Encapsulated Mixture of Methyl Salicylate and Tributyrin Modulates Intestinal Microbiota and Improves Growth Performance of Weaned Piglets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Diets
2.2. Collection of Blood and Intestinal Samples
2.3. Biochemical Analysis and Anti-Oxidatant Capacity Related Parameters of Serum
2.4. Morphological Analysis of Ileum
2.5. Analysis of Short-Chain Fatty Acid in Colonic Content
2.6. 16S rRNA Sequencing Analysis
2.7. GC-TOF-MS and Metabolomics Data Processing
2.8. Statistical Analysis
3. Results
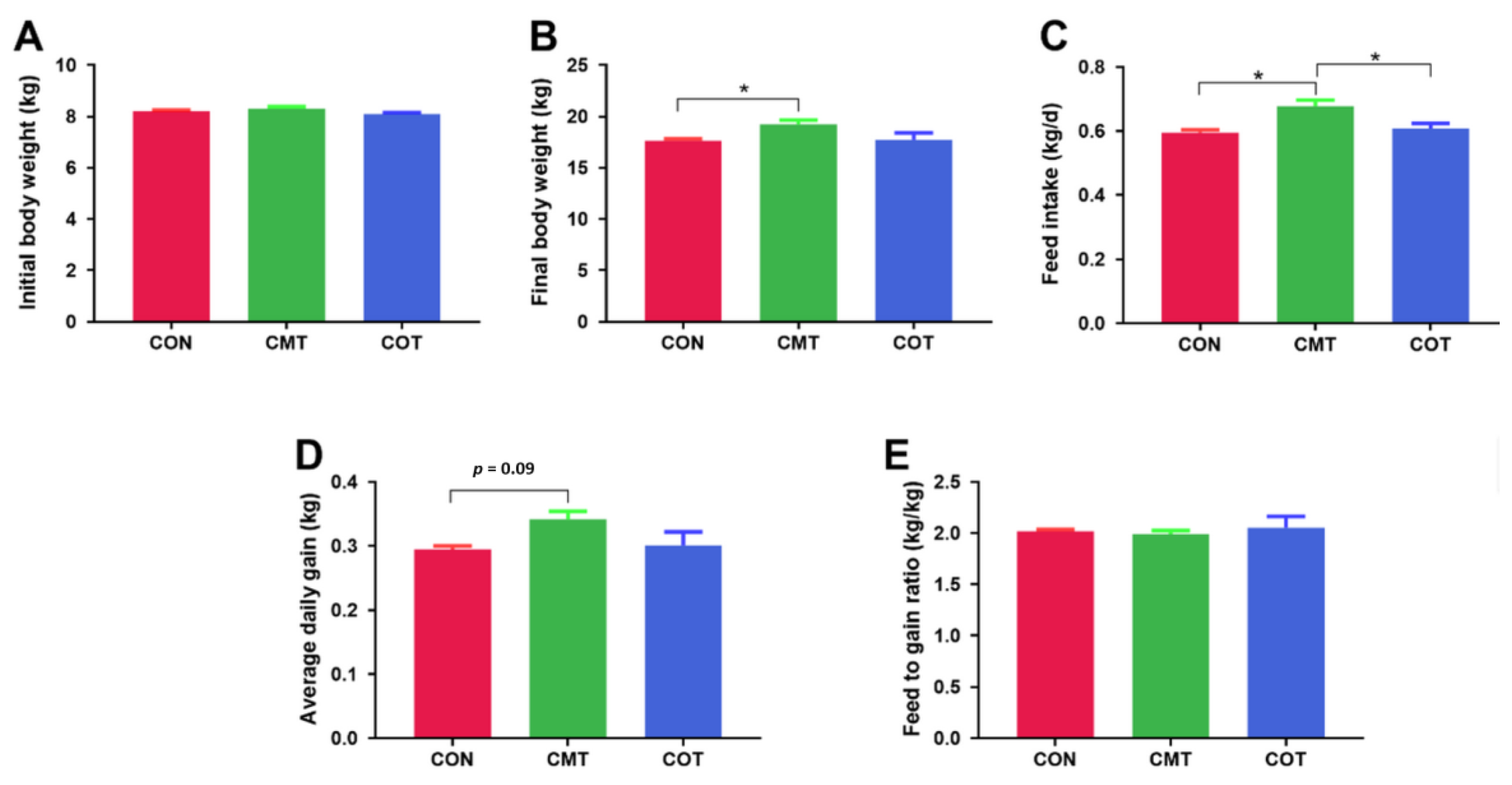
3.1. Growth Performance
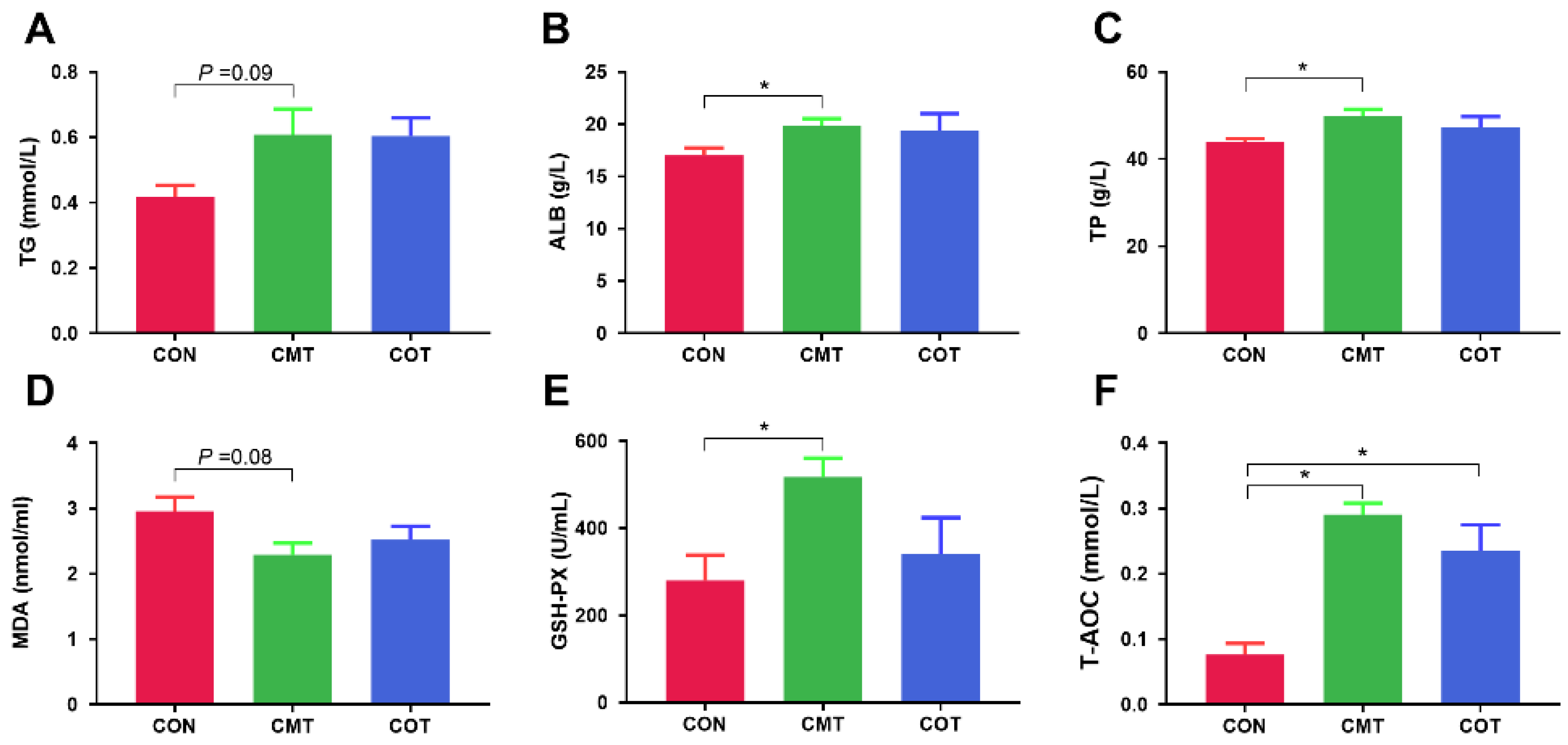
3.2. Serum Biochemical Indices and Anti-Oxidative Capacity Parameters
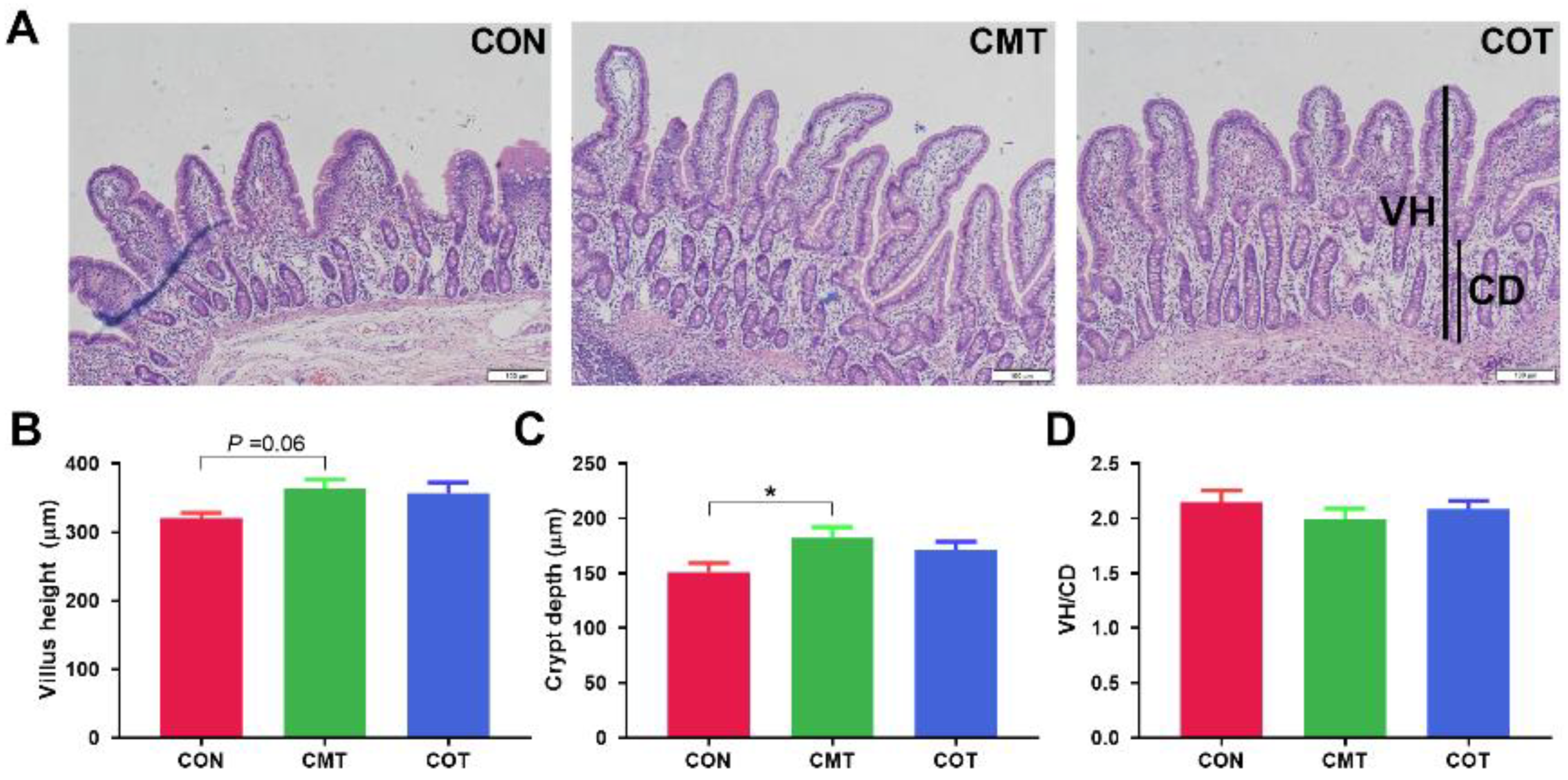
3.3. Ileum Morphological Analysis
3.4. Intestinal Content pH and Colonic SCFAs Profile
3.5. Compositional Profiles of the Intestinal Microbiota
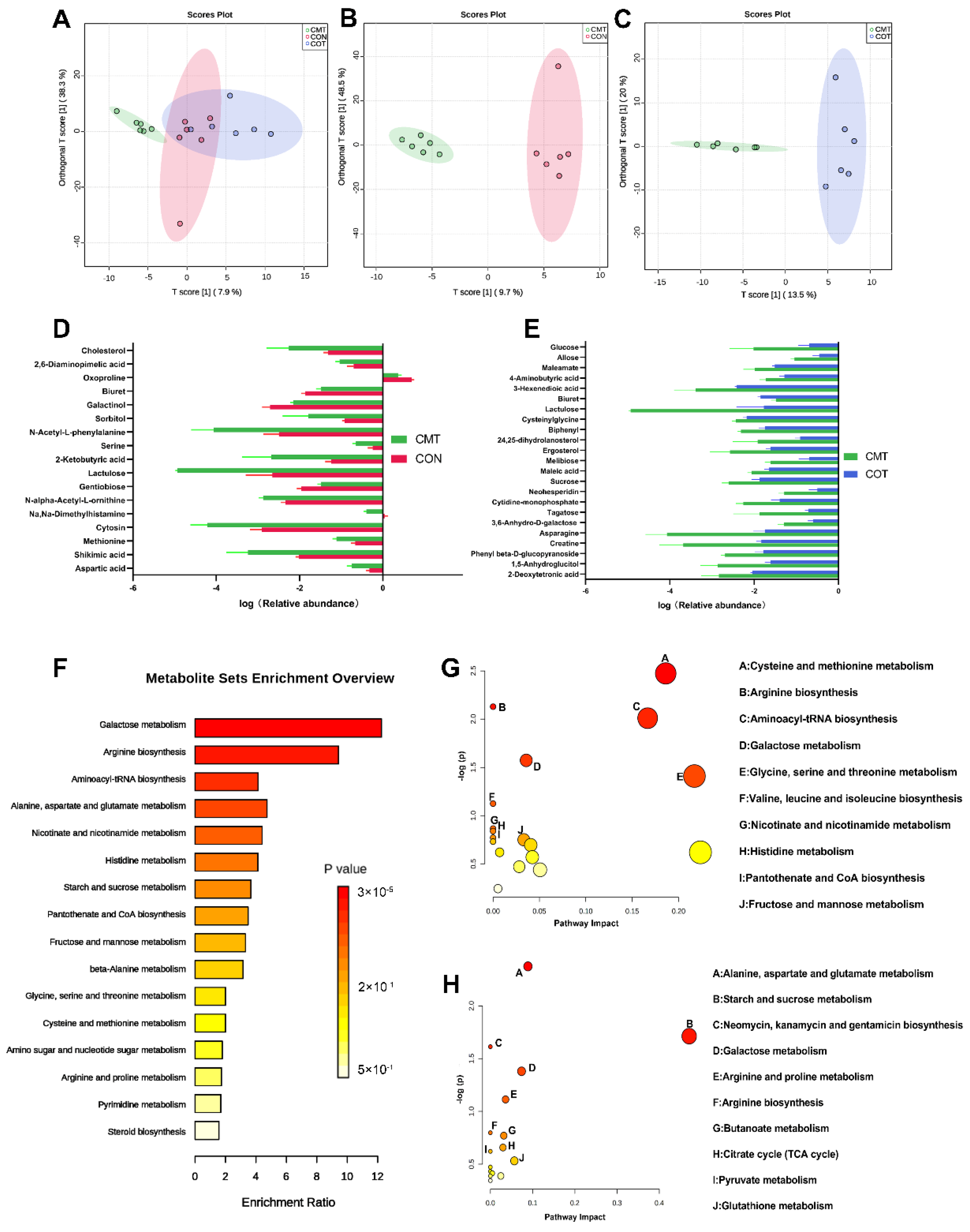
3.6. Overview of the Intestinal Metabolome
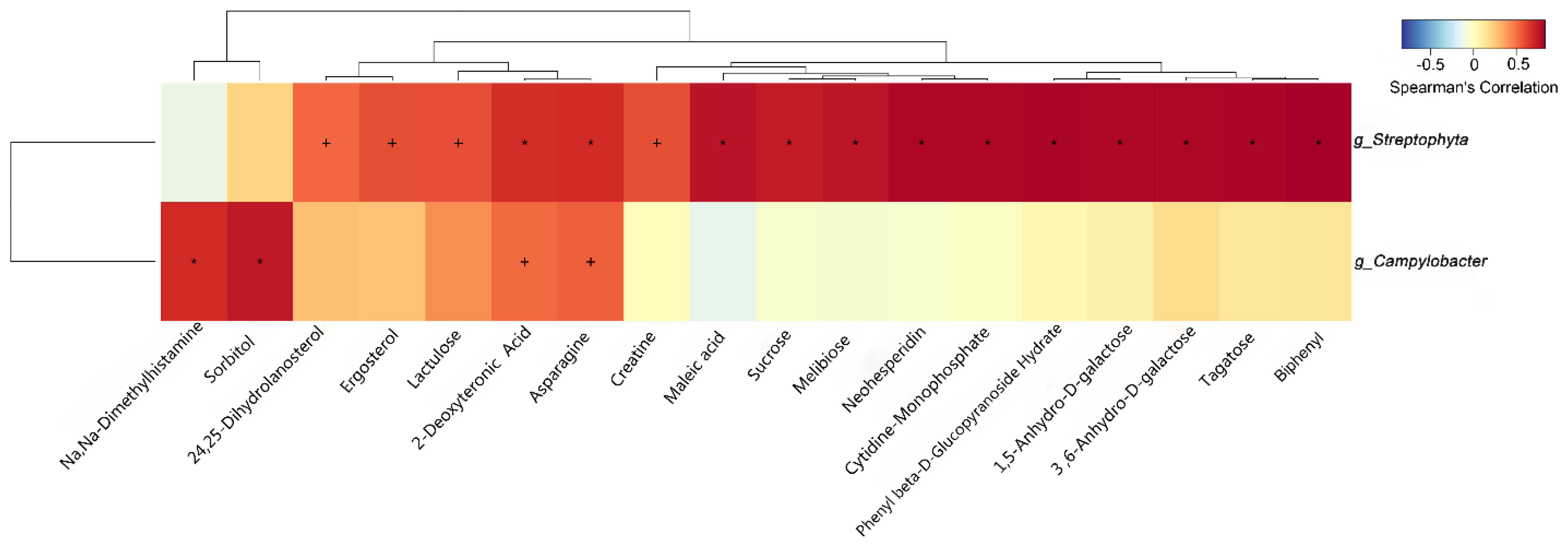
3.7. Correlation of Characteristic Gut Microbiota and Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cao, S.T.; Wang, C.C.; Wu, H.; Zhang, Q.H.; Jiao, L.F.; Hu, C.H. Weaning disrupts intestinal antioxidant status, impairs intestinal barrier and mitochondrial function, and triggers mitophagy in piglets. J. Anim. Sci. 2018, 96, 1073–1083. [Google Scholar] [CrossRef]
- Moeser, A.J.; Pohl, C.S.; Rajput, M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim. Nutr. 2017, 3, 313–321. [Google Scholar] [CrossRef]
