The Modulation of NADPH Oxidase Activity in Human Neutrophils by Moroccan Strains of Leishmania major and Leishmania tropica Is Not Associated with p47phox Phosphorylation
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethics Statements
2.2. Leishmania Strains
2.3. Soluble Leishmania Promastigote Antigens (SLAs) Preparation
2.4. Human PMN Isolation
2.5. In Vitro Infection of Human PMNs by L. major and L. tropica Promastigote Strains
2.6. Superoxide Anion Production Assay
2.7. Neutrophils Viability
2.8. Superoxide Anion Scavenging Assay
2.9. SDS–PAGE and Western Blotting for p47phox Phosphorylation Analysis
2.10. Statistical Analysis
3. Results
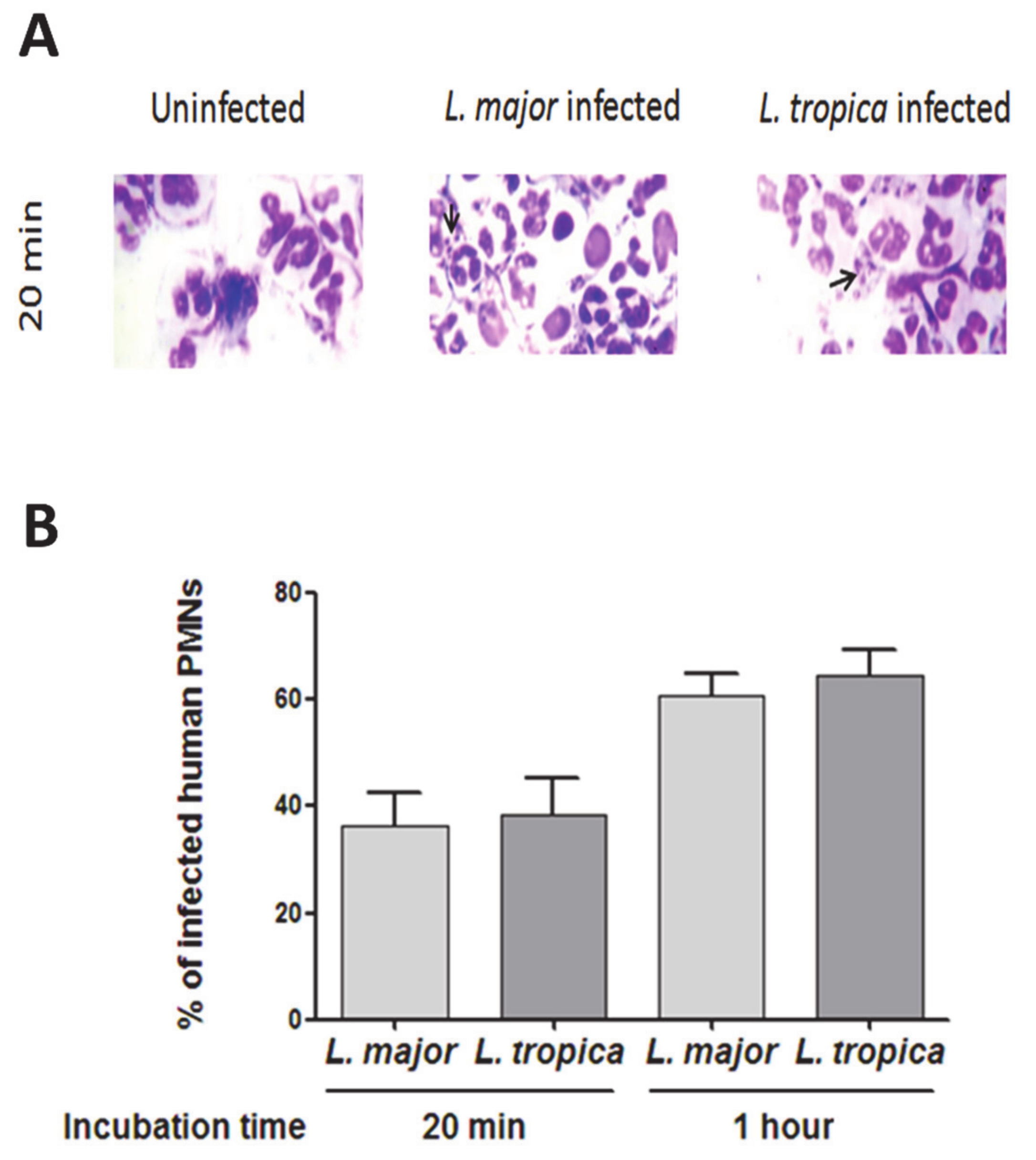
3.1. Infection of Human PMNs by L. major and L. tropica Primary Strains
3.2. Impact of L. major and L. tropica Promastigote Strains on O2− Production by Human PMNs
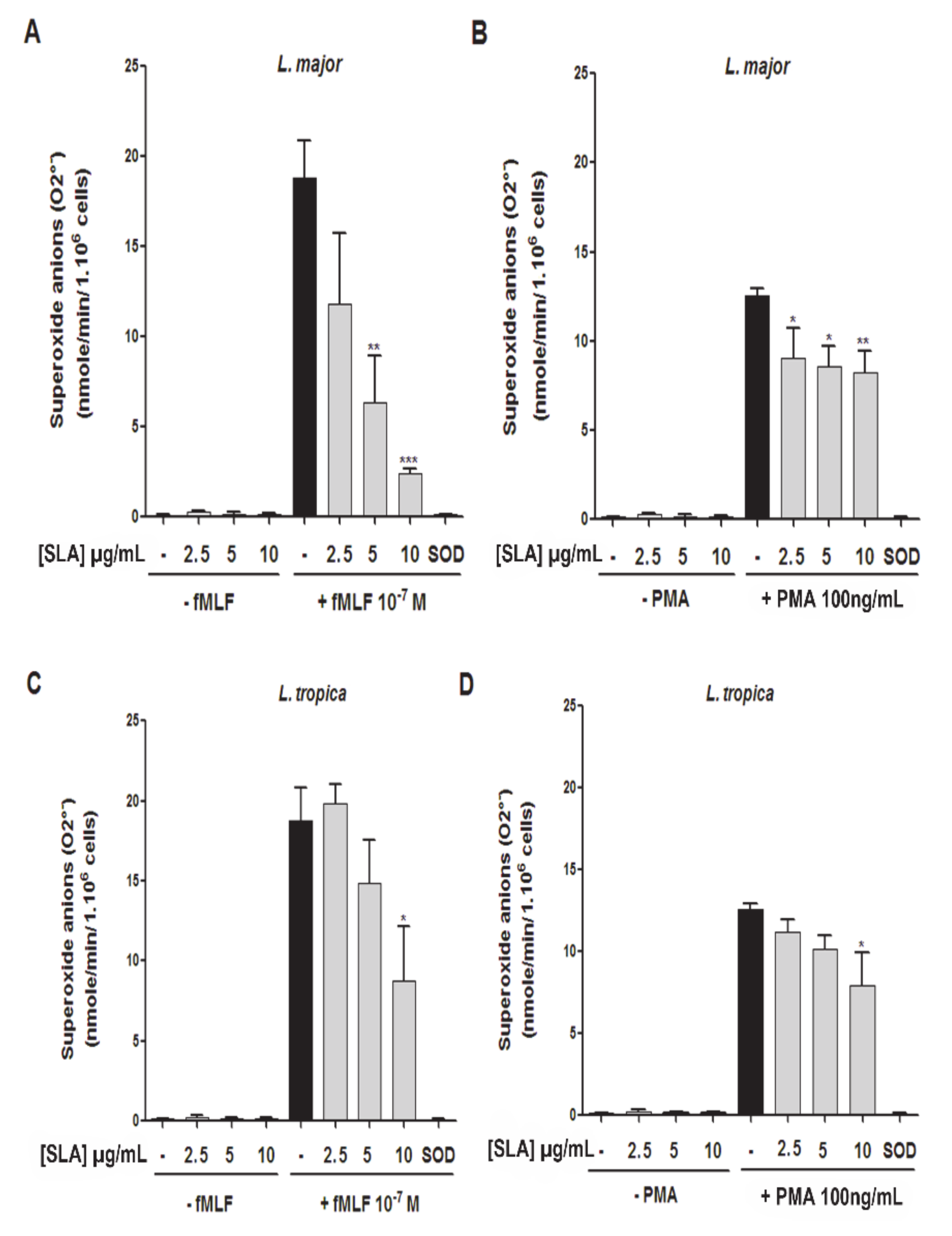
3.3. Impact of L. major and L. tropica SLAs on O2− Production by Human PMNs
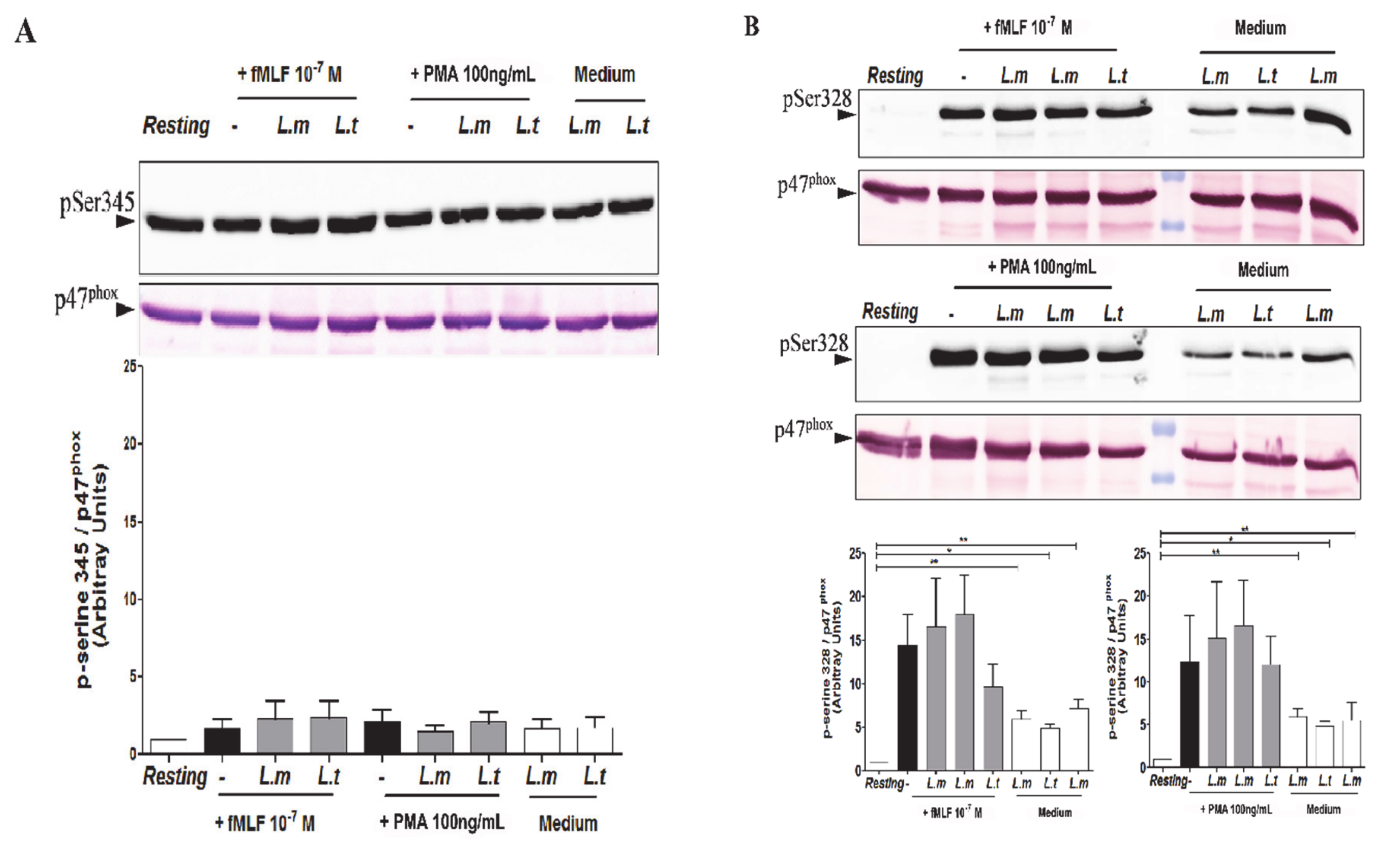
3.4. Impact of L. major and L. tropica Promastigotes on the Phosphorylation Status of p47phox in Human PMNs
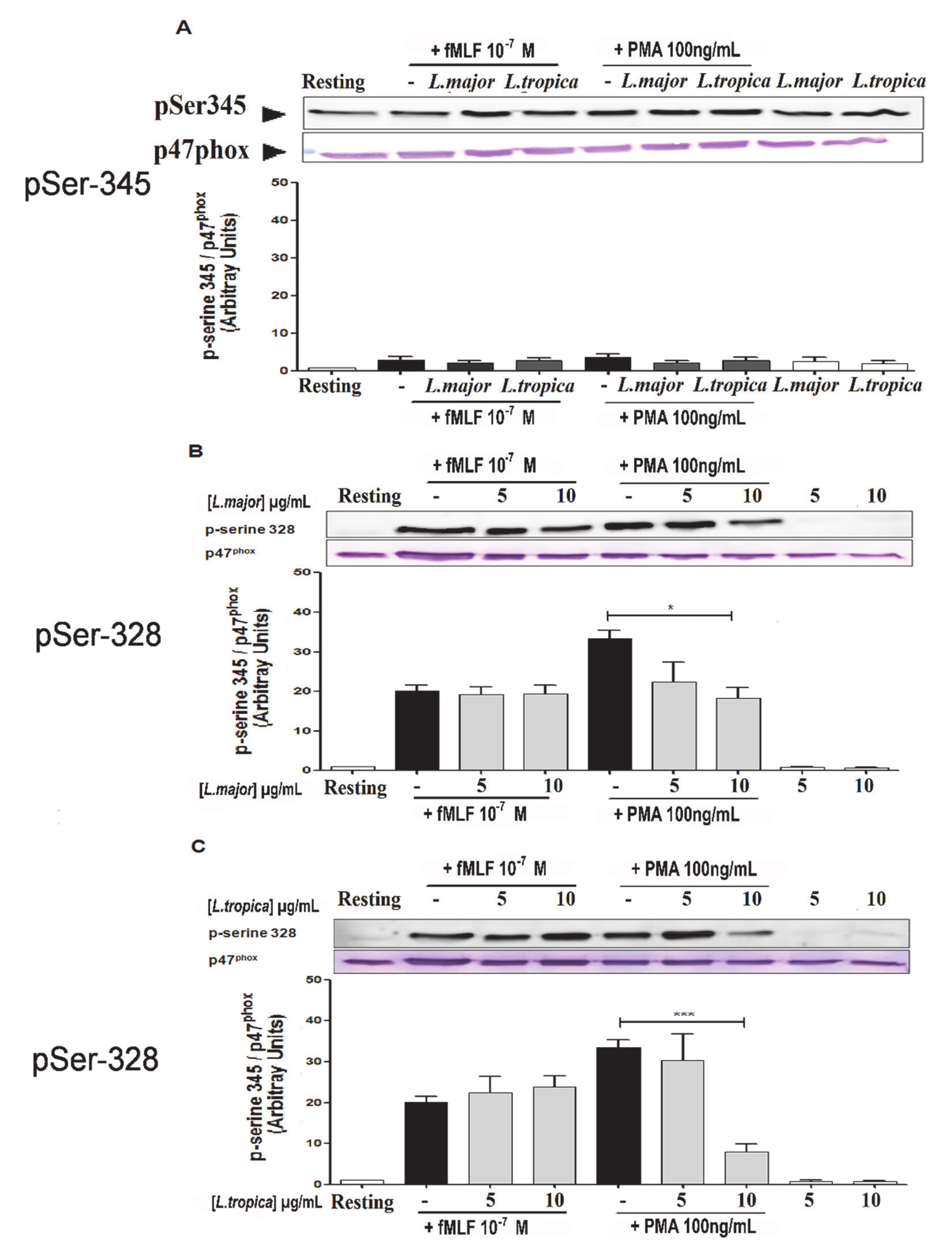
3.5. Impact of SLAs on p47phox Phosphorylation in Human PMNs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Georgiadou, S.P.; Makaritsis, K.P.; Dalekos, G.N. Leishmaniasis revisited: Current aspects on epidemiology, diagnosis and treatment. J. Transl. Intern. Med. 2015, 3, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef] [PubMed]
- Hakkour, M.; Hmamouch, A.; El Alem, M.M.; Rhalem, A.; Amarir, F.; Touzani, M.; Sadak, A.; Fellah, H.; Sebti, F. New epidemiological aspects of visceral and cutaneous leishmaniasis in Taza, Morocco. Parasites Vectors 2016, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rhajaoui, M.; Sebti, F.; Fellah, H.; Alam, M.; Nasereddin, A.; Abbasi, I.; Schönian, G. Identification