Gut Microbiota Dynamics, Growth Performance, and Gut Morphology in Broiler Chickens Fed Diets Varying in Energy Density with or without Bacitracin Methylene Disalicylate (BMD)
Abstract
1. Introduction
2. Materials and Methods
2.1. Management and Housing
2.2. Experimental Diets
2.3. Data and Sample Collection
2.4. Analysis of Diets and Histological Samples
2.4.1. Diet Analysis
2.4.2. Histological Measurements
2.5. DNA Extraction and 16S rRNA Gene Sequencing
2.6. Bioinformatics and Statistical Analyses
3. Results
3.1. Growth Performance, Organ Weight, and Jejunal Morphology
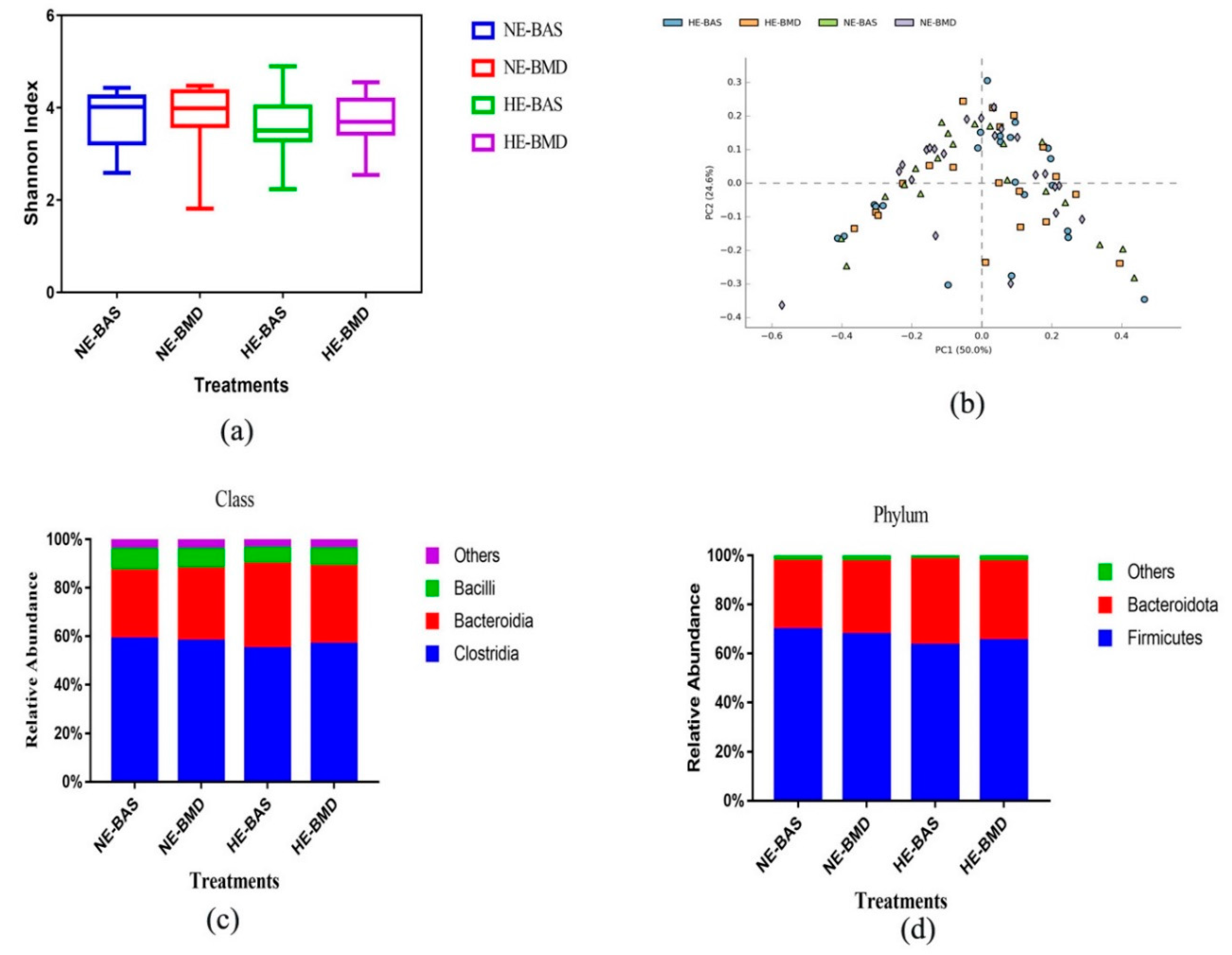
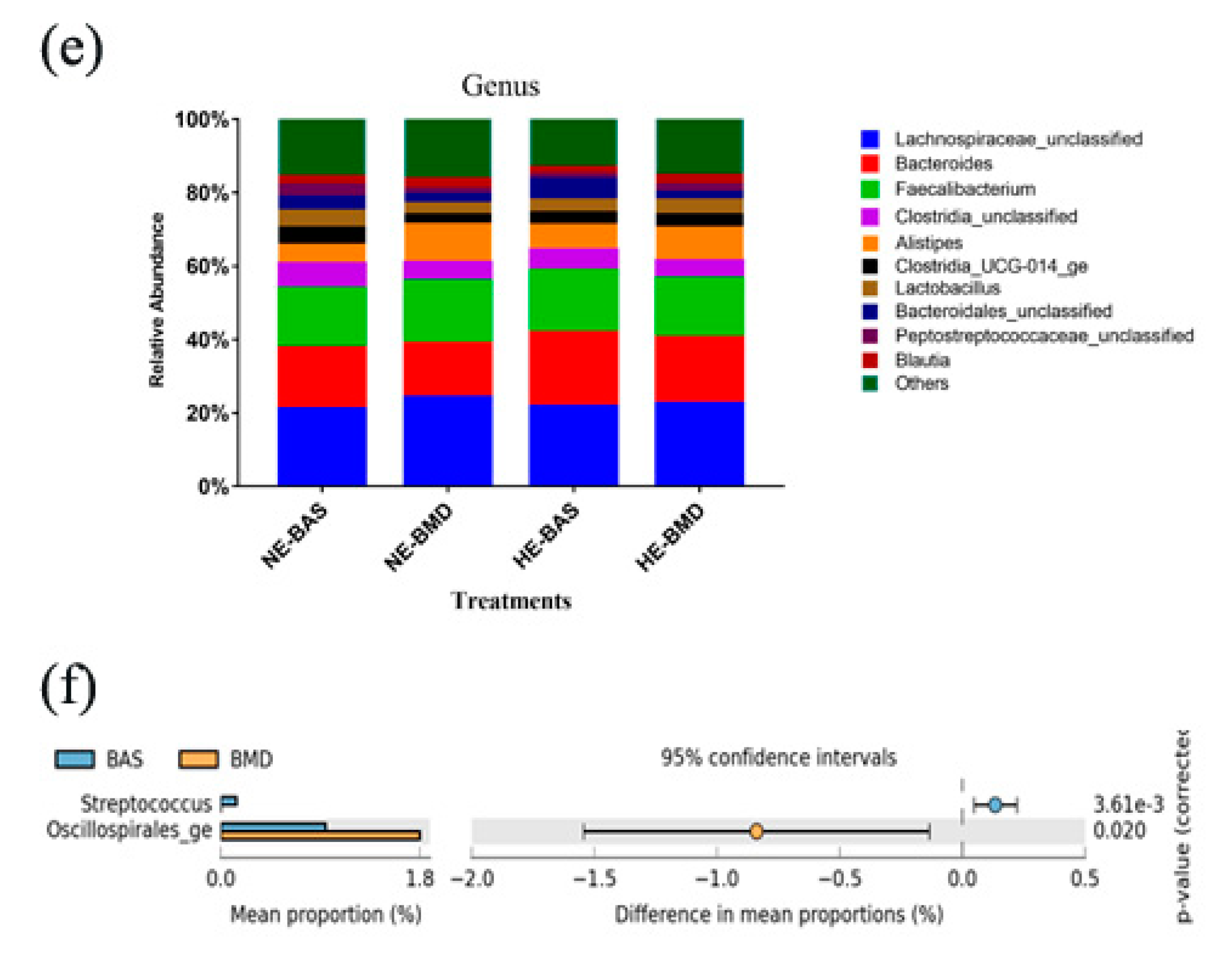
3.2. Composition of Chicken Gut Microbiota of Chickens Fed with or without BMD at Varying Energy Levels
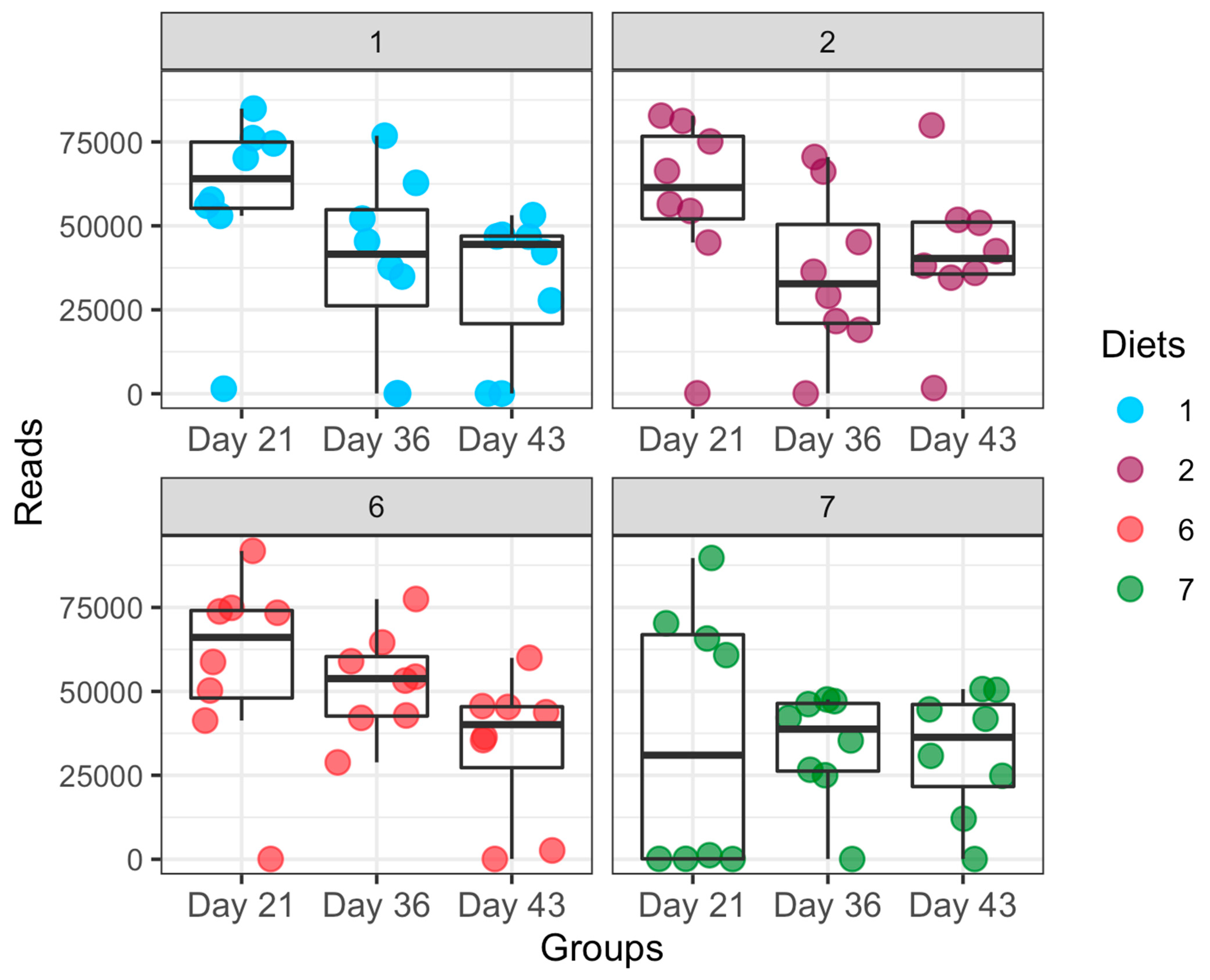
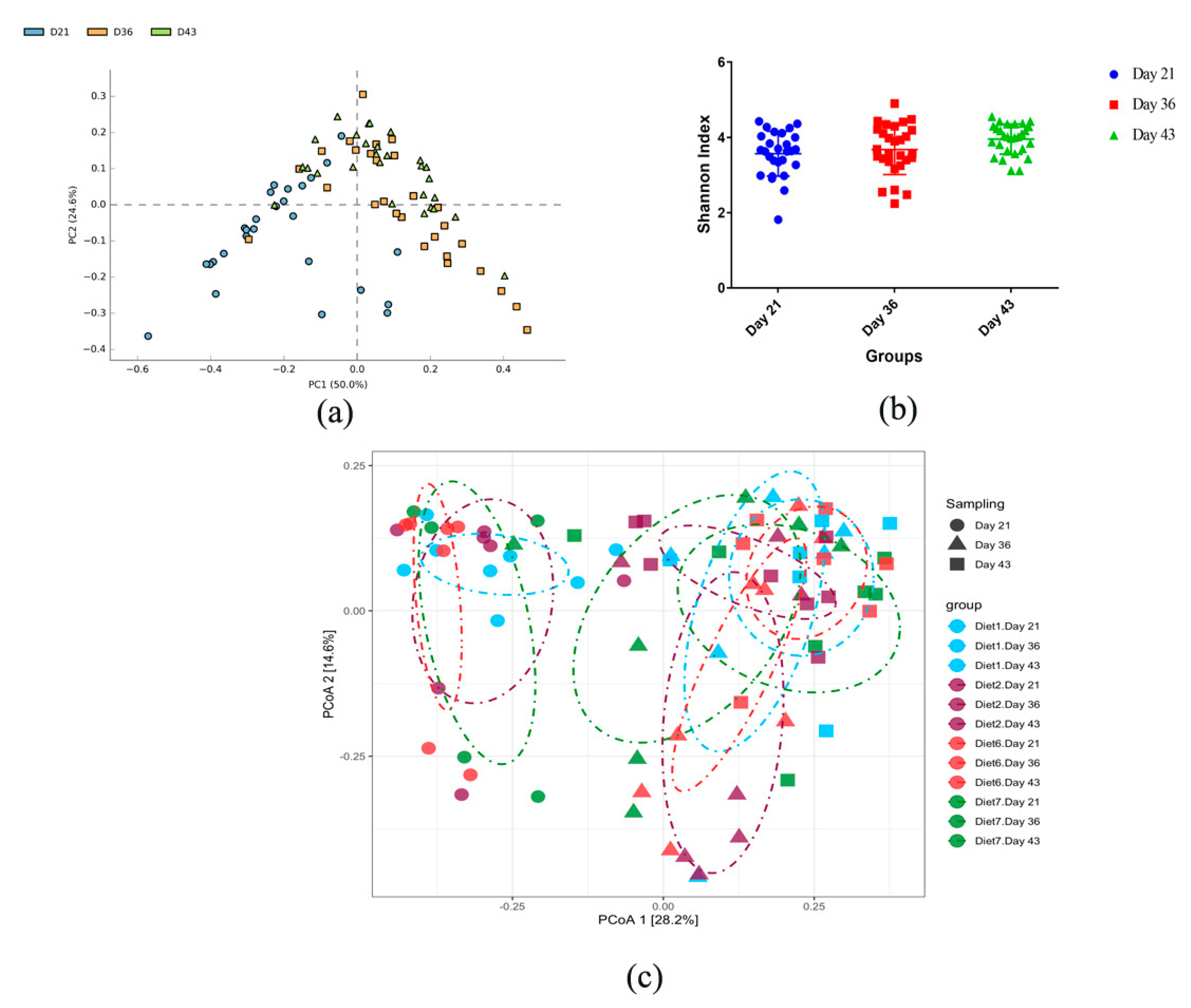
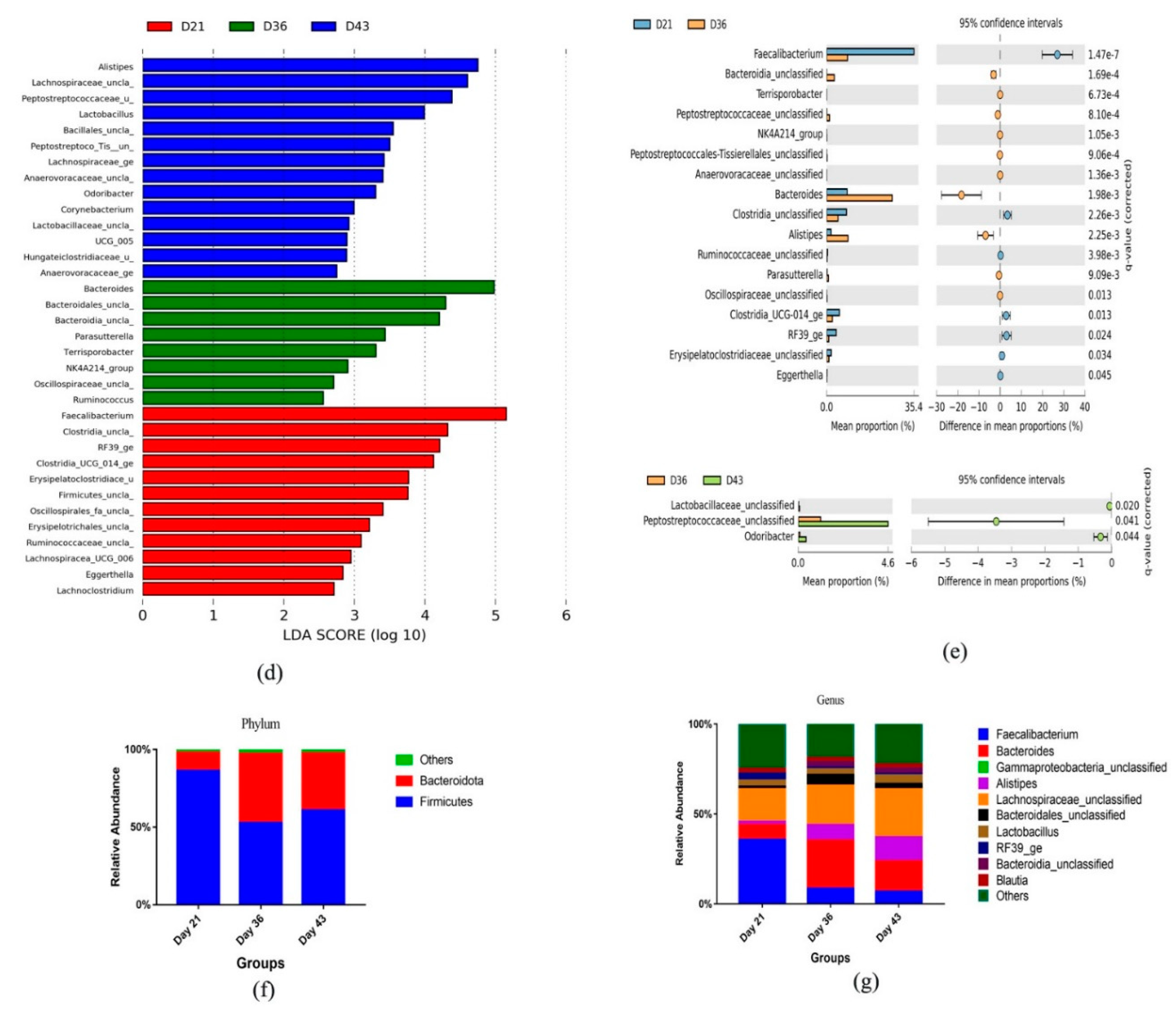
3.3. Dynamic Changes in Microbial Taxa across Growth Stages
4. Discussion
4.1. Effect of BMD and Energy Density on Growth Performance, Organ Weight, and Jejunal Morphology
4.2. Effect of BMD and Energy Density on Gut Microbiota Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drewnowski, A.; Almiron-Roig, E.; Marmonier, C.; Lluch, A. Dietary energy density and body weight: Is there a relationship? Nutr. Rev. 2004, 11, 403–413. [Google Scholar] [CrossRef]
