What Is in a Cat Scratch? Growth of Bartonella henselae in a Biofilm
Abstract
:1. Introduction
2. Clinical Importance
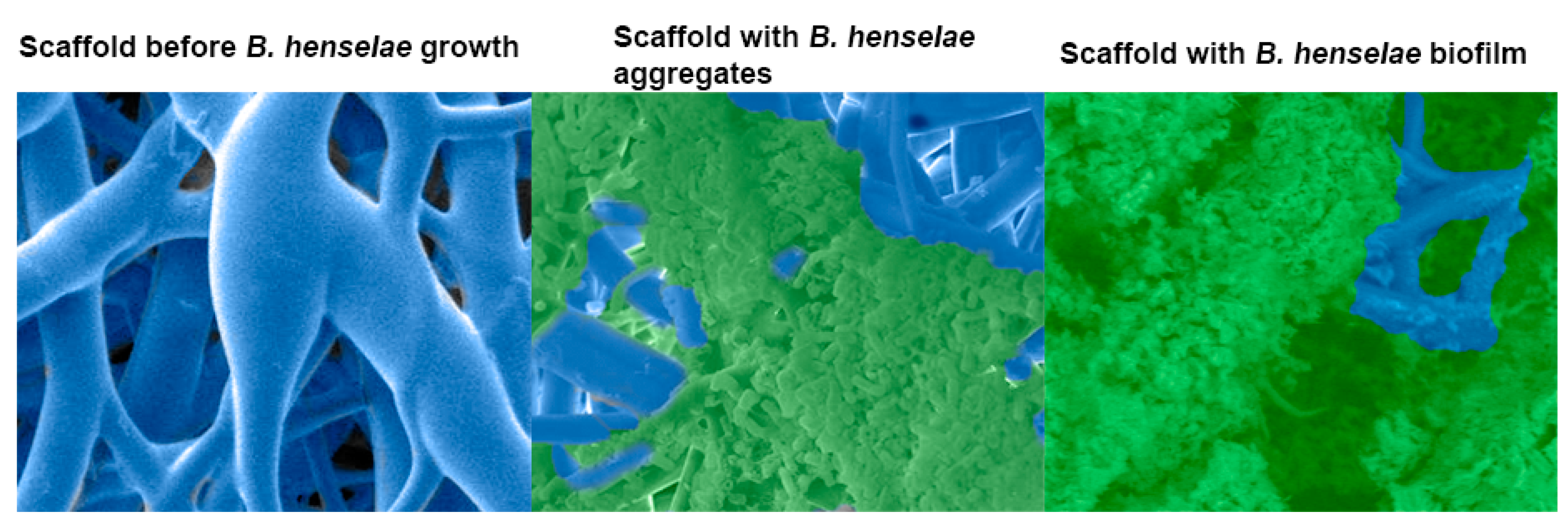
3. Biofilm Formation, Composition, and Life Cycle
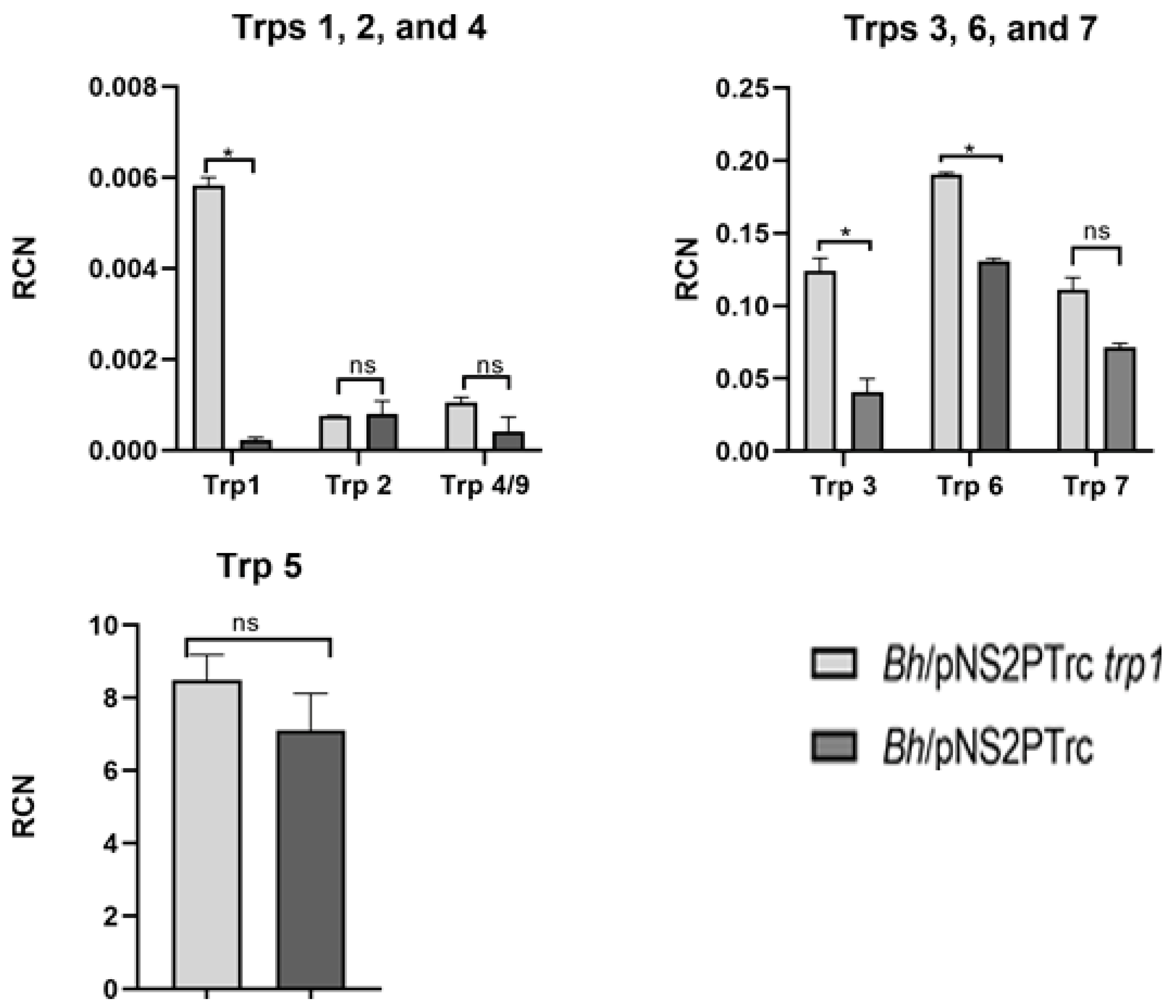
4. Genes Involved in Biofilm Regulation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Costerton, J.W.; Geesey, G.G.; Cheng, K.-J. How Bacteria Stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Lins, K.D.A.; Drummond, M.R.; Velho, P.E.N.F. Cutaneous manifestations of bartonellosis. An. Bras. de Dermatol. 2019, 94, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J. Bartonella quintana, past, present, and future of the scourge of World War, I. Acta Pathol. Microbiol. Immunol. Scand. 2018, 126, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Ruiz, J. Carrion’s disease: The sound of silence. Clin. Microbiol. Rev. 2017, 31, e00056-17. [Google Scholar] [CrossRef] [Green Version]
- Nelson, C.A.; Saha, S.; Mead, P.S. Cat-Scratch Disease in the United States, 2005–2013. Emerg. Infect. Dis. 2016, 22, 1741–1746. [Google Scholar] [CrossRef]
- Fournier, P.-E.; Robson, J.; Zeaiter, Z.; McDougall, R. Improved culture from lymph nodes of patients with cat scratch disease and genotypic characterization of Bartonella henselae isolates in Australia. J. Clin. Microbiol. 2002, 40, 3620–3624. [Google Scholar] [CrossRef] [Green Version]
- Kelly, P.J.; Meads, N.; Theobald, A.; Fournier, P.-E.; Raoult, D. Rickettsia felis, Bartonella henselae, and B. clarridgeiae, New Zealand. Emerg. Infect. Dis. 2004, 10, 967–968. [Google Scholar] [CrossRef]
- Maruyama, S.; Izumikawa, K.; Miyashita, M.; Kabeya, H.; Mikami, T.; Yamanouchi, H.; Sasaki, E.; Yoshida, H.; Izumikawa, K. First isolation of Bartonella henselae type I from a cat-scratch disease patient in Japan and its molecular analysis. Microbiol. Immunol. 2004, 48, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Valentine, K.H.; Harms, C.A.; Cadenas, M.B.; Birkenheuer, A.J.; Marr, H.S.; Braun-McNeill, J.; Maggi, R.G.; Breitschwerdt, E.B. Bartonella DNA in loggerhead sea turtles. Emerg. Infect. Dis. 2007, 13, 949–950. [Google Scholar] [CrossRef] [PubMed]
- Mascarelli, P.E.; Elmore, S.A.; Jenkins, E.J.; Alisauskas, R.T.; Walsh, M.; Breitschwerdt, E.B.; Maggi, R.G. Vector-borne pathogens in arctic foxes, Vulpes lagopus, from Canada. Res. Vet. Sci. 2015, 99, 58–59. [Google Scholar] [CrossRef] [PubMed]
- Mascarelli, P.E.; McQuillan, M.; Harms, C.A.; Harms, R.V.; Breitschwerdt, E.B. Bartonella henselae and B. koehlerae DNA in birds. Emerg. Infect. Dis. 2014, 20, 490–492. [Google Scholar] [PubMed]
- Okaro, U.; Addisu, A.; Casanas, B.; Anderson, B. Bartonella species, an emerging cause of blood-culture-negative endocarditis. Clin. Microbiol. Rev. 2017, 30, 709–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stützer, B.; Hartmann, K. Chronic Bartonellosis in cats: What are the potential implications? J. Feline Med. Surg. 2012, 14, 612–621. [Google Scholar] [CrossRef]
- Guru, P.K.; Agarwal, A.; Fritz, A. A miraculous recovery: Bartonella henselae infection following a red ant bite. BMJ Case Rep. 2018, 2018, bcr2017222326. [Google Scholar] [CrossRef]
- Rust, M.K. The Biology and ecology of cat fleas and advancements in their pest management: A review. Insects 2017, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Okaro, U.; Green, R.; Mohapatra, S.; Anderson, B. The trimeric autotransporter adhesin BadA is required for in vitro biofilm formation by Bartonella henselae. npj Biofilms Microbiomes 2019, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Riess, T.; Andersson, S.G.; Lupas, A.; Schaller, M.; Schäfer, A.; Kyme, P.; Martin, J.; Wälzlein, J.-H.; Ehehalt, U.; Lindroos, H.; et al. Bartonella Adhesin A mediates a proangiogenic host cell response. J. Exp. Med. 2004, 200, 1267–1278. [Google Scholar] [CrossRef]
- Szczesny, P.; Linke, D.; Ursinus, A.; Bär, K.; Schwarz, H.; Riess, T.M.; Kempf, V.A.J.; Lupas, A.N.; Martin, J.; Zeth, K. Structure of the head of the Bartonella Adhesin BadA. PLoS Pathog. 2008, 4, e1000119. [Google Scholar] [CrossRef]