- Hou, Y.Q.; Liu, Y.L.; Hu, J.; Shen, W.H. Effects of lactitol and tributyrin on growth performance, small intestinal morphology and enzyme activity in weaned pigs. Asian Australas. J. Anim. Sci. 2006, 19, 1470–1477. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.H.; Yang, G.Y.; Liu, X.; Xia, B.; Hu, X.; Su, J.H.; Wang, J.F. Lactobacillus rhamnosus GG affects microbiota and suppresses autophagy in the intestines of pigs challenged with salmonella infantis. Front. Microbiol. 2017, 8, 2705. [Google Scholar] [CrossRef] [Green Version]
- Diao, H.; Jiao, A.R.; Yu, B.; Mao, X.B.; Chen, D.W. Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets. Genes Nutr. 2019, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Sotira, S.; Dell’Anno, M.; Caprarulo, V.; Hejna, M.; Pirrone, F.; Callegari, M.L.; Tucci, T.V.; Rossi, L. Effects of tributyrin supplementation on growth performance, insulin, blood metabolites and gut microbiota in weaned piglets. Animals 2020, 10, 726. [Google Scholar] [CrossRef]
- Cresci, G.; Nagy, L.E.; Ganapathy, V. Lactobacillus GG and tributyrin supplementation reduce antibiotic-induced intestinal injury. J. Parenter. Enter. Nutr. 2013, 37, 763–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control. 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Michiels, J.; Missotten, J.; Dierick, N.; Fremaut, D.; Maene, P.; Smet, S.D. In vitro degradation and in vivo passage kinetics of carvacrol, thymol, eugenol and trans-cinnamaldehyde along the gastrointestinal tract of piglets. J. Sci. Food Agric. 2010, 88, 2371–2381. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, Y.F.; Zou, Y.; Hu, X.M.; Zheng, L.F.; Wei, H.K.; Giannenas, I.; Jin, L.Z.; Peng, J.; Jiang, S.W. Effects of dietary oregano essential oil supplementation on the stress response, antioxidative capacity, and HSPs mRNA expression of transported pigs. Livest. Sci. 2015, 180, 143–149. [Google Scholar] [CrossRef]