- Abdollahi, M.R.; Zaefarian, F.; Ravindran, V.; Selle, P.H. The Interactive Influence of Dietary Nutrient Density and Feed form on the Performance of Broiler Chickens. Anim. Feed Sci. Technol. 2018, 239, 33–43. [Google Scholar] [CrossRef]
- Brickett, K.E.; Dahiya, J.P.; Classen, H.L.; Gomis, S. Influence of Dietary Nutrient Density, Feed form, and Lighting on Growth and Meat Yield of Broiler Chickens. Poult. Sci. 2007, 86, 2172–2181. [Google Scholar] [CrossRef]
- Zeng, Q.F.; Cherry, P.; Doster, A.; Murdoch, R.; Adeola, O.; Applegate, T.J. Effect of Dietary Energy and Protein Content on Growth and Carcass Traits of Pekin Ducks. Poult. Sci. 2015, 94, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.P.; Xie, M.; Wang, W.W.; Hou, S.S.; Huang, W. Effects of Dietary Energy on Growth Performance and Carcass Quality of White Growing Pekin Ducks from Two to Six Weeks of Age. Poult. Sci. 2008, 87, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Grover, R.M.; Anderson, D.L.; Damon, R.A.; Ruggles, L.H. The Effects of Bird Density, Dietary Energy, Light Intensity, and Cage Level on the Reproductive Performance of Heavy Type Chickens in Wire Cages. Poult. Sci. 1972, 51, 565–575. [Google Scholar] [CrossRef]