of the causative agent of cutaneous leishmaniasis in Chichaoua province, Morocco. Parasite 2012, 19, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Christensen, S.M.; Belew, A.T.; El-Sayed, N.M.; Tafuri, W.L.; Silveira, F.T.; Mosser, D.M. Host and parasite responses in human diffuse cutaneous leishmaniasis caused by L. amazonensis. PLoS Negl. Trop. Dis. 2019, 13, e0007152. [Google Scholar] [CrossRef] [Green Version]
- Scorza, B.M.; Carvalho, E.M.; Wilson, M.E. Cutaneous Manifestations of Human and Murine Leishmaniasis. Int. J. Mol. Sci. 2017, 18, 1296. [Google Scholar] [CrossRef] [Green Version]
- Bañuls, A.L.; Bastien, P.; Pomares, C.; Arevalo, J.; Fisa, R.; Hide, M. Clinical pleiomorphism in human leishmaniases, with special mention of asymptomatic infection. Clin. Microbiol. Infect. 2011, 17, 1451–1461. [Google Scholar] [CrossRef] [Green Version]
- De Pablos, L.M.; Ferreira, T.R.; Walrad, P.B. Developmental differentiation in Leishmania lifecycle progression: Post-transcriptional control conducts the orchestra. Curr. Opin. Microbiol. 2016, 34, 82–89. [Google Scholar] [CrossRef]
- Hurrell, B.P.; Schuster, S.; Grün, E.; Coutaz, M.; Williams, R.A.; Held, W.; Malissen, B.; Malissen, M.; Yousefi, S.; Simon, H.-U.; et al. Rapid Sequestration of Leishmania mexicana by Neutrophils Contributes to the Development of Chronic Lesion. PLoS Pathog. 2015, 11, e1004929. [Google Scholar] [CrossRef]
- Ribeiro-Gomes, F.L.; Sacks, D. The influence of early neutrophil-Leishmania interactions on the host immune response to infection. Front. Cell. Infect. Microbiol. 2012, 2, 59. [Google Scholar] [CrossRef] [Green Version]
- Peters, N.C.; Egen, J.G.; Secundino, N.; Debrabant, A.; Kimblin, N.; Kamhawi, S.; Lawyer, P.; Fay, M.P.; Germain, R.N.; Sacks, D. In Vivo Imaging Reveals an Essential Role for Neutrophils in Leishmaniasis Transmitted by Sand Flies. Science 2008, 321, 970–974. [Google Scholar] [CrossRef] [Green Version]
