalence and incidence of yellow dwarf viruses across a climatic gradient: A four-year field study in south eastern Australia. Plant Dis. 2018, 102, 2465–2472. [Google Scholar] [CrossRef] [Green Version]
- Sward, R.J.; Lister, R.M. The incidence of barley yellow dwarf viruses in wheat in Victoria. Aust. J. Agric. Res. 1987, 38, 821–828. [Google Scholar] [CrossRef]
- Trębicki, P.; Nancarrow, N.; Bosque-Pérez, N.A.; Rodoni, B.; Aftab, M.; Freeman, A.; Yen, A.; Fitzgerald, G. Virus incidence in wheat increases under elevated CO2: A 4-year field study of yellow dwarf viruses from a free air carbon dioxide facility. Virus Res. 2017, 241, 137–144. [Google Scholar] [CrossRef]
- Gray, S.; Gildow, F.E. Luteovirus-aphid interactions. Annu. Rev. Phytopathol. 2003, 41, 539–566. [Google Scholar] [CrossRef]
- Oswald, J.W.; Houston, R. The yellow dwarf virus disease of cereal crops. Phytopathology 1953, 43, 128–136. [Google Scholar]
- Thackray, D.J.; Diggle, A.J.; Jones, R.A.C. BYDV PREDICTOR: A simulation model to predict aphid arrival, epidemics of barley yellow dwarf virus and yield losses in wheat crops in a Mediterranean-type environment. Plant Pathol. 2009, 58, 186–202. [Google Scholar] [CrossRef]
- Baltenberger, D.E.; Ohm, H.W.; Foster, J.E. Reactions of oat, barley and wheat to infection with Barley yellow dwarf virus isolates. Crop Sci. 1987, 27, 195–198. [Google Scholar] [CrossRef]
- Choudhury, S.; Larkin, P.; Meinke, H.; Hasanuzzaman, M.D.; Johnson, P.; Zhou, M. Barley yellow dwarf virus infection affects physiology, morphology, grain yield and flour pasting properties of wheat. Crop Pasture Sci. 2019, 70, 16–25. [Google Scholar] [CrossRef]
- Gildow, F.E.; Frank, J.A. Barley yellow dwarf virus in Pennsylvannia: Effect of the PAV isolate on yield components of Noble spring oats. Plant Dis. 1988, 72, 254–256. [Google Scholar] [CrossRef]
- Gill, C.C. Assessment of losses on spring wheat naturally infected with barley yellow dwarf virus. Plant Dis. 1980, 64, 197–203. [Google Scholar] [CrossRef]
- McKirdy, S.J.; Jones, R.A.C.; Nutter, F.W. Quantification of yield losses caused by barley yellow dwarf virus in wheat and oats. Plant Dis. 2002, 86, 769–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pike, K.S. A review of barley yellow dwarf virus grain yield losses. In World Perspectives on Barley Yellow Dwarf Virus; Burnett, P.A., Ed.; CIMMYT: Texcoco, Mexico, 1990; pp. 356–361. [Google Scholar]
- Thackray, D.J.; Ward, L.T.; Thomas-Carroll, M.L.; Jones, R.A.C. Role of winter-active aphids spreading barley yellow dwarf virus in decreasing wheat yields in a Mediterranean-type environment. Aust. J. Agric. Res. 2005, 56, 1089–1099. [Google Scholar] [CrossRef]
- Banttari, E.E. Occurrence of aster yellows in barley in the field and its comparison with barley yellow dwarf. Phytopathology 1965, 55, 838–843. [Google Scholar]
- Banks, P.M.; Davidson, J.L.; Bariana, H.; Larkin, P.J. Effects of barley yellow dwarf virus on the yield of winter wheat. Aust. J. Agric. Res. 1995, 45, 935–946. [Google Scholar] [CrossRef]
- El Yamani, M.; Hill, J.H. Identification and importance of barley yellow dwarf virus in Morocco. Plant Dis. 1990, 74, 291–294. [Google Scholar] [CrossRef]