- Adewole, D. Effect of Dietary Supplementation with Coarse or Extruded Oat Hulls on Growth Performance, Blood Biochemical Parameters, Ceca Microbiota and Short Chain Fatty Acids in Broiler Chickens. Animals 2020, 9, 1160. [Google Scholar] [CrossRef] [PubMed]
- Kuttappan, V.A.; Brewer, V.B.; Apple, J.K.; Waldroup, P.W.; Owens, C.M. Influence of Growth Rate on the Occurrence of White Striping in Broiler Breast Fillets. Poult. Sci. 2012, 91, 2677–2685. [Google Scholar] [CrossRef]
- Lamot, D.M.; Sapkota, D.; Wijtten, P.J.A.; Van Den Anker, I.; Heetkamp, M.J.W.; Kemp, B.; Van Den Brand, H. Diet Density during the First Week of Life: Effects on Growth Performance, Digestive Organ Weight, and Nutrient Digestion of Broiler Chickens. Poult. Sci. 2019, 98, 789–795. [Google Scholar] [CrossRef]
- Daniel, H.; Gholami, A.M.; Berry, D.; Desmarchelier, C.; Hahne, H.; Loh, G.; Mondot, S.; Lepage, P.; Rothballer, M.; Walker, A.; et al. High-Fat Diet Alters Gut Microbiota Physiology in Mice. ISME J. 2014, 8, 295–308. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-Fat Diet Determines the Composition of the Murine Gut Microbiome Independently of Obesity. Gastroenterology 2009, 137, 1716–1724.e2. [Google Scholar] [CrossRef]