- Tacchini-Cottier, F.; Zweifel, C.; Belkaid, Y.; Mukankundiye, C.; Vasei, M.; Launois, P.; Milon, G.; Louis, J.A. An Immunomodulatory Function for Neutrophils During the Induction of a CD4+Th2 Response in BALB/c Mice Infected withLeishmania major. J. Immunol. 2000, 165, 2628–2636. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, E.D.; Liang, Y.; Shelite, T.R.; Walker, D.H.; Melby, P.C.; Soong, L. Permissive and protective roles for neutrophils in leishmaniasis. Clin. Exp. Immunol. 2015, 182, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Laskay, T.; Van Zandbergen, G.; Solbach, W. Neutrophil granulocytes—Trojan horses for Leishmania major and other intracellular microbes? Trends Microbiol. 2003, 11, 210–214. [Google Scholar] [CrossRef]
- Maksouri, H.; Dang, P.M.-C.; Rodrigues, V.; Estaquier, J.; Riyad, M.; Akarid, K. Moroccan strains of Leishmania major and Leishmania tropica differentially impact on nitric oxide production by macrophages. Parasites Vectors 2017, 10, 506. [Google Scholar] [CrossRef] [Green Version]
- Horta, M.F.; Mendes, B.P.; Roma, E.H.; Noronha, F.S.M.; Macêdo, J.P.; Oliveira, L.S.; Duarte, M.M.; Vieira, L.Q. Reactive Oxygen Species and Nitric Oxide in Cutaneous Leishmaniasis. J. Parasitol. Res. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Re, D.; Cj, T.; Me, W. Infection and Activation of Human Neutrophils with Fluorescent Leishmania infantum. J. Immunol. Tech. Infect. Dis. 2016, 5, 1–13. [Google Scholar] [CrossRef]
- Gupta, G.; Oghumu, S.; Satoskar, A.R. Mechanisms of Immune Evasion in Leishmaniasis. In Advances in Virus Research Volume 23; Elsevier BV: Amsterdam, The Netherlands, 2013; Volume 82, pp. 155–184. [Google Scholar]