- Okaro, U.; George, S.; Valdes, S.; Macaluso, K.; Anderson, B. A non-coding RNA controls transcription of a gene encoding a DNA binding protein that modulates biofilm development in Bartonella henselae. Microb. Pathog. 2020, 147, 104272. [Google Scholar] [CrossRef]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. Acta Pathol. Microbiol. Immunol. Scand. 2013, 121, 1–58. [Google Scholar] [CrossRef]
- Florin, T.A.; Zaoutis, T.E.; Zaoutis, L.B. Beyond cat scratch disease: Widening spectrum of Bartonella henselae infection. Pediatrics 2008, 121, e1413–e1425. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Ma, X.; Li, T.; Shi, W.; Zhang, Y. Effect of different drugs and drug combinations on killing stationary phase and biofilms recovered cells of Bartonella henselae in vitro. BMC Microbiol. 2020, 20, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Raoult, D. Pathogenicity and treatment of Bartonella infections. Int. J. Antimicrob. Agents 2014, 44, 16–25. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karem, K.L.; Paddock, C.D.; Regnery, R.L. Bartonella henselae, B. quintana, and B. bacilliformis: Historical pathogens of emerging significance. Microbes Infect. 2000, 2, 1193–1205. [Google Scholar] [CrossRef]
- Resto-Ruiz, S.; Burgess, A.; Anderson, B.E. The role of the host immune response in pathogenesis of Bartonella henselae. DNA Cell Biol. 2003, 22, 431–440. [Google Scholar] [CrossRef]
- Jackson, L.A.; Perkins, B.A.; Wenger, J.D. Cat scratch disease in the United States: An analysis of three national databases. Am. J. Public Health 1993, 83, 1707–1711. [Google Scholar] [CrossRef] [Green Version]
- Debre, R. Cat scratch disease. Mars. Med. 1950, 87, 375–378. [Google Scholar]
- Klotz, S.A.; Ianas, V.; Elliott, S.P. Cat-scratch disease. Am. Fam. Physician 2011, 83, 152–155. [Google Scholar]
- Jacomo, V.; Kelly, P.J.; Raoult, D. Natural history of Bartonella infections (an exception to Koch’s postulate). Clin. Diagn. Lab. Immunol. 2002, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Stoler, M.H.; Bonfiglio, T.A.; Steigbigel, R.T.; Pereira, M. An atypical subcutaneous infection associated with acquired immune deficiency syndrome. Am. J. Clin. Pathol. 1983, 80, 714–718. [Google Scholar] [CrossRef]
- Chomel, B.B.; Kasten, R.W.; Sykes, J.E.; Boulouis, H.-J.; Breitschwerdt, E.B. Clinical impact of persistent Bartonella Bacteremia in humans and animals. Ann. N. Y. Acad. Sci. 2003, 990, 267–278. [Google Scholar] [CrossRef]
- Perkocha, L.A.; Geaghan, S.M.; Yen, T.B.; Nishimura, S.L.; Chan, S.P.; Garcia-Kennedy, R.; Honda, G.M.D.; Stoloff, A.C.M.D.; Klein, M.D.H.Z.; Goldman, M.D.R.L.; et al. Clinical and pathological features of bacillary peliosis hepatis in association with human immunodeficiency virus infection. N. Engl. J. Med. 1990, 323, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, T.; Warren, R.; Kass, M.; Brun, E.; Levy, C. Endocarditis caused by Rochalimaea henselae. Hum. Pathol. 1993, 24, 1140–1141. [Google Scholar] [CrossRef] [Green Version]
- Holmes, A.H.; Greenough, T.C.; Balady, G.J.; Regnery, R.L.; Anderson, B.E.; O’Keane, J.C.; Fonger, J.D.; McCrone, E.L. Bartonella henselae Endocarditis in an Immunocompetent Adult. Clin. Infect. Dis. 1995, 21, 1004–1007. [Google Scholar] [CrossRef]
- Chung, C.Y.; Kasten, R.W.; Paff, S.M.; Van Horn, B.A.; Vayssier-Taussat, M.; Boulouis, H.-J.; Chomel, B.B. Bartonella spp. DNA associated with biting flies from California. Emerg. Infect. Dis. 2004, 10, 1311–1313. [Google Scholar]
- Sanogo, Y.O.; Zeaiter, Z.; Caruso, G.; Merola, F.; Shpynov, S.; Brouqui, P.; Raoult, D. Bartonella henselae in Ixodes ricinus ticks (Acari: Ixodida) removed from humans, Belluno province, Italy. Emerg. Infect. Dis. 2003, 9, 329–332. [Google Scholar] [CrossRef]
- Chomel, B.B.; Kasten, R.W.; Floyd-Hawkins, K.; Chi, B.; Yamamoto, K.; Roberts-Wilson, J.; Koehler, J.E.; Pedersen, N.C.; Abbott, R.C. Experimental transmission of Bartonella henselae by the cat flea. J. Clin. Microbiol. 1996, 34, 1952–1956. [Google Scholar] [CrossRef] [Green Version]