- Minaya, D.M.; Turlej, A.; Joshi, A.; Nagy, T.; Weinstein, N.; DiLorenzo, P.; Hajnal, A.; Czaja, K. Consumption of a High Energy Density Diet Triggers Microbiota Dysbiosis, Hepatic Lipidosis, and Microglia Activation in the Nucleus of the Solitary Tract in Rats. Nutr. Diabetes 2020, 10, 20. [Google Scholar] [CrossRef]
- De La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to High-Fat Diet-Induced Obesity in Rats Is Associated with Changes in the Gut Microbiota and Gut Inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G440–G448. [Google Scholar] [CrossRef]
- Liu, J.B.; Yan, H.L.; Zhang, Y.; Hu, Y.D.; Zhang, H.F. Effects of Dietary Energy and Protein Content and Lipid Source on Growth Performance and Carcass Traits in Pekin Ducks. Poult. Sci. 2019, 98, 4829–4837. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. Categorization of Antimicrobial Drugs Based on Importance in Human Medicine. 2009. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/antimicrobial-resistance/categorization-antimicrobial-drugs-based-importance-human-medicine.html#shr-pg0 (accessed on 20 December 2020).
- Chicken Farmers of Canada Annual Report. 2019. Available online: https://www.chickenfarmers.ca/wp-content/uploads/2020/03/Annual-report-2019-%E2%80%93-E-Web.pdf (accessed on 20 December 2020).