- Oualha, R.; Barhoumi, M.; Marzouki, S.; Harigua-Souiai, E.; Ben Ahmed, M.; Guizani, I. Infection of Human Neutrophils with Leishmania infantum or Leishmania major Strains Triggers Activation and Differential Cytokines Release. Front. Cell. Infect. Microbiol. 2019, 9, 153. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, E.D.; Jie, Z.; Liang, Y.; Henard, C.A.; Hay, C.; Sun, J.; Guedes, H.D.M.; Soong, L. Interactions between Neutrophils and Leishmania braziliensis Amastigotes Facilitate Cell Activation and Parasite Clearance. J. Innate Immun. 2015, 7, 354–363. [Google Scholar] [CrossRef]
- El-Benna, J.; Hurtado-Nedelec, M.; Marzaioli, V.; Marie, J.-C.; Gougerot-Pocidalo, M.-A.; Dang, P.M.-C. Priming of the neutrophil respiratory burst: Role in host defense and inflammation. Immunol. Rev. 2016, 273, 180–193. [Google Scholar] [CrossRef]
- Belambri, S.A.; Rolas, L.; Raad, H.; Hurtado-Nedelec, M.; Dang, P.M.-C.; El-Benna, J. NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits. Eur. J. Clin. Investig. 2018, 48, e12951. [Google Scholar] [CrossRef] [Green Version]
- Mouttaki, T.; Morales-Yuste, M.; Merino-Espinosa, G.; Chiheb, S.; Fellah, H.; Martín-Sánchez, J.; Riyad, M. Molecular diagnosis of cutaneous leishmaniasis and identification of the causative Leishmania species in Morocco by using three PCR-based assays. Parasites Vectors 2014, 7, 420. [Google Scholar] [CrossRef] [Green Version]
- Dey, R.; Majumder, N.; Majumdar, S.B.; Bhattacharjee, S.; Banerjee, S.; Roy, S.; Majumdar, S. Induction of Host Protective Th1 Immune Response by Chemokines in Leishmania donovani-infected BALB/c Mice. Scand. J. Immunol. 2007, 66, 671–683. [Google Scholar] [CrossRef]
- Dang, P.M.-C.; Stensballe, A.; Boussetta, T.; Raad, H.; Dewas, C.; Kroviarski, Y.; Hayem, G.; Jensen, O.N.; Gougerot-Pocidalo, M.-A.; El-Benna, J. A specific p47phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J. Clin. Investig. 2006, 116, 2033–2043. [Google Scholar] [CrossRef] [Green Version]