- Zając, V.; Wójcik-Fatla, A.; Dutkiewicz, J.; Szymańska, J. Bartonella henselae in eastern Poland: The relationship between tick infection rates and the serological response of individuals occupationally exposed to tick bites. J. Vector Ecol. 2015, 40, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Cotté, V.; Bonnet, S.; Le Rhun, D.; Le Naour, E.; Chauvin, A.; Boulouis, H.J.; Lecuelle, B.; Lilin, T.; Vayssier-Taussat, M. Transmission of Bartonella henselae by Ixodes ricinus. Emerg. Infect. Dis. 2008, 14, 1074. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Billeter, S.A.; Breitschwerdt, E.B.; Chomel, B.B.; Raoult, D. Potential for Tick-borne Bartonelloses. Emerg. Infect. Dis. 2010, 16, 385–391. [Google Scholar] [CrossRef]
- McElroy, K.M.; Blagburn, B.L.; Breitschwerdt, E.B.; Mead, P.S.; McQuiston, J.H. Flea-associated zoonotic diseases of cats in the USA: Bartonellosis, flea-borne rickettsioses, and plague. Trends Parasitol. 2010, 26, 197–204. [Google Scholar] [CrossRef]
- Hutchinson, M.J.; Jacobs, D.E.; Mencke, N. Establishment of the cat flea (Ctenocephalides felis felis) on the ferret (Mustela putorius furo) and its control with imidacloprid. Med. Vet. Èntomol. 2001, 15, 212–214. [Google Scholar] [CrossRef]
- Araújo, F.R.; Silva, M.P.A.; Lopes, A.; Ribeiro, O.C.; Pires, P.P.; Carvalho, C.; Balbuena, C.B.A.; Villas, A.; Ramos, J. Severe cat flea infestation of dairy calves in Brazil. Vet. Parasitol. 1998, 80, 83–86. [Google Scholar] [CrossRef]
- Koehler, J.E.; Glaser, C.A.; Tappero, J.W. Rochalimaea henselae infection. A new zoonosis with the domestic cat as reservoir. J. Am. Med. Assoc. 1994, 271, 531–535. [Google Scholar] [CrossRef]
- Guptill, L.; Slater, L.; Wu, C.; Lin, T.; Glickman, L.T.; Welch, D.F.; HogenEsch, H. Experimental infection of young specific pathogen-free cats with Bartonella henselae. J. Infect. Dis. 1997, 176, 206–216. [Google Scholar] [CrossRef] [Green Version]
- O’Reilly, K.L.; Bauer, R.W.; Freeland, R.L.; Foil, L.D.; Hughes, K.J.; Rohde, K.R.; Roy, A.F.; Stout, R.W.; Triche, P.C. Acute clinical disease in cats following infection with a pathogenic strain of Bartonella henselae (LSU16). Infect. Immun. 1999, 67, 3066–3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouhsira, E.; Franc, M.; Boulouis, H.-J.; Jacquiet, P.; Raymond-Letron, I.; Liénard, E. Assessment of persistence of Bartonella henselae in Ctenocephalides felis. Appl. Environ. Microbiol. 2013, 79, 7439–7444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edouard, S.; Nabet, C.; Lepidi, H.; Fournier, P.E.; Raoult, D. Bartonella, a common cause of endocarditis: A report on 106 cases and review. J. Clin. Microbiol. 2015, 53, 824–829. [Google Scholar] [CrossRef] [Green Version]
- Chiaraviglio, L.; Duong, S.; Brown, D.A.; Birtles, R.J.; Kirby, J.E. An immunocompromised murine model of chronic Bartonella infection. Am. J. Pathol. 2010, 176, 2753–2763. [Google Scholar] [CrossRef] [PubMed]
- Spach, D.H.; Callis, K.P.; Paauw, D.S.; Houze, Y.B.; Schoenknecht, F.D.; Welch, D.F.; Rosen, H.; Brenner, D.J. Endocarditis caused by Rochalimaea quintana in a patient infected with human immunodeficiency virus. J. Clin. Microbiol. 1993, 31, 692–694. [Google Scholar] [CrossRef] [Green Version]
- Minnick, M.F.; Anderson, B.E. Bartonella. In Molecular Medical Microbiology, 2nd ed.; Tang, Y.-W., Sails, A., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 1911–1939. [Google Scholar]
- Koehler, J.E.; Quinn, F.D.; Berger, T.G.; LeBoit, P.E.; Tappero, J.W. Isolation of rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N. Engl. J. Med. 1992, 327, 1625–1631. [Google Scholar] [CrossRef]
- Houpikian, P.; Raoult, D. Blood culture-negative endocarditis in a reference center: Etiologic diagnosis of 348 cases. Medicine 2005, 84, 162–173. [Google Scholar] [CrossRef]
- Caponetti, G.C.; Pantanowitz, L.; Marconi, S.; Havens, J.M.; Lamps, L.W.; Otis, C.N. Evaluation of immunohistochemistry in identifying bartonella henselae in cat-scratch disease. Am. J. Clin. Pathol. 2009, 131, 250–256. [Google Scholar] [CrossRef]
- Relman, D.A.; Loutit, J.S.; Schmidt, T.M.; Falkow, S.; Tompkins, L.S. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N. Engl. J. Med. 1990, 323, 1573–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuneoka, H.; Yanagihara, M.; Nojima, J.; Ichihara, K. Antimicrobial susceptibility by Etest of Bartonella henselae isolated from cats and human in Japan. J. Infect. Chemother. 2010, 16, 446–448. [Google Scholar] [CrossRef]