- Sims, M.D.; Dawson, K.A.; Newman, K.E.; Spring, P.; Hooge, D.M. Effects of Dietary Mannan Oligosaccharide, Bacitracin Methylene Disalicylate, or Both on the Live Performance and Intestinal Microbiology of Turkeys. Poult. Sci. 2004, 83, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Huyghebaert, G.; Ducatelle, R.; Immerseel, F. Van. An Update on Alternatives to Antimicrobial Growth Promoters for Broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef]
- Crisol-Martínez, E.; Stanley, D.; Geier, M.S.; Hughes, R.J.; Moore, R.J. Understanding the Mechanisms of Zinc Bacitracin and Avilamycin on Animal Production: Linking Gut Microbiota and Growth Performance in Chickens. Appl. Microbiol. Biotechnol. 2017, 101, 4547–4559. [Google Scholar] [CrossRef] [PubMed]
- Engberg, R.M.; Hedemann, M.S.; Leser, T.D.; Jensen, B.B. Effect of Zinc Bacitracin and Salinomycin on Intestinal Microflora and Performance of Broilers. Poult. Sci. 2000, 79, 1311–1319. [Google Scholar] [CrossRef]
- Proctor, A.; Phillips, G.J. Differential Effects of Bacitracin Methylene Disalicylate (BMD) on the Distal Colon and Cecal Microbiota of Young Broiler Chickens. Front. Vet. Sci. 2019, 6, 114. [Google Scholar] [CrossRef]
- Gong, J.; Yu, H.; Liu, T.; Gill, J.J.; Chambers, J.R.; Wheatcroft, R.; Sabour, P.M. Effects of Zinc Bacitracin, Bird Age and Access to Range on Bacterial Microbiota in the Ileum and Caeca of Broiler Chickens. J. Appl. Microbiol. 2008, 104, 1372–1382. [Google Scholar] [CrossRef]
- Oladokun, S.; Koehler, A.; MacIsaac, J.; Ibeagha-Awemu, E.M.; Adewole, D.I. Bacillus Subtilis Delivery Route: Effect on Growth Performance, Intestinal Morphology, Cecal Short-Chain Fatty Acid Concentration and Cecal Microbiota in Broiler Chickens. Poult. Sci. 2021, 100, 100809. [Google Scholar] [CrossRef] [PubMed]
- CCAC. The Care and Use of Farm Animals in Research, Teaching and Testing; CCAC: Ottawa, ON, Canada, 2009.
- Kindlein, L.; Ferreira, T.Z.; Driemeier, D.; Nascimento, V.P.D.; Vieira, S.L.; Moraes, L.E.F.D.D.; King, A.; Sainz, R.D. Occurrence and Severity of White Striping in Broilers Until 50d of Age Fed with High and Low-Energy Diets: Body Weight, Histopathological Changes and Meat Quality. J. Vet. Sci. Technol. 2017, 8, 478. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; TrAC Trends Analytical Chemist: Washington, DC, USA, 1990. [Google Scholar]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illu- mina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Weaver, W. Recent Contributions to the Mathematical Theory of Communication. In The Mathematical Theory of Communication; Institute of General Semantics: Forest Hills, NY, USA, 1949. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Anderson, M.J.; Walsh, D.C.I. PERMANOVA, ANOSIM, and the Mantel Test in the Face of Heterogeneous Dispersions: What Null Hypothesis Are You Testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Welch, B.L. The Generalisation of Student’s Problems When Several Different Population Variances Are Involved. Biometrika 1947, 34, 28–35. [Google Scholar]
- Parks, D.H.; Beiko, R.G. Identifying Biologically Relevant Differences between Metagenomic Communities. Bioinformatics 2010, 26, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.P.; Chen, J.L.; Zhao, G.P.; Zheng, M.Q.; Jiang, R.R.; Wen, J. Live Performance, Carcass Composition, and Blood Metabolite Responses to Dietary Nutrient Density in Two Distinct Broiler Breeds of Male Chickens. Poult. Sci. 2009, 88, 2575–2584. [Google Scholar] [CrossRef]
- Perween, S.; Kumar, K.; Chandramoni; Kumar, S.; Singh, P.K.; Kumar, M.; Dey, A. Effect of Feeding Different Dietary Levels of Energy and Protein on Growth Performance and Immune Status of Vanaraja Chicken in the Tropic. Vet. World 2016, 9, 893–899. [Google Scholar] [CrossRef]
- Wang, X.; Peebles, E.D.; Zhai, W. Effects of Protein Source and Nutrient Density in the Diets of Male Broilers from 8 to 21 Days of Age on Their Subsequent Growth, Blood Constituents, and Carcass Compositions. Poult. Sci. 2014, 93, 1463–1474. [Google Scholar] [CrossRef]
- Coon, C.N.; Becker, W.A.; Spencer, J.V. The Effect of Feeding High Energy Diets Containing Supplemental Fat on Broiler Weight Gain, Feed Efficiency, and Carcass Composition. Poult. Sci. 1981, 60, 1264–1271. [Google Scholar] [CrossRef]
- Damron, B.L.; Wilson, H.R.; Fell, R.V. Growth and Performance of Broiler Breeders Fed Bacitracin Methylene Disalicylate and Zinc Bacitracin. Poult. Sci. 1991, 70, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Manafi, M.; Khalaji, S.; Hedayati, M.; Pirany, N. Efficacy of Bacillus Subtilis and Bacitracin Methylene Disalicylate on Growth Performance, Digestibility, Blood Metabolites, Immunity, and Intestinal Microbiota after Intramuscular Inoculation with Escherichia Coli in Broilers. Poult. Sci. 2017, 96, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kennes, Y.M.; Lepp, D.; Yin, X.; Wang, Q.; Yu, H.; Yang, C.; Gong, J.; Diarra, M.S. Effects of Encapsulated Cinnamaldehyde and Citral on the Performance and Cecal Microbiota of Broilers Vaccinated or Not Vaccinated against Coccidiosis. Poult. Sci. 2020, 99, 936–948. [Google Scholar] [CrossRef]