- Belambri, S.A.; Dang, P.M.-C.; El-Benna, J. Evaluation of p47phox Phosphorylation in Human Neutrophils Using Phospho-Specific Antibodies. Methods Mol. Biol. 2014, 1124, 427–433. [Google Scholar] [CrossRef]
- Chan, J.; Fujiwara, T.; Brennan, P.; McNeil, M.; Turco, S.J.; Sibille, J.C.; Snapper, M.; Aisen, P.; Bloom, B.R. Microbial glycolipids: Possible virulence factors that scavenge oxygen radicals. Proc. Natl. Acad. Sci. USA 1989, 86, 2453–2457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, A.; Singh, K.P.; Mandal, A.; Paswan, R.K.; Sinha, P.; Das, P.; Ali, V.; Bimal, S.; Lal, C.S. Intracellular zinc flux causes reactive oxygen species mediated mitochondrial dysfunction leading to cell death in Leishmania donovani. PLoS ONE 2017, 12, e0178800. [Google Scholar] [CrossRef]
- Lodge, R.; Diallo, T.O.; Descoteaux, A. Leishmania donovani lipophosphoglycan blocks NADPH oxidase assembly at the phagosome membrane. Cell. Microbiol. 2006, 8, 1922–1931. [Google Scholar] [CrossRef]
- Fontayne, A.; Dang, P.M.-C.; Gougerot-Pocidalo, A.M.-A.; El Benna, J. Phosphorylation of p47phoxSites by PKC α, βΙΙ, δ, and ζ: Effect on Binding to p22phoxand on NADPH Oxidase Activation. Biochemistry 2002, 41, 7743–7750. [Google Scholar] [CrossRef] [PubMed]
- Boussetta, T.; Gougerot-Pocidalo, M.-A.; Hayem, G.; Ciappelloni, S.; Raad, H.; Derkawi, R.A.; Bournier, O.; Kroviarski, Y.; Zhou, X.Z.; Malter, J.S.; et al. The prolyl isomerase Pin1 acts as a novel molecular switch for TNF-α–induced priming of the NADPH oxidase in human neutrophils. Blood 2010, 116, 5795–5802. [Google Scholar] [CrossRef] [Green Version]