- Biswas, S.; Maggi, R.G.; Papich, M.G.; Keil, D.; Breitschwerdt, E.B. Comparative activity of Pradofloxacin, Enrofloxacin, and Azithromycin against Bartonella henselae isolates collected from cats and a human. J. Clin. Microbiol. 2009, 48, 617–618. [Google Scholar] [CrossRef] [Green Version]
- Rolain, J.M.; Brouqui, P.; Koehler, J.E.; Maguina, C.; Dolan, M.J.; Raoult, D. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob. Agents Chemother. 2004, 48, 1921–1933. [Google Scholar] [CrossRef] [Green Version]
- Myers, W.F.; Grossman, D.M.; Wisseman, C.L. Antibiotic susceptibility patterns in Rochalimaea quintana, the agent of trench fever. Antimicrob. Agents Chemother. 1984, 25, 690–693. [Google Scholar] [CrossRef] [Green Version]
- Ives, T.J.; Manzewitsch, P.; Regnery, R.L.; Butts, J.D.; Kebede, M. In vitro susceptibilities of Bartonella henselae, B. quintana, B. elizabethae, Rickettsia rickettsii, R. conorii, R. akari, and R. prowazekii to macrolide antibiotics as determined by immunofluorescent-antibody analysis of infected Vero cell monolayers. Antimicrob. Agents Chemother. 1997, 41, 578–582. [Google Scholar] [CrossRef] [Green Version]
- Raoult, D.; Fournier, P.-E.; Vandenesch, F.; Mainardi, J.-L.; Eykyn, S.J.; Nash, J.; James, E.; Benoit-Lemercier, C.; Marrie, T.J. Outcome and treatment of Bartonella Endocarditis. Arch. Intern. Med. 2003, 163, 226–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, T.S.J.; Foweraker, J.; Gould, F.K.; Perry, J.D.; Sandoe, J.A.T. Guidelines for the antibiotic treatment of endocarditis in adults: Report of the Working Party of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 2004, 54, 971–981. [Google Scholar] [CrossRef]
- Barka, N.E.; Hadfield, T.; Patnaik, M.; Schwartzman, W.A.; Peter, J.B. EIA for detection of Rochalimaea henselae-Reactive IgG, IgM, and IgA antibodies in patients with suspected cat-scratch disease. J. Infect. Dis. 1993, 167, 1503–1504. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Chabane, Y.N.; Marti, S.; Rihouey, C.; Alexandre, S.; Hardouin, J.; Lesouhaitier, O.; Vila, J.; Kaplan, J.B.; Jouenne, T.; Dé, E. Characterisation of pellicles formed by acinetobacter baumannii at the air-liquid interface. PLoS ONE 2014, 9, e111660. [Google Scholar] [CrossRef]
- Paytubi, S.; Cansado, C.; Madrid, C.; Balsalobre, C. Nutrient composition promotes switching between pellicle and bottom biofilm in salmonella. Front. Microbiol. 2017, 8, 2160. [Google Scholar] [CrossRef]
- Barsoumian, A.E.; Mende, K.; Sanchez, C.J., Jr.; Beckius, M.L.; Wenke, J.C.; Murray, C.K.; Akers, K.S. Clinical infectious outcomes associated with biofilm-related bacterial infections: A retrospective chart review. BMC Infect. Dis. 2015, 15, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berne, C.; Ducret, A.; Hardy, G.G.; Brun, Y.V. Adhesins involved in attachment to abiotic surfaces by gram-negative bacteria. Microbiol. Spectr. 2015, 3, 101–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunne, W.M. Bacterial adhesion: Seen any good biofilms lately? Clin. Microbiol. Rev. 2002, 15, 155–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyme, P.; Dillon, B.; Iredell, J. Phase variation in Bartonella henselae. Microbiology 2003, 149, 621–629. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, S.M.; Michel, F.; Hogan, R.J.; Lafontaine, E.R. The autotransporter BpaB contributes to the virulence of burkholderia mallei in an aerosol model of infection. PLoS ONE 2015, 10, e0126437. [Google Scholar] [CrossRef] [Green Version]
- Totsika, M.; Wells, T.J.; Beloin, C.; Valle, J.; Allsopp, L.P.; King, N.P.; Ghigo, J.-M.; Schembri, M.A. Molecular characterization of the EhaG and UpaG trimeric autotransporter proteins from pathogenic escherichia coli. Appl. Environ. Microbiol. 2012, 78, 2179–2189. [Google Scholar] [CrossRef] [Green Version]
- Bentancor, L.V.; Camacho-Peiro, A.; Bozkurt-Guzel, C.; Pier, G.B.; Maira-Litrán, T. Identification of ata, a multifunctional trimeric autotransporter of acinetobacter baumannii. J. Bacteriol. 2012, 194, 3950–3960. [Google Scholar] [CrossRef] [Green Version]
- Tu, N.; Caroll, R.K.; Weiss, A.; Shaw, L.; Nicolas, G.; Thomas, S.; Lima, A.; Okaro, U.; Anderson, B. A family of genus-specific RNAs in tandem with DNA-binding proteins control expression of the badA major virulence factor gene in Bartonella henselae. Microbiologyopen 2016, 6, e00420. [Google Scholar] [CrossRef] [PubMed]