- Bai, W.Q.; Zhang, K.Y.; Ding, X.M.; Bai, S.P.; Wang, J.P.; Peng, H.W.; Zeng, Q.F. High Dietary Energy Content Increases Inflammatory Markers after Lipopolysaccharide Challenge in Meat Ducks. Poult. Sci. 2019, 98, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Schat, K.A.; Skinner, M.A. Avian Immunosuppressive Diseases and Immunoevasion. In Avian Immunology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 275–297. [Google Scholar]
- Grond, K.; Sandercock, B.K.; Jumpponen, A.; Zeglin, L.H. The Avian Gut Microbiota: Community, Physiology and Function in Wild Birds. J. Avian Biol. 2018, 49, e01788. [Google Scholar] [CrossRef]
- Cho, H.J.; Zhao, P.; Kim, I.H. Effects of Emulsifier and Multi-Enzyme in Different Energy Densitydiet on Growth Performance, Blood Profiles, and Relative Organ Weight in Broiler Chickens. J. Agric. Sci. 2012, 4, 161. [Google Scholar] [CrossRef]
- Hussein, E.O.S.; Suliman, G.M.; Alowaimer, A.N.; Ahmed, S.H.; Abd El-Hack, M.E.; Taha, A.E.; Swelum, A.A. Growth, Carcass Characteristics, and Meat Quality of Broilers Fed a Low-Energy Diet Supplemented with a Multienzyme Preparation. Poult. Sci. 2020, 99, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Adebowale, T. The Effect of Dietary High Energy Density and Carbohydrate Energy Ratio on Digestive Enzymes Activity, Nutrient Digestibility, Amino Acid Utilization and Intestinal Morphology of Weaned Piglets. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1492–1502. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, G.R.; Syed, B.; Haldar, S.; Pender, C. Phytogenic Feed Additives as an Alternative to Antibiotic Growth Promoters in Broiler Chickens. Front. Vet. Sci. 2015, 2, 21. [Google Scholar]
- Abdelqader, A.; Al-Fataftah, A.R. Effect of Dietary Butyric Acid on Performance, Intestinal Morphology, Microflora Composition and Intestinal Recovery of Heat-Stressed Broilers. Livest. Sci. 2016, 183, 78–83. [Google Scholar] [CrossRef]
- Broom, L.J.; Kogut, M.H. The Role of the Gut Microbiome in Shaping the Immune System of Chickens. Vet. Immunol. Immunopathol. 2018, 204, 44–51. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The Effect of Antibiotics on the Composition of the Intestinal Microbiota—A Systematic Review. J Infect. 2019, 79, 471–489. [Google Scholar] [CrossRef]
- Dal Pont, G.C.; Farnell, M.; Farnell, Y.; Kogut, M.H. Dietary Factors as Triggers of Low-Grade Chronic Intestinal Inflammation in Poultry. Microorganisms 2020, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Angelakis, E.; Raoult, D. The Increase of Lactobacillus Species in the Gut Flora of Newborn Broiler Chicks and Ducks Is Associated with Weight Gain. PLoS ONE 2010, 5, e10463. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Eck, A.; Rutten, N.B.M.M.; Singendonk, M.M.J.; Rijkers, G.T.; Savelkoul, P.H.M.; Meijssen, C.B.; Crijns, C.E.; Oudshoorn, J.H.; Budding, A.E.; Vlieger, A.M. Neonatal Microbiota Development and the Effect of Early Life Antibiotics Are Determined by Two Distinct Settler Types. PLoS ONE 2020, 15, e0228133. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Shanker, E.; Federle, M.J. Quorum Sensing Regulation of Competence and Bacteriocins in Streptococcus Pneumoniae and Mutans. Genes 2017, 8, 15. [Google Scholar] [CrossRef]
- Matsumoto-Nakano, M. Role of Streptococcus Mutans Surface Proteins for Biofilm Formation. Jpn. Dent. Sci. Rev. 2018, 54, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Zheng, H.M.; Zhang, G.X.; Chen, F.L.; Chen, L.D.; Yang, Z.C. High Oscillospira Abundance Indicates Constipation and Low BMI in the Guangdong Gut Microbiome Project. Sci. Rep. 2020, 10, 9364. [Google Scholar] [CrossRef] [PubMed]
- García-Bayona, L.; Comstock, L.E. Bacterial Antagonism in Host-Associated Microbial Communities. Science 2018, 361, eaat2456. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Dawid, S. Mobilization of Bacteriocins during Competence in Streptococci. Trends Microbiol. 2018, 26, 389–391. [Google Scholar] [CrossRef]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci. 2018, 5, 254. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Avershina, E.; Ross, R.P.; Murphy, K.; Stanton, C.; Rudi, K.; Kober, O.I.; Marchesi, J.R.; Narbad, A.; Juge, N.; et al. The Composition of the Gut Microbiota throughout Life, with an Emphasis on Early Life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef]