- Kahime, K.; Boussaa, S.; Idrissi, A.L.-E.; Nhammi, H.; Boumezzough, A. Epidemiological study on acute cutaneous leishmaniasis in Morocco. J. Acute Dis. 2016, 5, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Regli, I.B.; Passelli, K.; Hurrell, B.P.; Tacchini-Cottier, F. Survival Mechanisms Used by Some Leishmania Species to Escape Neutrophil Killing. Front. Immunol. 2017, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Ricci-Azevedo, R.; Oliveira, A.F.; Conrado, M.C.A.V.; Carvalho, F.C.; Roque-Barreira, M.C. Neutrophils Contribute to the Protection Conferred by ArtinM against Intracellular Pathogens: A Study on Leishmania major. PLoS Negl. Trop. Dis. 2016, 10, e0004609. [Google Scholar] [CrossRef] [Green Version]
- Salei, N.; Hellberg, L.; Köhl, J.; Laskay, T. Enhanced survival of Leishmania major in neutrophil granulocytes in the presence of apoptotic cells. PLoS ONE 2017, 12, e0171850. [Google Scholar] [CrossRef] [Green Version]
- Ago, T.; Nunoi, H.; Ito, T.; Sumimoto, H. Mechanism for Phosphorylation-induced Activation of the Phagocyte NADPH Oxidase Protein p47. J. Biol. Chem. 1999, 274, 33644–33653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollinedo, F.; Janssen, H.; de la Iglesia-Vicente, J.; Villa-Pulgarin, J.A.; Calafat, J. Selective Fusion of Azurophilic Granules with Leishmania-containing Phagosomes in Human Neutrophils. J. Biol. Chem. 2010, 285, 34528–34536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho-Finamore, J.; Freitas, V.; Assis, R.; Melo, M.; Novozhilova, N.; Secundino, N.; Pimenta, P.; Turco, S.; Soares, R. Leishmania infantum: Lipophosphoglycan intraspecific variation and interaction with vertebrate and invertebrate hosts. Int. J. Parasitol. 2011, 41, 333–342. [Google Scholar] [CrossRef] [Green Version]
- McConville, M.J.; Schnur, L.F.; Jaffe, C.; Schneider, P. Structure of Leishmania lipophosphoglycan: Inter- and intra-specific polymorphism in Old World species. Biochem. J. 1995, 310, 807–818. [Google Scholar] [CrossRef] [Green Version]