- Wilson, C.L.; Franklin, J.D.; Otto, B.E. Fruit volatiles inhibitory to monilinia-fructicola and botrytis-cinerea. Plant Disease. 1987, 71, 316–319. [Google Scholar] [CrossRef]
- Zhang, W.X.; Zhang, Y.; Zhang, X.W.; Deng, Z.X.; Liu, J.X.; He, M.L.; Wang, H.F. Effects of dietary supplementation with combination of tributyrin and essential oil on gut health and microbiota of weaned piglets. Animals 2020, 10, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabuurs, M.J.A.; Hoogendoorn, A.; Vandermolen, E.J.; Vanosta, A.L.M. Villus height and crypt depth in weaned and unweaned pigs, reared under various circumstances in the Netherlands. Res. Vet. Sci. 1993, 55, 78–84. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, M.; Yang, Y.; Mu, C.; Su, Y.; Zhu, W. Differential effect of early antibiotic intervention on bacterial fermentation patterns and mucosal gene expression in the colon of pigs under diets with different protein levels. Appl. Microbiol. Biotechnol. 2017, 101, 2493–2505. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, 141–145. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal.Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef] [Green Version]
- Omonijo, F.A.; Ni, L.; Gong, J.; Wang, Q.; Lahaye, L.; Yang, C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018, 4, 126–136. [Google Scholar] [CrossRef]