- Dehio, C. Bartonella–host-cell interactions and vascular tumour formation. Nat. Rev. Genet. 2005, 3, 621–631. [Google Scholar] [CrossRef]
- Salvatore, P. Detrimental effects of Bartonella henselae are counteracted by L-arginine and nitric oxide in human endothelial progenitor cells. Proc. Natl. Acad. Sci. USA 2008, 105, 9427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mändle, T.; Einsele, H.; Schaller, M.; Neumann, D.; Vogel, W.; Autenrieth, I.B.; Kempf, V.A.J. Infection of human CD34+ progenitor cells with Bartonella henselae results in intraerythrocytic presence of B henselae. Blood 2005, 106, 1215–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvatore, P.; Zullo, A.; Sommese, L.; Colicchio, R.; Picascia, A.; Schiano, C.; Mancini, F.P.; Napoli, C. Infections and cardiovascular disease: Is Bartonella henselae contributing to this matter? J. Med. Microbiol. 2015, 64, 799–809. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, J.L.; Brown, T.P.; O’reilly, K.L.; Wedincamp, J., Jr.; Foil, L.D. Studies on the growth of Bartonella henselae in the cat flea (Siphonaptera: Pulicidae). J. Med. Entomol. 2002, 39, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Fazli, M.; Almblad, H.; Rybtke, M.L.; Givskov, M.; Eberl, L.; Tolker-Nielsen, T. Regulation of biofilm formation in Pseudomonas and Burkholderia species. Environ. Microbiol. 2014, 16, 1961–1981. [Google Scholar] [CrossRef]
- Van Puyvelde, S.; Steenackers, H.P.; Vanderleyden, J. Small RNAs regulating biofilm formation and outer membrane homeostasis. RNA Biol. 2013, 10, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Bak, G.; Lee, J.; Suk, S.; Kim, D.; Lee, J.Y.; Kim, K.-S.; Choi, B.-S.; Lee, Y. Identification of novel sRNAs involved in biofilm formation, motility and fimbriae formation in Escherichia coli. Sci. Rep. 2015, 5, 15287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kievit, T.R.; Gillis, R.; Marx, S.; Brown, C.; Iglewski, B.H. Quorum-sensing genes in pseudomonas aeruginosa biofilms: Their role and expression patterns. Appl. Environ. Microbiol. 2001, 67, 1865–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuqua, C.; Greenberg, E.P. Self-perception in bacteria: Quorum sensing with acylated homoserine lactones. Curr. Opin. Microbiol. 1998, 1, 183–189. [Google Scholar] [CrossRef]
- Hammer, B.K.; Bassler, B.L. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 2003, 50, 101–104. [Google Scholar] [CrossRef]
- Sharma, C.; Heidrich, N. Small RNAs and virulence in bacterial pathogens. RNA Biol. 2012, 9, 361–363. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Lesnik, E.A.; Hall, T.A.; Sampath, R.; Griffey, R.H.; Ecker, D.J.; Blyn, L.B. A bioinformatics based approach to discover small RNA genes in the Escherichia coli genome. BioSystems 2002, 65, 157–177. [Google Scholar] [CrossRef]
- Wassarman, K.M. Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev. 2001, 15, 1637–1651. [Google Scholar] [CrossRef] [Green Version]
- Lenz, D.H.; Mok, K.C.; Lilley, B.N.; Kulkarni, R.V.; Wingreen, N.S.; Bassler, B.L. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in vibrio harveyi and vibrio cholerae. Cell 2004, 118, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, C.; Papenfort, K.; Hentrich, K.; Ahmad, I.; Le Guyon, S.; Reimann, R.; Römling, U.; Grantcharova, N. Hfq and Hfq-dependent small RNAs are major contributors to multicellular development in Salmonella enterica serovar Typhimurium. RNA Biol. 2012, 9, 489–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmqvist, E.; Reimegård, J.; Sterk, M.; Grantcharova, N.; Römling, U.; Wagner, E.G.H. Two antisense RNAs target the transcriptional regulator CsgD to inhibit curli synthesis. EMBO J. 2010, 29, 1840–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mika, F.; Busse, S.; Possling, A.; Berkholz, J.; Tschowri, N.; Sommerfeldt, N.; Pruteanu, M.; Hengge, R. Targeting of csgD by the small regulatory RNA RprA links stationary phase, biofilm formation and cell envelope stress in Escherichia coli. Mol. Microbiol. 2012, 84, 51–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, Ã.; Lvaro, D.; Quereda, J.J.; Pucciarelli, M.G.; Portillo, F.G.-D. Non-coding RNA regulation in pathogenic bacteria located inside eukaryotic cells. Front. Cell. Infect. Microbiol. 2014, 4, 162. [Google Scholar] [CrossRef] [PubMed]