- Dang, P.M.-C.; Fontayne, A.; Hakim, J.; El Benna, J.; Périanin, A. Protein Kinase C ζ Phosphorylates a Subset of Selective Sites of the NADPH Oxidase Component p47phoxand Participates in Formyl Peptide-Mediated Neutrophil Respiratory Burst. J. Immunol. 2001, 166, 1206–1213. [Google Scholar] [CrossRef] [Green Version]
- Hassani, K.; Shio, M.T.; Martel, C.; Faubert, D.; Olivier, M. Absence of Metalloprotease GP63 Alters the Protein Content of Leishmania Exosomes. PLoS ONE 2014, 9, e95007. [Google Scholar] [CrossRef] [Green Version]
- Akarid, K.; Arnoult, D.; Micic-Polianski, J.; Sif, J.; Estaquier, J.; Ameisen, J.C. Leishmania major-mediated prevention of programmed cell death induction in infected macrophages is associated with the repression of mitochondrial release of cytochrome c. J. Leukoc. Biol. 2004, 76, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Moreira, D.; Rodrigues, V.; Abengozar, M.; Rivas, L.; Rial, E.; LaForge, M.; Li, X.; Foretz, M.; Viollet, B.; Estaquier, J.; et al. Leishmania infantum Modulates Host Macrophage Mitochondrial Metabolism by Hijacking the SIRT1-AMPK Axis. PLoS Pathog. 2015, 11, e1004684. [Google Scholar] [CrossRef] [Green Version]
- Descoteaux, A.; Matlashewski, G.; Turco, S.J. Inhibition of macrophage protein kinase C-mediated protein phosphorylation by Leishmania donovani lipophosphoglycan. J. Immunol. 1992, 149, 3008–3015. [Google Scholar]
- Pitale, D.M.; Gendalur, N.S.; Descoteaux, A.; Shaha, C. Leishmania donovani Induces Autophagy in Human Blood–Derived Neutrophils. J. Immunol. 2019, 202, 1163–1175. [Google Scholar] [CrossRef] [Green Version]
- Baghad, B.; Razanapinaritra, R.; Maksouri, H.; El Bouri, H.; Outlioua, A.; Fellah, H.; Lemrani, M.; Akarid, K.; Martin-Sanchez, J.; Chiheb, S.; et al. Possible introduction of Leishmania tropica to urban areas determined by epidemiological and clinical profiles of patients with cutaneous leishmaniasis in Casablanca (Morocco). Parasite Epidemiol. Control 2020, 9, e00129. [Google Scholar] [CrossRef]