- Wang, C.C.; Shen, Z.J.; Cao, S.T.; Zhang, Q.H.; Peng, Y.; Hong, Q.H.; Feng, J.; Hu, C.H. Effects of tributyrin on growth performance, intestinal microflora and barrier function of weaned pigs. Anim. Feed. Sci. Technol. 2019, 258, 114311. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, T.B.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Munro, I.C.; Portoghese, P.S.; Smith, R.L.; Waddell, W.J.; et al. The FEMA GRAS assessment of hydroxy- and alkoxy-substituted benzyl derivatives used as flavor ingredients. Food Chem. Toxicol. 2005, 43, 1241–1271. [Google Scholar] [CrossRef] [PubMed]
- Rattaz, C.; Goubet, N.; Bullinger, A. The calming effect of a familiar odor on full-term newborns. J. Dev. Behav. Pediatr. 2005, 26, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Stelter, K.; Frahm, J.; Paulsen, J.; Berk, A.; Kleinwachter, M.; Selmar, D.; Danicke, S. Effects of oregano on performance and immunmodulating factors in weaned piglets. Arch. Anim. Nutr. 2013, 67, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Giannenas, I.; Florou-Paneri, P.; Papazahariadou, M.; Christaki, E.; Botsoglou, N.A.; Spais, A.B. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Arch. Tierernahr. 2003, 57, 99–106. [Google Scholar] [PubMed]
- Fuhrman, M.P.; Charney, P.; Mueller, C.M. Hepatic proteins and nutrition assessment. J. Am. Diet. Assoc. 2004, 104, 1258–1264. [Google Scholar] [CrossRef]
- Konstantinos, G.; Dimitrios, S.; Konstantinos, P.; Stylianos, K.; Dimitrios, K.; Panagiotis, G.; Dimitrios, K. Feed supplemented with polyphenolic by product from olive mill wastewater processing improves the redox status in blood and tissues of piglets. Food Chem. Toxicol. 2015, 86, 319–327. [Google Scholar]