- Wachter, S.; Hicks, L.D.; Raghavan, R.; Minnick, M.F. Novel small RNAs expressed by Bartonella bacilliformis under multiple conditions reveal potential mechanisms for persistence in the sand fly vector and human host. PLoS Negl. Trop. Dis. 2020, 14, e0008671. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, F.; Kumar, V.; Gao, Y.-G.; Zhang, L.-H. BswR controls bacterial motility and biofilm formation in Pseudomonas aeruginosa through modulation of the small RNA rsmZ. Nucleic Acids Res. 2014, 42, 4563–4576. [Google Scholar] [CrossRef]
- Artsimovitch, I. A processive riboantiterminator seeks a switch to make biofilms. Mol. Microbiol. 2010, 76, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Abduljalil, J.M. Bacterial riboswitches and RNA thermometers: Nature and contributions to pathogenesis. Non Coding RNA Res. 2018, 3, 54–63. [Google Scholar] [CrossRef]
- Hertig, M. Carrion’s disease. V. Studies on phlebotomus as the possible vector. Proc. Soc. Exp. Biol. Med. 1937, 37, 598–600. [Google Scholar] [CrossRef]
- de Silva, A.M.; Fikrig, E. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J. Clin. Investig. 1997, 99, 377–379. [Google Scholar] [CrossRef] [Green Version]
- Schotthoefer, A.M.; Bearden, S.W.; Holmes, J.L.; Vetter, S.M.; Montenieri, J.A.; Williams, S.K.; Graham, C.B.; Woods, E.M.; Eisen, R.J.; Gage, K.L. Effects of temperature on the transmission of Yersinia Pestis by the flea, Xenopsylla Cheopis, in the late phase period. Parasites Vectors 2011, 4, 191. [Google Scholar] [CrossRef] [Green Version]
- Silverman, J.; Rust, M.K. Some abiotic factors affecting the survival of the cat flea, Ctenocephalides felis (Siphonaptera: Pulicidae). Environ. Entomol. 1983, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, J.S.; Ward, R.E.; Lacroix, J.; Hébert, P.C.; Barnes, M.A.; Bohn, D.J.; Dirks, P.B.; Doucette, S.; Fergusson, D.; Gottesman, R.; et al. Hypothermia therapy after traumatic brain injury in children. N. Engl. J. Med. 2008, 358, 2447–2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, D.W.; Miller, W.H.; Griffin, C.E. Structure and function of the skin. In Muller & Kirk’s Small Animal Dermatology, 6th ed.; W.B. Saunders: Philadelphia, PA, USA, 2001; pp. 1–70. [Google Scholar]
- Jin, K.A. Acidosis. Compr. Pediatr. Hosp. Med. 2007, 125–132. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2018, 47, D427–D432. [Google Scholar] [CrossRef]
- Galperin, M.Y. Structural classification of bacterial response regulators: Diversity of output domains and domain combinations. J. Bacteriol. 2006, 188, 4169. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Rasko, D.A.; Lockatell, C.V.; Johnson, D.E.; Mobley, H.L. Repression of bacterial motility by a novel fimbrial gene product. EMBO J. 2001, 20, 4854–4862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riess, T.; Raddatz, G.; Linke, D.; Schäfer, A.; Kempf, V.A.J. Analysis of Bartonella adhesin a expression reveals differences between various B. henselae strains. Infect. Immun. 2006, 75, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, P.O.; Linke, D.; Schwarz, H.; Leo, J.C.; Kempf, V.A.J. Analysis of the BadA stalk from Bartonella henselae reveals domain-specific and domain-overlapping functions in the host cell infection process. Cell Microbiol. 2011, 14, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.C.; Schulein, R.; Dehio, M.; Denecker, G.; Carena, I.; Dehio, C. The VirB type IV secretion system of Bartonella henselae mediates invasion, proinflammatory activation and antiapoptotic protection of endothelial cells. Mol. Microbiol. 2004, 52, 81–92. [Google Scholar] [CrossRef]
- Alvarez-Martinez, C.E.; Christie, P.J. Biological diversity of prokaryotic type IV secretion systems. Microbiol. Mol. Biol. Rev. 2009, 73, 775. [Google Scholar] [CrossRef] [Green Version]