- Rostamian, M.; Jafari, D.; Abolghazi, M.; Farahani, H.; Niknam, H.M. Leishmania tropica: Suggestive evidences for the effect of infectious dose on pathogenicity and immunogenicity in an experimental model. Parasitol. Res. 2018, 117, 2949–2956. [Google Scholar] [CrossRef]
- Rodrigues, V.; André, S.; Maksouri, H.; Mouttaki, T.; Chiheb, S.; Riyad, M.; Akarid, K.; Estaquier, J. Transcriptional Analysis of Human Skin Lesions Identifies Tryptophan-2,3-Deoxygenase as a Restriction Factor for Cutaneous Leishmania. Front. Cell. Infect. Microbiol. 2019, 9, 338. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksouri, H.; Darif, D.; Estaquier, J.; Riyad, M.; Desterke, C.; Lemrani, M.; Dang, P.M.-C.; Akarid, K. The Modulation of NADPH Oxidase Activity in Human Neutrophils by Moroccan Strains of Leishmania major and Leishmania tropica Is Not Associated with p47phox Phosphorylation. Microorganisms 2021, 9, 1025. https://doi.org/10.3390/microorganisms9051025
Maksouri H, Darif D, Estaquier J, Riyad M, Desterke C, Lemrani M, Dang PM-C, Akarid K. The Modulation of NADPH Oxidase Activity in Human Neutrophils by Moroccan Strains of Leishmania major and Leishmania tropica Is Not Associated with p47phox Phosphorylation. Microorganisms. 2021; 9(5):1025. https://doi.org/10.3390/microorganisms9051025
Chicago/Turabian StyleMaksouri, Hasnaa, Dounia Darif, Jerome Estaquier, Myriam Riyad, Christophe Desterke, Meryem Lemrani, Pham My-Chan Dang, and Khadija Akarid. 2021. "The Modulation of NADPH Oxidase Activity in Human Neutrophils by Moroccan Strains of Leishmania major and Leishmania tropica Is Not Associated with p47phox Phosphorylation" Microorganisms 9, no. 5: 1025. https://doi.org/10.3390/microorganisms9051025