- Ji, F.J.; Wang, L.X.; Yang, H.S.; Hu, A.; Yin, Y.L. Review: The roles and functions of glutamine on intestinal health and performance of weaning pigs. Animal. 2019, 13, 2727–2735. [Google Scholar] [CrossRef] [Green Version]
- Kotunia, A.; Wolinski, J.; Laubitz, D.; Jurkowska, M.; Rome, V.; Guilloteau, P.; Zabielski, R. Effect of sodium butyrate on the small intestine development in neonatal piglets fed [correction of feed] by artificial sow. J. Physiol. Pharmacol. 2004, 55 Suppl 2, 59–68. [Google Scholar]
- Droy-Lefaix, M.T.; Drouet, Y.; Geraud, G.; Hosford, D.; Braquet, P. Superoxide dismutase (SOD) and the paf-Antagonist (BN 52021) reduce small intestinal damage induced by ischemia-reperfusion. Taylor & Francis 2009, 13, 725–735. [Google Scholar]
- Omonijo, F.A.; Liu, S.; Hui, Q.; Zhang, H.; Lahaye, L.; Bodin, J.-C.; Gong, J.; Nyachoti, M.; Yang, C. Thymol improves barrier function and attenuates inflammatory responses in porcine intestinal epithelial cells during lipopolysaccharide (LPS)-induced inflammation. J. Agric. Food Chem. 2018, 67, 615–624. [Google Scholar] [CrossRef]
- Kang, S.N.; Lee, E.; Lee, M.K.; Lim, S.J. Preparation and evaluation of tributyrin emulsion as a potent anti-cancer agent against melanoma. Drug Deliv. 2011, 18, 143–149. [Google Scholar] [CrossRef]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, M.D.; Gordon, D.T.; Pace, L.W.; Garleb, K.A.; Kerley, M.S. Effects of dietary supplementation with fructooligosaccharides on colonic microbiota populations and epithelial cell proliferation in neonatal pigs. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.T.; Liu, L.; Long, S.F.; Pan, L.; Piao, X.S. Effect of organic acids and essential oils on performance, intestinal health and digestive enzyme activities of weaned pigs. Anim. Feed. Sci. Technol. 