- Perry, K.L.; Kolb, F.L.; Sammons, B.; Lawson, C.; Cisar, G.; Ohm, H. Yield effects of barley yellow dwarf virus in soft red winter wheat. Phytopathology 2000, 90, 1043–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.R.; Sward, R.J. Crop loss assessment studies on the effects of barley yellow dwarf virus in wheat in Victoria. Aust. J. Agric. Res. 1982, 33, 179–185. [Google Scholar] [CrossRef]
- Edwards, M.C.; Fetch, T.G.J.; Schwarz, P.B.; Steffenson, B.J. Effect of barley yellow dwarf virus infection on yield and malting quality of barley. Plant Dis. 2001, 85, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Rochow, W.F. Biological properties of four isolates of barley yellow dwarf virus. Phytopathology 1969, 59, 1580–1589. [Google Scholar] [PubMed]
- Jeffrey, S.J.; Carter, J.O.; Moodie, K.B.; Beswick, A.R. Using spatial interpolation to construct a comprehensive archive of Australian climate data. Environ. Model. Softw. 2001, 16, 309–330. [Google Scholar] [CrossRef]
- Nancarrow, N.; Constable, F.; Finlay, K.; Freeman, A.; Rodoni, B.; Trębicki, P.; Vassiliades, S.; Yen, A.; Luck, J. The effect of elevated temperature on barley yellow dwarf virus-PAV in wheat. Virus Res. 2014, 186, 97–103. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Scholthof, K.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef]
- Fitzgerald, P.J.; Stoner, W.N. Barley yellow dwarf studies in wheat (Triticum aestivum L). Crop Sci. 1967, 7, 337–341. [Google Scholar] [CrossRef]
- Gill, C.C. Epidemiology of barley yellow dwarf virus in Manitoba and effect of the virus on yield of cereals. Phytopathology 1970, 60, 1826–1830. [Google Scholar] [CrossRef]
- Yount, D.J.; Martin, J.M.; Carroll, T.W.; Zaske, S.K. Effects of barley yellow dwarf virus on growth and yield of small grains in Montana. Plant Dis. 1985, 69, 487–491. [Google Scholar] [CrossRef]
- Jensen, S.G.; Fitzgerald, P.J.; Thysell, J.R. Physiology and field performance of wheat infected with barley yellow dwarf virus. Crop Sci. 1971, 11, 775–780. [Google Scholar] [CrossRef]
- Davis, T.S.; Bosque-Pérez, N.A.; Foote, N.E.; Magney, T.; Eigenbrode, S. Environmentally dependent host-pathogen and vector-pathogen interactions in the Barley yellow dwarf virus pathosystem. J. Appl. Ecol. 2015, 52, 1392–1401. [Google Scholar] [CrossRef]
- Roossinck, M.J. Plant Virus Ecology. PLoS Pathog. 2013, 9, e1003304. [Google Scholar] [CrossRef]
- Xu, P.; Chen, F.; Mannas, J.P.; Feldman, T.; Sumner, L.W.; Roossinck, M.J. Virus infection improves drought tolerance. New Phytol. 2008, 180, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, T.K.; Kolb, F.L. Effects of barley yellow dwarf virus on yield and yield components of drilled winter wheat. Plant Dis. 1998, 82, 620–624. [Google Scholar] [CrossRef]
- Monneveux, P.; St-Pierre, C.A.; Comeau, A.; Makkouk, K.M. Barley yellow dwarf virus tolerance in drought situations. In Barley Yellow Dwarf in West Asia and North Africa; Comeau, A., Makkouk, K.M., Eds.; ICARDA: Aleppo, Syria, 1992; pp. 209–220. [Google Scholar]
- Erion, G.G.; Riedell, W.E. Barley yellow dwarf virus effects on cereal plant growth and transpiration. Crop Sci. 2012, 52, 2794–2799. [Google Scholar] [CrossRef]