- Donaldson, E.E.; Stanley, D.; Hughes, R.J.; Moore, R.J. The Time-Course of Broiler Intestinal Microbiota Development after Administration of Cecal Contents to Incubating Eggs. PeerJ 2017, 5, e3587. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, N.; Wang, X.; Chi, Y.; Zhang, Y.; Qiu, X.; Hu, Y.; Li, J.; Liu, Y. Dysbiosis Gut Microbiota Associated with Inflammation and Impaired Mucosal Immune Function in Intestine of Humans with Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2015, 5, 8096. [Google Scholar] [CrossRef]
- Graziani, F.; Pujol, A.; Nicoletti, C.; Dou, S.; Maresca, M.; Giardina, T.; Fons, M.; Perrier, J. Ruminococcus Gnavus E1 Modulates Mucin Expression and Intestinal Glycosylation. J. Appl. Microbiol. 2016, 120, 1403–1417. [Google Scholar] [CrossRef]
- Song, Y.; Malmuthuge, N.; Steele, M.A.; Guan, L.L. Shift of Hindgut Microbiota and Microbial Short Chain Fatty Acids Profiles in Dairy Calves from Birth to Pre-Weaning. FEMS Microbiol. Ecol. 2018, 94, fix179. [Google Scholar] [CrossRef]
- Hollister, E.B.; Gao, C.; Versalovic, J. Compositional and Functional Features of the Gastrointestinal Microbiome and Their Effects on Human Health. Gastroenterology 2014, 146, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Neal-McKinney, J.M.; Lu, X.; Duong, T.; Larson, C.L.; Call, D.R.; Shah, D.H.; Konkel, M.E. Production of Organic Acids by Probiotic Lactobacilli Can Be Used to Reduce Pathogen Load in Poultry. PLoS ONE 2012, 7, e43928. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, Y.; Xiao, K.; Jiang, F.; Wang, H.; Tang, D.; Liu, D.; Liu, B.; Liu, Y.; He, X.; et al. The Chicken Gut Metagenome and the Modulatory Effects of Plant-Derived Benzylisoquinoline Alkaloids 06 Biological Sciences 0605 Microbiology. Microbiome 2018, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Becker, S.; Xiao, Y.; Lyu, W.; Yang, Q.; Zhu, H.; Yang, H.; Zhao, J.; Zhang, G. Differential Impact of Subtherapeutic Antibiotics and Ionophores on Intestinal Microbiota of Broilers. Microorganisms 2019, 7, 282. [Google Scholar] [CrossRef] [PubMed]

| Starter | Grower | Finisher | ||||
|---|---|---|---|---|---|---|
| Normal Energy | High Energy | Normal Energy | High Energy | Normal Energy | High Energy | |
| Ingredient composition | ||||||
| Corn | 41.33 | 42.66 | 44.32 | 39.68 | 48.48 | 44.11 |
| Soybean meal (46.5% CP) | 40.17 | 36.43 | 36.48 | 38.70 | 31.52 | 33.52 |
| Wheat | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Vegetable Oil | 3.43 | 5.87 | 4.59 | 7.00 | 5.67 | 8.04 |
| Limestone | 1.80 | 1.80 | 1.65 | 1.64 | 1.52 | 1.51 |
| Dicalcium phosphate | 1.23 | 1.21 | 1.06 | 1.05 | 0.93 | 0.92 |
| Pellet Binding Agent Y | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| DL-Methionine Premix X | 0.61 | 0.63 | 0.53 | 0.56 | 0.49 | 0.52 |
| Vitamin–Mineral Premix W,V | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Salt | 0.40 | 0.40 | 0.37 | 0.37 | 0.38 | 0.38 |
| HCL Lys | 0.03 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 |
| Calculated composition | ||||||
| Folic acid (ppm) | 2.20 | 2.20 | 2.20 | 2.20 | 2.20 | 2.20 |
| Digestible Trp | 0.25 | 0.26 | 0.23 | 0.24 | 0.21 | 0.22 |
| Digestible Thr | 0.87 | 0.90 | 0.82 | 0.84 | 0.74 | 0.76 |
| Digestible Met + Cys | 0.95 | 0.98 | 0.87 | 0.90 | 0.80 | 0.83 |
| Digestible Lys | 1.28 | 1.32 | 1.16 | 1.21 | 1.03 | 1.07 |
| ME, kcal/kg | 3000 | 3100 | 3100 | 3200 | 3200 | 3300 |
| Crude protein | 23.0 | 23.8 | 21.5 | 22.2 | 19.5 | 20.1 |
| Calcium | 0.96 | 0.96 | 0.87 | 0.87 | 0.79 | 0.79 |
| Available P | 0.48 | 0.48 | 0.44 | 0.44 | 0.40 | 0.40 |
| Sodium | 0.19 | 0.19 | 0.18 | 0.18 | 0.18 | 0.18 |
| ME/CP | 130 | 130 | 144 | 144 | 164 | 164 |
| ME/Digestible Lys | 2344 | 2349 | 2672 | 2645 | 3107 | 3084 |
| ME/Met + Cys | 3158 | 3163 | 3563 | 3556 | 4000 | 3976 |
| ME/Digestible Thr | 3448 | 3444 | 3781 | 3810 | 4324 | 4342 |
| ME/Trp | 12,000 | 11,923 | 13,478 | 13,333 | 15,238 | 15,000 |
| Analyzed composition (%, except where otherwise stated) | ||||||
| ME, kcal/kg | 3022 | 3145 | 3109 | 3203 | 3220 | 3317 |
| Crude protein | 24.2 | 25.2 | 21.7 | 22.9 | 20.2 | 20.3 |
| Crude fat | 6.11 | 8.03 | 6.41 | 8.69 | 7.04 | 6.70 |
| Dry matter | 88.8 | 89.1 | 86.2 | 87.9 | 87.7 | 86.7 |
| Normal Energy | High Energy | SEM 1 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Basal | BMD 2 | Basal | BMD | BMD | Energy | BMD × Energy | ||
| Feed intake, g/bird | ||||||||
| Days 0–7 | 164 | 164 | 167 | 171 | 5.07 | 0.670 | 0.363 | 0.724 |
| Days 8–14 | 354 | 362 | 350 | 351 | 9.91 | 0.636 | 0.448 | 0.727 |
| Days 15–24 | 1129 | 1068 | 1288 | 1059 | 97.8 | 0.149 | 0.447 | 0.398 |
| Days 25–35 | 1837 | 1795 | 1824 | 1753 | 25.4 | 0.035 | 0.279 | 0.574 |
| Days 35–42 | 1186 | 1402 | 1198 | 1187 | 83.8 | 0.232 | 0.235 | 0.186 |
| Days 0–42 | 4670 | 4791 | 4826 | 4520 | 137 | 0.507 | 0.679 | 0.130 |
| Body weight, g | ||||||||
| Day 0 | 43.6 | 43.3 | 43.2 | 43.2 | 0.29 | 0.658 | 0.390 | 0.658 |
| Day 7 | 186 a | 177 ab | 177 ab | 171 b | 3.70 | 0.131 | 0.010 | 0.892 |