2018, 235, 110–119. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Yun, X.; Kelsy, R.; Junjun, W.; Guolong, Z.; Jiangchao, Z.; Yingping, X. Gut microbiota is a major contributor to adiposity in pigs. Front. Microbiol. 2018, 9, 3045. [Google Scholar]
- Yang, C.; Zhang, L.; Cao, G.; Feng, J.; Yue, M.; Xu, Y.; Dai, B.; Han, Q.; Guo, X. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J. Anim. Sci. 2018, 97, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.B.; Chowdhury, M.A.K.; Hou, Y.Q.; Gong, J. Phytogenic compounds as alternatives to in-feed antibiotics: Potentials and challenges in application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.S.; Wu, C.M.; Wang, Y.; Jeon, B.; Shen, Z.Q.; Wang, Y.; Zhang, Q.; Shen, J.Z. Antimicrobial resistance in Campylobacter coli isolated from pigs in two provinces of China. Int. J. Food Microbiol. 2011, 146, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Schautteet, K.; Vanrompay, D. Chlamydiaceae infections in pig. Vet. Res. 2011, 42, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchandin, H.; Jumas-Bilak, E. The Family Veillonellaceae. In The Prokaryotes; Rosenberg, E., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg Germany, 2014; pp. 433–453. [Google Scholar]
- McCormack, U.M.; Curiao, T.; Buzoianu, S.G.; Prieto, M.L.; Ryan, T.; Varley, P.; Crispie, F.; Magowan, E.; Metzler-Zebeli, B.U.; Berry, D.; et al. Exploring a possible link between the intestinal microbiota and feed efficiency in pigs. Appl. Environ. Microbiol. 2017, 83, e00380-00317. [Google Scholar] [CrossRef] [Green Version]