- Quebatte, M.; Dehio, M.; Tropel, D.; Basler, A.; Toller, I.; Raddatz, G.; Engel, P.; Huser, S.; Schein, H.; Lindroos, H.L.; et al. The BatR/BatS two-component regulatory system controls the adaptive response of Bartonella henselae during human endothelial cell infection. J. Bacteriol. 2010, 192, 3352–3367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, N.; Lima, A.; Bandeali, Z.; Anderson, B. Characterization of the general stress response in Bartonella henselae. Microb. Pathog. 2016, 92, 1–10. [Google Scholar] [CrossRef] [Green Version]
- O’Rourke, F.; Schmidgen, T.; Kaiser, P.O.; Linke, D.; Kempf, V.A. Adhesins of Bartonella spp. Adv. Exp. Med. Biol. 2011, 715, 51–70. [Google Scholar]
- Raghunathan, D.; Wells, T.J.; Morris, F.C.; Shaw, R.K.; Bobat, S.; Peters, S.E.; Paterson, G.K.; Jensen, K.T.; Leyton, D.L.; Blair, J.M.A.; et al. SadA, a trimeric autotransporter from salmonella enterica serovar typhimurium, can promote biofilm formation and provides limited protection against infection. Infect. Immun. 2011, 79, 4342–4352. [Google Scholar] [CrossRef] [Green Version]
- Fialho, A.; Mil-Homens, D. Trimeric autotransporter adhesins in members of the Burkholderia Cepacia complex: A multifunctional family of proteins implicated in virulence. Front. Cell. Infect. Microbiol. 2011, 13, 2235–2988. [Google Scholar] [CrossRef] [Green Version]
- Chomel, B.B.; Boulouis, H.J.; Breitschwerdt, E.B.; Kasten, R.W.; Vayssier-Taussat, M.; Birtles, R.J.; Dehio, C. Ecological fitness and strategies of adaptation of Bartonella species to their hosts and vectors. Vet. Res. 2009, 40, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foil, L.; Andress, E.; Freeland, R.L.; Roy, A.F.; Rutledge, R.; Triche, P.C.; O’Reilly, K.L. Experimental infection of domestic cats with Bartonella henselae by inoculation of Ctenocephalides felis (Siphonaptera: Pulicidae) feces. J. Med. Entomol. 1998, 35, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.M.; Franc, M.; Davoust, B.; Raoult, D. Molecular detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in cat fleas, France. Emerg. Infect. Dis. 2003, 9, 338–342. [Google Scholar] [CrossRef]
- Spach, D.H. Bacillary angiomatosis. Int. J. Dermatol. 1992, 31, 19–24. [Google Scholar] [CrossRef]
- Garcia, F.U.; Wojta, J.; Broadley, K.N.; Davidson, J.M.; Hoover, R.L. Bartonella bacilliformis stimulates endothelial cells in vitro and is angiogenic in vivo. Am. J. Pathol. 1990, 136, 1125–1135. [Google Scholar]
- Cockerell, C.J.; LeBoit, P.E. Bacillary angiomatosis: A newly characterized, pseudoneoplastic, infectious, cutaneous vascular disorder. J. Am. Acad. Dermatol. 1990, 22, 501–512. [Google Scholar] [CrossRef]
- Fournier, P.E.; Lelievre, H.; Eykyn, S.J.; Mainardi, J.L.; Marrie, T.J.; Bruneel, F.; Roure, C.; Nash, J.; Clave, D.; James, E.; et al. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: A study of 48 patients. Medicine 2001, 80, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.; Fournier, P.E.; Dauge, M.C.; Gallien, S.; Raoult, D.; Andremont, A.; Ruimy, R. Bartonella quintana coinfection in Staphylococcus aureus endocarditis: Usefulness of screening in high-risk patients? Clin. Infect. Dis. 2009, 48, 1332–1333. [Google Scholar] [CrossRef] [Green Version]
- Tattevin, P.; Watt, G.; Revest, M.; Arvieux, C.; Fournier, P.-E. Update on blood culture-negative endocarditis. Méd. Mal. Infect. 2015, 45, 1–8. [Google Scholar] [CrossRef]
- Schülein, R.; Seubert, A.; Gille, C.; Lanz, C.; Hansmann, Y.; Piémont, Y.; Dehio, C. Invasion and persistent intracellular colonization of erythrocytes. J. Exp. Med. 2001, 193, 1077–1086. [Google Scholar] [CrossRef] [Green Version]
- Harms, A.; Dehio, C. Intruders below the radar: Molecular pathogenesis of Bartonella spp. Clin. Microbiol. Rev. 2012, 25, 42–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battisti, J.M.; Sappington, K.N.; Smitherman, L.S.; Parrow, N.L.; Michael, F.M. Environmental signals generate a differential and coordinated expression of the heme receptor gene family of Bartonella quintana. Infect. Immun. 2006, 74, 3251–3261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Biville, F. Managing iron supply during the infection cycle of a flea borne pathogen, Bartonella henselae. Front. Cell. Infect. Microbiol. 2013, 3, 60. [Google Scholar] [CrossRef] [Green Version]