| Day 14 | 501 | 492 | 502 | 506 | 13.5 | 0.830 | 0.573 | 0.611 |
| Day 24 | 1310 | 1290 | 1284 | 1287 | 13.6 | 0.517 | 0.289 | 0.404 |
| Day 35 | 2471 | 2453 | 2457 | 2477 | 23.0 | 0.966 | 0.829 | 0.433 |
| Day 42 | 3233 | 3247 | 3246 | 3291 | 38.2 | 0.447 | 0.464 | 0.681 |
| Body weight gain, g/bird | ||||||||
| Days 0–7 | 142 a | 138 ab | 134 ab | 127 b | 3.73 | 0.151 | 0.012 | 0.842 |
| Days 8–14 | 317 | 314 | 326 | 332 | 13.2 | 0.929 | 0.332 | 0.733 |
| Days 15–24 | 807 | 795 | 781 | 785 | 15.9 | 0.789 | 0.276 | 0.639 |
| Days 25–35 | 1161 | 1164 | 1173 | 1190 | 15.9 | 0.545 | 0.228 | 0.675 |
| Days 25–42 | 762 | 794 | 789 | 814 | 22.0 | 0.204 | 0.297 | 0.902 |
| Days 0–42 | 3190 | 3204 | 3203 | 3248 | 38.2 | 0.446 | 0.455 | 0.687 |
| Feed conversion ratio | ||||||||
| Days 0–7 | 1.15 b | 1.19 ab | 1.25 ab | 1.38 a | 0.06 | 0.194 | 0.017 | 0.609 |
| Days 8–14 | 1.13 | 1.16 | 1.09 | 1.07 | 0.05 | 0.871 | 0.173 | 0.663 |
| Days 15–24 | 1.41 | 1.34 | 1.67 | 1.35 | 0.13 | 0.135 | 0.360 | 0.414 |
| Days 25–35 | 1.59 a | 1.54 a | 1.56 a | 1.47 b | 0.02 | 0.003 | 0.009 | 0.286 |
| Days 25–42 | 1.55 | 1.80 | 1.52 | 1.46 | 0.12 | 0.667 | 0.117 | 0.220 |
| Days 0–42 | 1.47 | 1.50 | 1.51 | 1.39 | 0.01 | 0.335 | 0.378 | 0.099 |
| Parameter | Normal Energy | High Energy | SEM 1 | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Basal | BMD 2 | Basal | BMD | BMD | Energy | BMD × Energy | ||
| Spleen | 0.74 | 0.86 | 0.83 | 0.76 | 0.07 | 0.738 | 0.890 | 0.148 |
| Ceca | 5.70 | 4.55 | 4.61 | 4.60 | 0.51 | 0.260 | 0.314 | 0.264 |
| Liver | 15.8 | 15.5 | 15.4 | 15.0 | 0.53 | 0.484 | 0.417 | 0.883 |
| Bursa | 1.48 | 1.28 | 1.65 | 1.72 | 0.14 | 0.608 | 0.022 | 0.281 |
| Heart | 4.80 | 5.20 | 4.80 | 4.99 | 0.21 | 0.175 | 0.609 | 0.622 |
| Parameter | Normal Energy | High Energy | SEM 1 | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Basal | BMD 2 | Basal | BMD | BMD | Energy | BMD × Energy | ||
| Villus height | 1.51 ab | 1.33 b | 1.51 ab | 1.63 a | 0.04 | 0.630 | 0.019 | 0.051 |
| Villus width | 0.18 a | 0.19 a | 0.12 b | 0.19 a | 0.01 | 0.007 | 0.519 | 0.017 |
| Crypt depth | 0.17 | 0.18 | 0.15 | 0.17 | 0.01 | 0.179 | 0.552 | 0.666 |
| VH/CD 3 | 9.72 | 7.78 | 9.63 | 9.36 | 0.39 | 0.121 | 0.062 | 0.453 |
| log2 Fold Change | p-Value | Padj | Phylum | Genus | |
|---|---|---|---|---|---|
| Otu00013 | 10.27628 | 3.77 × 10−104 | 3.76 × 10−101 | Bacteroidota | Bacteroidia_unclassified |
| Otu00008 | 12.16469 | 1.10 × 10−52 | 5.50 × 10−50 | Bacteroidota | Alistipes |
| Otu00210 | 6.015742 | 2.53 × 10−34 | 8.40 × 10−32 | Bacteroidota | Bacteroidia_unclassified |
| Otu00016 | 12.06219 | 1.26 × 10−29 | 3.15 × 10−27 | Firmicutes | Peptostreptococcaceae_unclassified |
| Otu00081 | −5.50531 | 5.47 × 10−23 | 1.09 × 10−20 | Firmicutes | Faecalibacterium |
| Otu00004 | 12.52144 | 6.05 × 10−21 | 1.00 × 10−18 | Bacteroidota | Bacteroides |
| Otu00309 | 6.301178 | 1.10 × 10−20 | 1.57 × 10−18 | Firmicutes | Ruminococcus |
| Otu00209 | 5.807156 | 1.99 × 10−19 | 2.47 × 10−17 | Bacteroidota | Alistipes |
| Otu00001 | −3.82554 | 4.49 × 10−19 | 4.96 × 10−17 | Firmicutes | Faecalibacterium |
| Otu00039 | 6.494405 | 2.03 × 10−16 | 2.02 × 10−14 | Bacteroidota | Bacteroides |
| Otu00280 | −5.7675 | 2.99 × 10−16 | 2.70 × 10−14 | Firmicutes | Faecalibacterium |
| Otu00071 | 10.03166 | 1.45 × 10−15 | 1.21 × 10−13 | Firmicutes | Bacillales_unclassified |
| Otu00079 | 8.499449 | 1.78 × 10−15 | 1.36 × 10−13 | Firmicutes | Lachnospiraceae_unclassified |
| Otu00229 | 6.941029 | 1.93 × 10−15 | 1.37 × 10−13 | Firmicutes | Firmicutes_unclassified |
| Otu00150 | 3.574264 | 2.30 × 10−15 | 1.53 × 10−13 | Firmicutes | Lachnospiraceae_unclassified |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adewole, D.; Akinyemi, F. Gut Microbiota Dynamics, Growth Performance, and Gut Morphology in Broiler Chickens Fed Diets Varying in Energy Density with or without Bacitracin Methylene Disalicylate (BMD). Microorganisms 2021, 9, 787. https://doi.org/10.3390/microorganisms9040787
Adewole D, Akinyemi F. Gut Microbiota Dynamics, Growth Performance, and Gut Morphology in Broiler Chickens Fed Diets Varying in Energy Density with or without Bacitracin Methylene Disalicylate (BMD). Microorganisms. 2021; 9(4):787. https://doi.org/10.3390/microorganisms9040787
Chicago/Turabian StyleAdewole, Deborah, and Fisayo Akinyemi. 2021. "Gut Microbiota Dynamics, Growth Performance, and Gut Morphology in Broiler Chickens Fed Diets Varying in Energy Density with or without Bacitracin Methylene Disalicylate (BMD)" Microorganisms 9, no. 4: 787. https://doi.org/10.3390/microorganisms9040787
APA StyleAdewole, D., & Akinyemi, F. (2021). Gut Microbiota Dynamics, Growth Performance, and Gut Morphology in Broiler Chickens Fed Diets Varying in Energy Density with or without Bacitracin Methylene Disalicylate (BMD). Microorganisms, 9(4), 787. https://doi.org/10.3390/microorganisms9040787