- Aar, P.; Molist, F.; Klis, J. The central role of intestinal health on the effect of feed additives on feed intake in swine and poultry. Anim. Feed Sci. Technol. 2017, 233, 64–75. [Google Scholar]
- Fiorucci, S.; Distrutti, E. Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends Mol. Med. 2015, 21, 702–714. [Google Scholar] [CrossRef]
- Pina, M.M.; Giuseppina, M.R.; Gina, C.; Giovanna, T.; Chiara, D.F.; Serena, A.; Marina, P.; Claudio, P.; Francesca, D.G.; Adriano, L.; et al. Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes 2017, 66, 1405–1418. [Google Scholar]
- Zhou, L.; Zheng, H.; Tang, Y.; Yu, W.; Gong, Q. Eugenol inhibits quorum sensing at sub-inhibitory concentrations. Biotechnol. Lett. 2012, 35, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Geng, M.M.; Li, T.J.; Kong, X.F.; Song, X.Y.; Chu, W.Y.; Huang, R.L.; Yin, Y.L.; Wu, G.Y. Reduced expression of intestinal N-acetylglutamate synthase in suckling piglets: A novel molecular mechanism for arginine as a nutritionally essential amino acid for neonates. Amino Acids. 2011, 40, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, T.; Hebbel, R.P.; Kaul, D.K. Protective effect of arginine on oxidative stress in transgenic sickle mouse models. Free Radic. Biol. Med. 2006, 41, 1771–1780. [Google Scholar] [CrossRef] [Green Version]


| Items, g/kg | |
|---|---|
| Ingredients | |
| Corn | 597 |
| Soybean meal | 105 |
| Fermented soybean meal | 55 |
| Extruded soybean | 100 |
| Fish meal | 40 |
| Whey powder | 30 |
| Glucose | 20 |
| Wheat flour | 4.4 |
| Soybean oil | 10 |
| L-Lysine hydrochloride | 3 |