- Roden, J.A.; Wells, D.H.; Chomel, B.B.; Kasten, R.W.; Koehler, J.E. Hemin binding protein C is found in outer membrane vesicles and protects Bartonella henselae against toxic concentrations of hemin. Infect. Immun. 2012, 80, 929–942. [Google Scholar] [CrossRef] [Green Version]
- Dewitte, A.; Bouvenot, T.; Pierre, F.; Ricard, I.; Pradel, E.; Barois, N.; Hujeux, A.; Bontemps-Gallo, S.; Sebbane, F. A refined model of how Yersinia pestis produces a transmissible infection in its flea vector. PLoS Pathog. 2020, 16, e1008440. [Google Scholar] [CrossRef] [Green Version]
- Hinkle, N.C.; Koehler, P.G.; Kern, W.H.; Patterson, R.S. Hematophagous strategies of the cat flea (Siphonaptera: Pulicidae). Fla. Èntomol. 1991, 74, 377. [Google Scholar] [CrossRef]
- Waters, L.S.; Storz, G. Regulatory RNAs in bacteria. Cell 2009, 136, 615–628. [Google Scholar] [CrossRef] [Green Version]
- Raina, M.; King, A.; Bianco, C.; Vanderpool, C.K. Dual-Function RNAs. Regul. RNA Bact. Archaea 2019, 6, 471–485. [Google Scholar] [CrossRef]
- Caron, M.-P.; Bastet, L.; Lussier, A.; Simoneau-Roy, M.; Massé, E.; Lafontaine, D.A. Dual-acting riboswitch control of translation initiation and mRNA decay. Proc. Natl. Acad. Sci. USA 2012, 109, E3444–E3453. [Google Scholar] [CrossRef] [Green Version]
- Bobrovskyy, M.; Vanderpool, C.K. Diverse mechanisms of post-transcriptional repression by the small RNA regulator of glucose-phosphate stress. Mol. Microbiol. 2016, 99, 254–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novick, R.P.; Ross, H.F.; Projan, S.J.; Kornblum, J.; Kreiswirth, B.; Moghazeh, S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993, 12, 3967–3975. [Google Scholar] [CrossRef] [PubMed]
- Vuong, C.; Götz, F.; Otto, M. Construction and characterization of an agr deletion mutant of Staphylococcus epidermidis. Infect. Immun. 2000, 68, 1048–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitam, I.; Dittmar, K.; Parola, P.; Whiting, M.F.; Raoult, D. Fleas and flea-borne diseases. Int. J. Infect. Dis. 2010, 14, e667–e676. [Google Scholar] [CrossRef] [Green Version]
- Otranto, D.; Dantas-Torres, F. Canine and feline vector-borne diseases in Italy: Current situation and perspectives. Parasites Vectors 2010, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- W.H.O. Vector-Borne Diseases. 2 March 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 12 March 2021).
- Mayer, S.V.; Tesh, R.B.; Vasilakis, N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017, 166, 155–163. [Google Scholar] [CrossRef]
- McTavish, H.; LaQuier, F.; Arciero, D.; Logan, M.; Mundfrom, G.A.; Fuchs, J.; Hooper, A.B. Multiple copies of genes coding for electron transport proteins in the bacterium Nitrosomonas europaea. J. Bacteriol. 1993, 175, 2445–2447. [Google Scholar] [CrossRef] [Green Version]
- Klappenbach, J.A.; Dunbar, J.M.; Schmidt, T.M. rRNA operon copy number reflects ecological strategies of bacteria. Appl. Environ. Microbiol. 2000, 66, 1328–1333. [Google Scholar] [CrossRef] [Green Version]
- Sievers, S.; Lund, A.; Menendez-Gil, P.; Nielsen, A.; Storm Mollerup, M.; Lambert Nielsen, S.; Larsson, P.B.; Borch-Jensen, J.; Johansson, J.; Kallipolitis, B.H. The multicopy sRNA LhrC controls expression of the oligopeptide-binding protein OppA in Listeria monocytogenes. RNA Biol. 2015, 12, 985–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouikha, I.; Hinnebusch, B.J. Yersinia-flea interactions and the evolution of the arthropod-borne transmission route of plague. Curr. Opin. Microbiol. 2012, 15, 239–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jefferson, K.K. What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 2004, 236, 163–173. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okaro, U.; George, S.; Anderson, B. What Is in a Cat Scratch? Growth of Bartonella henselae in a Biofilm. Microorganisms 2021, 9, 835. https://doi.org/10.3390/microorganisms9040835
Okaro U, George S, Anderson B. What Is in a Cat Scratch? Growth of Bartonella henselae in a Biofilm. Microorganisms. 2021; 9(4):835. https://doi.org/10.3390/microorganisms9040835
Chicago/Turabian StyleOkaro, Udoka, Sierra George, and Burt Anderson. 2021. "What Is in a Cat Scratch? Growth of Bartonella henselae in a Biofilm" Microorganisms 9, no. 4: 835. https://doi.org/10.3390/microorganisms9040835