- Hoffman, T.K.; Kolb, F.L. Effects of barley yellow dwarf virus on root and shoot growth of winter wheat seedlings grown in aeroponic culture. Plant Dis. 1997, 81, 497–500. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Rashidi, M.; Rogers, C.; Marshall, J.; Price, W.J.; Rashed, A. Winter wheat (Triticum aestivum) response to Barley yellow dwarf virus at various nitrogen application rates in the presence and absence of its aphid vector, Rhopalosiphum padi. Entomol. Exp. Appl. 2019, 167, 98–107. [Google Scholar] [CrossRef]
- Malmstrom, C.M.; Bigelow, P.; Trębicki, P.; Busch, A.K.; Friel, C.; Cole, E.; Abdel-Azim, H.; Phillippo, C.; Alexander, H.M. Crop-associated virus reduces the rooting depth of non-crop perennial native grass more than non-crop associated virus with known viral suppressor of RNA silencing (VSR). Virus Res. 2017, 241, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Comeau, A.; Jedlinski, H. Successful breeding for barley yellow dwarf virus resistance or tolerance: A systemic approach related to other agronomic characteristics. In World Perspectives on Barley Yellow Dwarf Virus; Burnett, P.A., Ed.; CIMMYT: Texcoco, Mexico, 1990; pp. 441–451. [Google Scholar]
- Comeau, A.; Pelletier, G.J. Predisposition to Septoria leaf blotch in oats affected by barley yellow dwarf virus. Can. J. Plant Sci. 1976, 56, 13–19. [Google Scholar] [CrossRef] [Green Version]




| Year | Jan–Mar | Apr–Jun | Jul–Sep | Oct–Dec | |
|---|---|---|---|---|---|
| Rainfall (mm) | 2014 | 36 | 133 | 64 | 33 |
| 2015 | 88 | 77 | 60 | 28 | |
| 2016 | 69 | 110 | 222 | 108 | |
| 2017 | 56 | 135 | 128 | 112 | |
| 2018 | 24 | 82 | 79 | 42 | |
| Long-term mean | 1961–2018 | 67 | 107 | 132 | 97 |
| Mean maximum temperature (°C) | 2014 | 30.9 | 18.4 | 16.4 | 27.3 |
| 2015 | 29.1 | 17.1 | 15.4 | 29.3 | |
| 2016 | 30.2 | 18.4 | 14.8 | 24.0 | |
| 2017 | 30.3 | 17.9 | 15.3 | 27.2 | |
| 2018 | 30.6 | 19.0 | 16.0 | 26.7 | |
| Long-term mean | 1961–2018 | 28.9 | 17.8 | 15.2 | 24.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nancarrow, N.; Aftab, M.; Hollaway, G.; Rodoni, B.; Trębicki, P. Yield Losses Caused by Barley Yellow Dwarf Virus-PAV Infection in Wheat and Barley: A Three-Year Field Study in South-Eastern Australia. Microorganisms 2021, 9, 645. https://doi.org/10.3390/microorganisms9030645
Nancarrow N, Aftab M, Hollaway G, Rodoni B, Trębicki P. Yield Losses Caused by Barley Yellow Dwarf Virus-PAV Infection in Wheat and Barley: A Three-Year Field Study in South-Eastern Australia. Microorganisms. 2021; 9(3):645. https://doi.org/10.3390/microorganisms9030645
Chicago/Turabian StyleNancarrow, Narelle, Mohammad Aftab, Grant Hollaway, Brendan Rodoni, and Piotr Trębicki. 2021. "Yield Losses Caused by Barley Yellow Dwarf Virus-PAV Infection in Wheat and Barley: A Three-Year Field Study in South-Eastern Australia" Microorganisms 9, no. 3: 645. https://doi.org/10.3390/microorganisms9030645
APA StyleNancarrow, N., Aftab, M., Hollaway, G., Rodoni, B., & Trębicki, P. (2021). Yield Losses Caused by Barley Yellow Dwarf Virus-PAV Infection in Wheat and Barley: A Three-Year Field Study in South-Eastern Australia. Microorganisms, 9(3), 645. https://doi.org/10.3390/microorganisms9030645