| L-Threonine | 1.5 |
| DL-Methionine | 0.6 |
| Choline chloride | 1 |
| Sodium chloride | 3.5 |
| Calcium hydrophosphate | 9 |
| Limestone | 10 |
| Vitamin-mineral premix 1 | 10 |
| Nutrition composition | |
| Digestible energy †, MJ/kg (calculated) | 143.96 |
| Crude protein | 190.05 |
| Calcium | 10.15 |
| Lysine | 13.37 |
| Methionine | 4.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Mao, J.; Liu, J.; Zhang, Y.; Deng, Z.; Lv, J.; He, M.; Liu, J.; Wang, H. Encapsulated Mixture of Methyl Salicylate and Tributyrin Modulates Intestinal Microbiota and Improves Growth Performance of Weaned Piglets. Microorganisms 2021, 9, 1342. https://doi.org/10.3390/microorganisms9061342
Wei Y, Mao J, Liu J, Zhang Y, Deng Z, Lv J, He M, Liu J, Wang H. Encapsulated Mixture of Methyl Salicylate and Tributyrin Modulates Intestinal Microbiota and Improves Growth Performance of Weaned Piglets. Microorganisms. 2021; 9(6):1342. https://doi.org/10.3390/microorganisms9061342
Chicago/Turabian StyleWei, Yusen, Jiangdi Mao, Jingliang Liu, Yu Zhang, Zhaoxi Deng, Jiaqi Lv, Maolong He, Jianxin Liu, and Haifeng Wang. 2021. "Encapsulated Mixture of Methyl Salicylate and Tributyrin Modulates Intestinal Microbiota and Improves Growth Performance of Weaned Piglets" Microorganisms 9, no. 6: 1342. https://doi.org/10.3390/microorganisms9061342
APA StyleWei, Y., Mao, J., Liu, J., Zhang, Y., Deng, Z., Lv, J., He, M., Liu, J., & Wang, H. (2021). Encapsulated Mixture of Methyl Salicylate and Tributyrin Modulates Intestinal Microbiota and Improves Growth Performance of Weaned Piglets. Microorganisms, 9(6), 1342. https://doi.org/10.3390/microorganisms9061342







